Abstract
Background and objectives: Actinic keratosis (AK) is a frequent cutaneous lesion usually developing on sun-exposed skin and may be evolving into invasive squamous cell carcinoma requiring expensive therapy. Size, site or number of lesions limits the efficacy and/or acceptability of surgical and chemical therapies. Up to now photodynamic therapy (PDT) is recommended as treatment of choice. Material and methods: A patient (75y, m) suffered from recalcitrant AK lesions on the scalp, forearms and face for more than 20y. Various treatments including PDT, ablative laser and cryotherapy or chemical treatments failed to eradicate these lesions especially on the scalp. We used a CE-certified cold atmospheric plasma (CAP) jet, the Maxium® electrosurgery unit with maxium® beamer (KLS Martin GmbH + Co. KG), to treat one scalp lesion in one session (60 s, 20 W, 6 L/min). Results: CAP was able to eradicate AK of the patient in one session and was well tolerated. Histologic examination showed complete cure of AK. Control histology and visits up to 26 months after treatment do not show relapse or other skin deterioration. Conclusion: CAP seems to be an effective, curative and economic (one single treatment) alternative to conventional treatment of recalcitrant AK with field cancerization with excellent tolerability.
Public Interest Statement
Actinic keratosis (AK) is a cutaneous lesion. Up to 10% progress to carcinoma which requires surgical intervention. In patients with field cancerization photodynamic ALA-therapy is recommended but its limitations (pain, tumor resistance) underline the need for alternatives. Since cold plasma (CAP) was shown to kill tumor cells and significant responsive against malignant melanoma in a mouse model we wanted to trial its efficacy against AK in a 75 years old male patient. We treated one scalp lesion in a single 60s session. The treated area showed local circumscribed inflammation over 1 week, than the wound showed undisturbed healing without relapsing lesions. No unexpected local or systemic side effects occurred. The patient reported only a slight pain immediately after treatment and no pain afterwards. So CAP seems suited for curative treatment of AK and showed excellent tolerability. It seems an effective and economic alternative to conventional treatment of AK.
Competing Interests
The authors declare no competing interest.
1. Introduction
Among the most frequent cancer types in the Caucasian population, the non-melanoma skin cancer (NMSC), basal cell carcinoma (BCC) and squamous cell carcinoma (SCC) are most common (Fernández-Figueras et al., Citation2015). Actinic keratosis (AK) typically develops after long term UV exposure as hyperkeratotic, often erosive single or multiple (field cancerization) lesions of sun exposed areas like scalp, face, forearms, upper breast and back. AK can be seen as special form of SCC but at a different stage of evolution (Fernández-Figueras et al., Citation2015; Patel, Frankel, & Goldenberg, Citation2011). AK lesions are treated because they may be precursors of invasive SCC. Between 5 and 10% of AK progress to SCC and/or seldom Bowen’s disease (BD) requiring extensive and expensive therapy (Ericson, Wennberg, & Larkö, Citation2008; Patel et al., Citation2011).
Surgical interventions are the most chosen approaches to treat AK, SCC and BCC if there are single tumor entitites. For multiple tumor, larger surfaces and especially patients with field cancerization non-invasive approaches like cryotherapy, topical 5-fluorouracil (5%, 1%, 0.5%), imiquimod (5%, 3.75%), ingenol mebutate (0.05%, 0.015%), diclofenac, topical retinoids, chemical peels and PDT are more attractive. Among them PDT actually seems the most promising alternative (Eisemann et al., Citation2014; Morton et al., Citation2015; Sidoroff & Thaler, Citation2010).
PDT works via activation of a photosensitizer (i.e. 5-aminolevulinic acid [ALA] or its methylated derivate [MAL]) by photons (of adequate wavelength) causing the production of singlet oxygen and other reactive oxygen species (ROS). These ROS selectively kill all those cells incorporating the photosensitizer. Following topical or systemic ALA application Protoporphyrin IX (PpIX) selectively accumulates in the irradiated (tumor) cells and delivers cytotoxic ROS. PDT allows repeated non-invasive treatment, reaches significant skin surface (field treatment) with one single treatment (i.e. total scalp or forearm) and provides optimum cosmetic outcomes. In case of recalcitrant tumor growth PDT can be combined with other therapeutic modalities like immunomodulation (imiquimod), chemotherapeutics (5-fluorouracil, ingenol mebutate, methotrexate, diclofenac) and in some cases also radiotherapy (Morton et al., Citation2015).
However PDT may have some disadvantages. One could be that patients probably feel strong discomfort during the irradiation and the consecutive inflammation phase of the treatment. Albeit strong supportive means like cooling, pain relief and skin care, these patient suffer sometimes over several days and finally refuse further treatment. This is a particular disadvantage since relapses under PDT may occur hence cutting the option for further PDT in these patients. Meanwhile a completely new therapeutic approach, the cold plasma therapy (CAP) was introduced in tumor therapy. Since we were able to show significant antitumor efficacy by CAP against malignant Melanoma in a mouse model (Daeschlein et al., Citation2013), we can deduce also CAP-efficacy against NMSC. To proof, whether CAP may be able to overcome the described disadvantages of PDT we treated one patient with recalcitrant AK with CAP in a single treatment session and monitored clinical and histological outcome.
2. Materials and methods
2.1. Patient
The treatment was conducted January 2014 at the Dermatology Service, Greifswald University, Germany. The patient conditions were the following. Male, 75y, more than 20 clinical grade I and/or II AK located on the scalp for over 20y, no skin lesions within the target area interfering with next clinical evaluations, no AK treatment during the last 3 month, no immunosuppression. Treatment was conducted in accordance with the Declaration of Helsinki. The patient provided written informed consent before treatment initiation.
2.2. Plasma source
We used a CE-certified commercial CAP jet, the Maxium® electrosurgery unit with maxium® beamer and beam electrode (Gebrüder Martin GmbH + Co. KG), within its intended use (monopolar argon-assisted coagulation).
A handpiece is electrically connected to the maxium® beamer for the argon supply. After starting the high-frequency current, the argon gas is propelled through a window integrated in the feedline and reaches the operating site via the tube electrode in the handpiece. The high voltage of the maxium® allows the ignition of the Argon-air mixture at the nozzle of the beam-electrode, ionizing the gas to produce the conductible CAP, which is finally emitted onto the operation field. The settings used were: Cutting and plasma beam, power 20 W, argon gas-flow rate 6 L/min. The visible diameter of the plasma beam on the target takes approximately 5 mm (corresponding distance between wound surface to the tip of the device about 0.5 to 1 cm). Temperature at the tip of the device was measured using a laser- assisted infrared thermometer (model VA 6520, Komerci oHG, Ebern, Germany) and averaged 25.1°C.
2.3. Therapeutic protocol
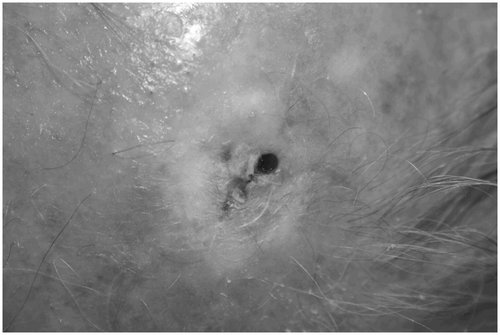
The target area with multiple AK lesions (field cancerization) was digitally photographed and the most active lesion, a hyperkeratotic and erosive lesion of 10 × 10 mm, was selected for treatment. Prior to treatment scales and crusts were gently removed by a wetted (sterile saline) swab after performing skin antisepsis with octenidin dihydrochloride. Subsequently, local anaesthesia was applied by infiltration of a sterile solution consisting of 0.5 ml of adrenaline (1/1,000), 20 ml of ropivacaine hydrochloride (10%), and 20 ml of lidocaine hydrochloride (2%). Then, we removed hyperkeratotic material by curettage. Afterwards a punch biopsy (3 mm) was taken and directly sealed by one stitch suture. After the suture of the punch biopsy had been covered by a non-transparent wound plaster, the neighbouring skin lesion was treated by CAP which was performed at a distance of 1 to 2 cm above the skin surface by slowly moving the plasma beam over the wound surface in a meandering pattern (Figure ). The CAP treatment took about one minute and the patient was asked during and after therapy for pain and satisfaction with the treatment (VAS). The patient underwent one single-session of CAP treatment.
After treatment the patient was advised to present in any case of new symptoms like pain, sensations or wound deterioration like bleeding.
The wound was visited daily for one week after treatment. Further controls were done after one, two, three, 6, 9, 12, 15, 24 and 26 months after treatment. We checked for relapse, cosmetic outcome and patient preference. Pain was asked during treatment and daily up to one week. (NAS score 1 to 10/10). Directly before and 9 weeks after treatment a biopsy was taken and conventional histology performed (H&E stain).
3. Results
The area treated with CAP showed local circumscribed inflammation over 1 week followed by undisturbed wound healing without relapsing actinic lesions until the last visit up to now (26 month). In Figures – the lesions area before, directly after, and 26 months after treatment is shown. Nine weeks after treatment scarring was observed and histologically proven.
Regarding tolerability, no unexpected local or systemic treatment-related side effects obtained. Pain was scored 3/10 (NAS) directly after treatment and 0/10 afterwards. Afterwards no pain was scored. Furthermore CAP was associated with good cosmetic outcome, and the tissue became inconspicuously scarred. Concerning patient satisfaction the VAS satisfaction score was 100% (10/10).
3.1. Histologic examination (right parietal capillitium)
3.1.1. One week before CAP treatment
Hyperparakeratosis, irregular cell stratification, abundant and pronounced atypical nuclei, intradermal lymphocytic inflammation and infiltration with plasma cells, abundant elastosis. Diagnosis: progressed AK.
3.1.2. Nine weeks after CAP treatment
Regular epidermal stratification, orthokeratosis, in the corium grouped bundles of collagenous fibers with increased numbers of fibrocytes, some mononuclear infiltrates. Diagnosis: Non disturbed scarification.
4. Discussion
The repertoire to treat isolated or multiple lesions of AK includes surgical excision, electrosurgery, laser ablation, cryotherapy and PDT but also chemical and pharmacological treatments (Ferrándiz et al., Citation2014).
Additionally these treatments may be combined. Regarding efficacy, tolerability, cosmetic outcome and acceptance by patients PDT can be highlighted as the most convenient alternative among all cited treatments up to now (Borroni, Carugno, Rivetti, Arbustini, & Brazzelli, Citation2013; Braathen et al., Citation2007; Lehmann, Citation2007; Marmur, Schmults, & Goldberg, Citation2004; Morton, Citation2004; Steinbauer et al., Citation2010; Zhao & He, Citation2010).
The reported complete response rates of (MAL-) PDT are 91% in BCC at three months and 76% at five years of follow up, and 86 to 93% in patients with Bowen’s disease (BD) at three months and 68 to 71% at 24 months (Morton et al., Citation2006).
Further advantages of PDT include the possibility of combining it with other therapies and repeating the process until satisfying cosmetic results are obtained. As mentioned in the introduction section, PDT has some limitations, mainly pain during and after irradiation and the possibility of resistance (Attili, Dawe, & Ibbotson, Citation2011; Gholam, Denk, Sehr, Enk, & Hartmann, Citation2010; Kasche, Luderschmidt, Ring, & Hein, Citation2006). Another treatment option, radiotherapy, which was used in cases of recalcitrant AK with SCC development over decades, is now abandoned because of the side effects of ionizing radiation in favour of combined modern therapies. Accordingly, radiotherapy today is restricted to some rare patients i.e. with SCC, which cannot be operated or with extended age (Rong, Zuo, Shang, & Bazan, Citation2015).
It should be emphasized that pain may be involved in the development of increasing blood pressure sometimes associated even with postoperative hypertension and hypertensive crisis (Borroni et al., Citation2013).
Twenty percent of patients report intense and 80% moderate pain under PDT (Kasche et al., Citation2006). This pain seems to be a consequence from nerve hyperstimulation by irradiation and heat development.
There may be different reasons concerning the negative response of AK to PDT. The tumor thickness as a limiting factor is one of them and well known i.e. for BCC (McKay et al., Citation2013) with tumor thickness ≥ 0.4 mm. Nodular BCC, which are deeper than 2 mm, are even excluded for PDT (Braathen et al., Citation2007).
CAP could act more independently from those limitations. We could show well responsiveness to CAP in a mouse melanoma model (Daeschlein et al., Citation2013) while directly treating the tumor without prior reduction of the tissue between tumor and plasma source. Finally it was another tumor entity (melanoma), another CAP device and another mode of therapy and we cannot exclude that i.e. a bulk reduction prior to CAP treatment would have caused an even more pronounced antitumor effect. Therefore and for better comparison with PDT we removed hyperkeratotic plaques (curettage) in our patient prior to CAP-treatment. In contrary to PDT we are also not excluding thicker tumor for CAP treatment. Regarding the limitations for PDT associated with special localizations like limbs (more resistance of AK lesions in comparison with face and skin) (Kaufmann et al., Citation2008; Morton, Szeimies, Sidoroff, & Braathen, Citation2013) and fingers (more resistance of BD) no such data exist for CAP treatment up to now. Since CAP effects do not base upon the same principles like PDT, except the ROS production, we do not expect these limitations for CAP treatment.
Anti-tumor effects of PDT depend on three mechanisms, the direct cytotoxicity on tumor cells, and two indirect effects. Those are the damage of tumor vasculature, and the tumor inactivation via the activation of the cellular immune response. The cytotoxic effects mainly depend on three factors-1. Selective uptake of the photosensitizer by tumor cells, 2. Accessibility of sufficient oxygen levels in the treated tissue and 3. The adequate dosage of irradiation. In our patient with recalcitrant AK, at least one of these factors seems responsible for PDT failure but interestingly not for CAP failure. Accordingly we can assume that CAP is suitable for the treatment of recalcitrant AK after PDT. The background of this hypothesis needs clarification in order to optimize CAP and PDT. The action of ROS (and singlet oxygen by PDT) is the working principle of both therapies. The other effects are quite distinct. While ROS generation is the main principle killing the tumor cells after PDT, the antitumor repertoire of CAP is much more complex. Besides ROS production electrical fields, electrons, reactive gas species, ozone, UV and RNS (reactive nitrogen species) (Tiede et al., Citation2014) were produced.
However, the mechanisms by which tumor tissue can be affected by CAP are not fully understood yet. Moreover it is not known if CAP may induce tumor cell resistance as it was reported for PDT (Bardazzi et al., Citation2015; Fiechter et al., Citation2012; Maydan, Nooothet, & Goldman, Citation2006).
Recently daylight PDT (D-PDT) was introduced to simplify the PDT procedure and to overcome some of the above mentioned limitations (pain and inflammation). Hereby sunlight is used as radiation source. With this innovative development preliminary occlusion is not necessary. Furthermore significantly reduced pain and inflammation during therapy is reported. Another option for this purpose is the use of MAL- instead of ALA-PDT (Rubel et al., Citation2014; Wiegell et al., Citation2012).
Although tumor therapy undoubtedly benefits from these modifications of PDT, CAP treatment has the potential to overcome the limitations of all current treatment options including D-PDT. These potential advantages are exceptional short duration of treatment (1 min for 1 × 1 cm surface), no extensive pre-treatment (topical ALA or MAL ointment), minimal pain during (after local anaesthesia injection) and no pain after treatment and finally curative treatment after one session. The excellent tolerability, during and after treatment, can be explained partially by favourable healing characteristics with non-complicated wound closure and minimal inflammation. This may be due to the former described beneficial effects of CAP regarding wound healing (Daeschlein et al., Citation2015).
Altogether, CAP has the potential to significantly shorten and simplify the therapy of AK, what has to be evaluated and verified in clinical trials. Up to now despite an impressive number of reports regarding CAP susceptibility of all kind of human tumor cells in vitro, no data upon clinical effectiveness are reported. In our patient CAP was shown the first successful AK treatment without relapse up to 26 month after one application. The AK of the patient was recalcitrant over years after several treatments like PDT (2 sessions) and ingenol mebutate (applicated over years), diclofenac (3%, 2 times a day for 5 years), CO2-laser ablation and cryotherapy (one treatment each).
In summary, to overcome the limitations of particular treatments i.e. the development of resistance and the toxicity followed by adverse effects, diverse therapeutic modalities are combined, thus enhancing oncologic treatments. Among a bundle of different treatment modalities PDT seems more beneficial in patients in whom the number of lesions, their size, and the site limit the efficacy and acceptability of conventional therapies. Thus, PDT is perhaps the best therapeutic option, especially in patients with field cancerization (3–5) up to now. However, PDT has some limitations, mainly pain and obviously tumor resistance. To overcome those limitations D-PDT was introduced. However, CAP as new therapeutic non-invasive antitumor treatment could overcome therapeutic failure under conventional AK treatment with best tolerability. Its antitumor efficacy is flanked by healing supporting properties.
5. Conclusion
In this case report we could show that CAP seems to be a promising candidate in AK therapy. After only one treatment with CAP recalcitrant AK located at the scalp of the patient no actinic lesions relapse until last visit (26 months after treatment) and scar formation could be proven. In opposite to PDT treatment the patient does not claim any relevant pain. Evaluation and verification of these results during clinical trials is the next necessary step.
| Abbreviations | ||
| CAP | = | cold atmospheric plasma |
| AK | = | actinic keratosis |
| PDT | = | photodynamic therapy |
| D-PDT | = | daylight photodynamic therapy |
| SCC | = | squamous cell carcinoma |
| NMSC | = | non-melanoma skin cancer |
| BCC | = | basal cell carcinoma |
| BD | = | Bowen´s disease |
| ALA | = | 5-aminolevulinic acid |
| MAL | = | methylated derivate of ALA |
| ROS | = | reactive oxygen species |
| RNS | = | reactive nitrogen species |
| PpIX | = | protoporphyrin IX |
| VAS | = | visual anlalogue scale |
Funding
The cold plasma units and consumables used were kindly lent by KLS Martin GmbH + Co. KG, Germany GmbH.
Additional information
Notes on contributors
Georg Daeschlein
The head of the working group PD Dr med Georg Daeschlein is a long-term employee at the Dermatology Clinic of the University Medicine Greifswald and Head of the research group for Plasma Medicine and Antimicrobial Methods. He is a specialist in laboratory medicine, for medical microbiology and infection epidemiology, for hygiene and environmental medicine and for dermatology and venereology.
References
- Attili, S. K., Dawe, R., & Ibbotson, S. (2011). A review of pain experienced during topical photodynamic therapy – Our experience in Dundee. Photodiagnosis and Photodynamic Therapy, 8, 53–57.10.1016/j.pdpdt.2010.12.008
- Bardazzi, F., Loi, C., Magnano, M., Burtica, E. C., Giordano, F., & Patrizi, A. (2015). Methyl-aminolevulinic acid photodynamic therapy for actinic keratoses: A useful treatment or a risk factor? A retrospective study Journal of Dermatological Treatment, 26, 168–170.10.3109/09546634.2014.915004
- Borroni, R. G., Carugno, A., Rivetti, N., Arbustini, E., & Brazzelli, V. (2013). Risk of acute postoperative hypertension after topical photodynamic therapy for non-melanoma skin cancer. Photodermatology, Photoimmunology & Photomedicine, 29, 73–77.10.1111/phpp.2013.29.issue-2
- Braathen, L. R., Szeimies, R. M., Basset-Seguin, N., Bissonnette, R., Foley, P., Pariser, D., … Morton, C. A. (2007). Guidelines on the use of photodynamic therapy for nonmelanoma skin cancer: An international consensus. Journal of the American Academy of Dermatology, 56, 125–143.10.1016/j.jaad.2006.06.006
- Daeschlein, G., Napp, M., Lutze, S., Arnold, A., Podewils, S., Guembel, D., & Jünger, M. (2015). Skin and wound decontamination of multidrug-resistant bacteria by cold atmospheric plasma coagulation. Journal der Deutschen Dermatologischen Gesellschaft, 13(2), 143–149.
- Daeschlein, G., Scholz, S., Lutze, S., Arnold, A., Podewils, S., Kiefer, T., … Langner, S. (2013). Comparison between cold plasma, electrochemotherapy and combined therapy in a melanoma mouse model. Experimental Dermatology, 22(9), 582–586.10.1111/exd.12201
- Eisemann, N., Waldmann, A., Geller, A. C., Weinstock, M. A., Volkmer, B., Greinert, R., … Katalinic, A. (2014). Non-melanoma skin cancer incidence and impact of skin cancer screening on incidence. Journal of Investigative Dermatology, 134, 43–50.10.1038/jid.2013.304
- Ericson, M. B., Wennberg, A. M., & Larkö, O. (2008). Review of photodynamic therapy in actinic keratosis and basal cell carcinoma. Therapeutics and Clinical Risk Management, 4, 1–9.
- Fernández-Figueras, M. T., Carrato, C., Sáenz, X., Puig, L., Musulen, E., Ferrándiz, C., & Ariza, A. (2015). Actinic keratosis with atypical basal cells (AK I) is the most common lesion associated with invasive squamous cell carcinoma of the skin. Journal of the European Academy of Dermatology and Venereology, 29, 991–997.10.1111/jdv.2015.29.issue-5
- Ferrándiz, C., Fonseca-Capdevila, E., García-Diez, A., Guillén-Barona, C., Belinchón-Romero, L., Redondo-Bellón, P., … Senán, R. (2014). Spanish adaptation of the European guidelines for the evaluation and treatment of actinic keratosis. Actas Dermo-Sifiliográficas, 105, 378–393.10.1016/j.ad.2013.11.013
- Fiechter, S., Skaria, A., Nievergelt, H., Anex, R., Borradori, L., & Parmentier, L. (2012). Facial basal cell carcinomas recurring after photodynamic therapy: A retrospective analysis of histological subtypes. Dermatology, 224, 346–351.10.1159/000339335
- Gholam, P., Denk, K., Sehr, T., Enk, A., & Hartmann, M. (2010). Factors influencing pain intensity during topical photodynamic therapy of complete cosmetic units for actinic keratoses. Journal of the American Academy of Dermatology, 63, 213–218.10.1016/j.jaad.2009.08.062
- Kasche, A., Luderschmidt, S., Ring, J., & Hein, R. (2006). Photodynamic therapy induces less pain in patients treated with methyl aminolevulinate compared to aminolevulinic acid. Journal of Drugs in Dermatology: JDD, 5, 353–356.
- Kaufmann, R., Spelman, L., Weightman, W., Reifenberger, J., Szeimies, R. M., Verhaeghe, E., … Rhodes, L. E. (2008). Multicentre intraindividual randomized trial of topical methyl aminolaevulinate-photodynamic therapy vs. cryotherapy for multiple actinic keratoses on the extremities. British Journal of Dermatology, 158, 994–999.10.1111/j.1365-2133.2008.08488.x
- Lehmann, P. (2007). Methyl aminolaevulinate? Photodynamic therapy: A review of clinical trials in the treatment of actinic keratoses and nonmelanoma skin cancer. British Journal of Dermatology, 156, 793–801.10.1111/bjd.2007.156.issue-5
- Marmur, E. S., Schmults, C. D., & Goldberg, D. J. (2004). A review of laser and photodynamic therapy for the treatment of nonmelanoma skin cancer. Dermatol Surg., 30, 264–271.
- Maydan, E., Nooothet, P. K., & Goldman, M. P. (2006). Case reports: Development of a keratoacanthoma aftertopical photodynamic therapy with 5-aminolevolunic acid. Journal of Drugs in Dermatology, 5, 804–806.
- McKay, K. M., Sambrano, B. L., Fox, P. S., Bassett, R. L., Chon, S., & Prieto, V. G. (2013). Thickness of superficial basal cell carcinoma (sBCC) predicts imiquimod efficacy: A proposal for a thickness-based definition of sBCC. British Journal of Dermatology, 169, 549–554.10.1111/bjd.2013.169.issue-3
- Morton, C. A. (2004). Photodynamic therapy for nonmelanoma skin cancer – And more? Archives of Dermatology, 140, 116–120.
- Morton, C. A., Campbell, S., Gupta, G., Keohane, S., Lear, J., Zaki, I., … Soto, P. (2006). Intraindividual, right?left comparison of topical methyl aminolaevulinate-photodynamic therapy and cryotherapy in subjects with actinic keratoses: A multicentre, randomized controlled study. British Journal of Dermatology, 155, 1029–1036.10.1111/bjd.2006.155.issue-5
- Morton, C. A., Szeimies, R. M., Sidoroff, A., & Braathen, L. R. (2013). European guidelines for topical photodynamic therapy part 1: Treatment delivery and current indications - actinic keratoses, Bowen’s disease, basal cell carcinoma. Journal of the European Academy of Dermatology and Venereology, 27, 536–544.10.1111/jdv.2013.27.issue-5
- Morton, C., Szeimies, R. M., Sidoroff, A., Wennberg, A. M., Basset-Seguin, N., Calzavara-Pinton, P., … Lehmann, P. (2015). European dermatology forum guidelines on topical photodynamic therapy. European Journal of Dermatology, 25, 296–311.
- Patel, R. V., Frankel, A., & Goldenberg, G. (2011). An update on nonmelanoma skin cancer. The Journal of Clinical and Aesthetic Dermatology, 4, 20–27.
- Rong, Y., Zuo, L., Shang, L., & Bazan, J. G. (2015). Radiotherapy treatment for nonmelanoma skin cancer. Expert Review of Anticancer Therapy, 15, 765–776.10.1586/14737140.2015.1042865
- Rubel, D. M., Spelman, L., Murrell, D. F., See, J. A., Hewitt, D., Foley, P., … Shumack, S. (2014). Daylight photodynamic therapy with methyl aminolevulinate cream as a convenient, similarly effective, nearly painless alternative to conventional photodynamic therapy in actinic keratosis treatment: A randomized controlled trial. British Journal of Dermatology, 171(5), 1164–1171.10.1111/bjd.2014.171.issue-5
- Sidoroff, A., & Thaler, P. (2010). Taking treatment decisions in non-melanoma skin cancer – The place for topical photodynamic therapy (PDT). Photodiagnosis and Photodynamic Therapy, 7, 24–32.10.1016/j.pdpdt.2009.12.004
- Steinbauer, J. M., Schreml, S., Kohl, E. A., Karrer, S., Landthaler, M., Szeimies, R. M. (2010). Photodynamic therapy in dermatology. Journal der Deutschen Dermatologischen Gesellschaft, 8, 454–464.
- Tiede, R., Hirschberg, J., Daeschlein, G., von Woedtke, T., Vioel, W., & Emmert, S. (2014). Plasma applications: A dermatological view. Contributions to Plasma Physics, 54(2), 118–130.10.1002/ctpp.v54.2
- Wiegell, S. R., Fabricius, S., Gniadecka, M., Stender, I. M., Berne, B., Kroon, S., … Jemec, G. B. E. (2012). Daylight mediated photodynamic therapy of moderate to thick actinic keratoses of the face and scalp: A randomized multicentre study. British Journal of Dermatology, 166(6), 1327–1332.10.1111/bjd.2012.166.issue-6
- Zhao, B., & He, Y. Y. (2010). Recent advances in the prevention and treatment of skin cancer using photodynamic therapy. Expert Review of Anticancer Therapy, 10, 1797–1809.10.1586/era.10.154