Abstract
This study was intended to evaluate the hair growth activity of Zataria multiflora Boiss. (ZM) and Matricaria chamomilla (MC) for androgenetic alopecia following a six-month clinical trial. In vitro studies were conducted to measure the inhibitory effects of MC and ZM against 5α-reductase (5αR). In this regard, five samples were prepared: NADPH + enzyme + testosterone + extract (n = 2); enzyme blank (n = 1); positive and negative controls (n = 2). Thereafter, 60 patients with androgenetic alopecia were recruited from Imam Khomeini Hospital, Tehran, Iran. They were divided in a 1:1:1 ratio to Groups A, B, and C. The first two were instructed to use the topical extract twice daily for three months. Group C was treated with placebo in the same instruction. All groups also received 5% topical minoxidil. The outcome measures were patch size, terminal hair count, and grown hair count. No complications were observed during the treatment. There were significant improvements in the patch size and terminal hair count of Groups A and B as compared with those of Group C (p < 0.05). Moreover, the non-considerable promotion of grown hair count showed possible synergistic effects. It could be concluded that ZM and MC extracts can afford to enhance hair growth by inhibiting the 5αR activity and might thus be potent antiandrogen agents.
Public Interest Statement
Herbs are invaluable source with a vast range of features that unleash the potential of new safe treatments for various medical conditions. Of which, the present study attempts to investigate the anti-androgenic activity of two well-known herbs, Zataria multiflora Boiss. and Matricaria chamomilla, rich in phenolics. Both experimental and clinical studies were performed on their ethanolic extracts. The plants have great potential to inhibit the enzyme activity of 5α-reductase and yet enhance human hair growth.
Competing interests
The authors declare no competing interest.
1. Introduction
Steroid-5α-reductase (5α-R) as an androgen metabolizing enzyme exerts its cellular influence to adjust paramount processes associated with the prostate, such as the NADPH-dependent local conversion of testosterone to the more potent androgen dihydrotestosterone (DHT) (Krieg, Weisser, & Tunn, Citation1995; Levy et al., Citation1995). It has been demonstrated that high levels of DHT are correlated to different medical conditions, namely prostate carcinogenesis, benign prostatic hyperplasia, androgenetic alopecia, hirsutism, acne, male pattern of baldness, and seborrhoea (Dallob et al., Citation1994; Geller, Citation1992; Labrie, Belanger, Simard, Labrie, & Dupont, Citation1993; Sansone & Reisner, Citation1971; Tolino et al., Citation1996; Zouboulis et al., Citation1998). Androgenetic alopecia is considered as the main kind of scalp hair loss irrespective of gender (Jain & De-Eknamkul, Citation2014). This medical condition is becoming a paramount issue across the globe, with 30–50% of men being afflicted by age 50. Besides, it comes about in a highly reproducible pattern, greatly involving the temples, vertex and mid frontal scalp. However it mostly appears as a virtually minor dermatological condition, hair loss carries adverse effects on self-image and accounts for anxiety and depression in a number of men. Of note, it can affect the likelihood of arterial stiffness and cardiovascular disease. It has been documented that elevated androgen levels not only end up with androgenetic alopecia, but also account for atherosclerotic, thrombosis, and coronary heart disease (Mansouri, Mortazavi, Eslami, & Mazinani, Citation2005; Sharma, Dubey, Gupta, & Agrawal, Citation2013; Sharma & Jindal, Citation2014). The predisposing factors to androgenetic alopecia include a familial tendency and race (Cranwell & Sinclair, Citation2000/2016). An epidemiological study was found androgenetic alopecia as the second prevalent skin disorder in northern Iran (Hajheydari & Golpour, Citation2007). Therefore, 5α-R appears promising for treating such disease through suppression of DHT synthesis (Brawley, Citation2003; Lieberman, Citation2003). Two distinct isozymes – termed type I (5α-RI) and type II (5α-RII) – are identified with different biochemical features, tissue distribution patterns as well as respond to pharmaceutical agents (Andersson, Berman, Jenkins, & Russell, Citation1991; Andersson & Russell, Citation1990; Li, Chen, Singh, Labire, & Labire, Citation1995). As 5α-R type II is the chief isoform in human prostate, selective anti-androgenic agents might yield good outcomes for the treatment of DHT. Finasteride and minoxidil are widely-used drug candidates as a 5α-R inhibitor across the globe. Nevertheless, they has shown multiple undesirable effects. Phytogenic substances for 5α-R inhibition might be a key addressing this problem.
Zataria multiflora Boiss. (ZM) (synonyms: Avishan-e-Shirazi in Persian, Thyme in English; ID: K000193689 at Royal Botanic Gardens, Kew) from the Lamiaceae family distributes geographically in central and southern Iran, Pakistan and Afghanistan (Hosseinzadeh, Ramezani, & Salmani, Citation2000). It has been shown that ZM has notable antinociceptive, antimicrobial, spasmolytic and anti-inflammatory potentials. This plant has been recently available in the form of oral drops, soft capsules and vaginal creams for special medical necessities. ZM was associated with the oxidative stress and genotoxicity arising from cyclophosphamide in mouse bone marrow cells (Hosseinimehr et al., Citation2011). Moreover, it has been reported that ZM is composed of phenolic compounds mainly carvacrol (Gupta & Gupta, Citation1972). In this regard, (Saleem, Nazli, Afza, Sami, & Ali, Citation2004) identified thymol as the chief constituent of the fresh plant (73.21%) whereas the dried plant substantially contains carvacrol (62.87%). The Labiatae family is reported to be used for hair loss traditionally (Naghibi, Mosaddegh, Mohammadi Motamed, & Ghorbani, Citation2010). Bahney (Citation2009) utilized a remedy containing Rosemary, Thyme, Lavender, and CedarWood to stimulate hair growth.
On the other hand, Matricaria chamomilla L. (MC) (synonyms: Babooneh in Persian, Chamomile in English) extends all the world around from Europe in Germany and Hungary to North Africa, Asia, North and South America, Australia, and New Zealand (Ivens, Citation1979). It contains a great breadth of therapeutically active compounds such as sesquiterpenes, flavonoids, coumarins, and polyacetylenes (Schilcher & Kamille, Citation1987). As many as 11 phenolic constituents were characterized in chamomile extract (Gupta, Mittal, Bansal, Khokra, & Kaushik, Citation2010). It has been vastly applied for medicinal purposes such as selective COX-2 inhibitor owing to its anti-inflammatory, antimicrobial, antioxidant, antiplatelet, chemopreventive potentials (Duke, Citation2001; McKay & Blumberg, Citation2006; Srivastava, Pandey, & Gupta, Citation2009). Gowda, Farooqi, Subbaiah, and Raju (Citation1991) reported that the essential oil taken from MC heads includes azulene which can have applications in perfumery, cosmetic creams, hair preparations, and the like. Additionally, the use of MC has been recommended in scalp burnout to wash hair due to its anti-irritant activity (Trüeb, Citation2015).
Despite the extant evidence in favor of protective effects of MC and ZM on hair, the scientific basis and clinical outcomes are yet to be explored. With a view towards plant-derived 5α-R inhibitors, we attempted to investigate two indigenous herbs of Iran for their potential against 5α-RII in both in vitro and clinical settings.
2. Materials and methods
2.1. Preparation of extracts
Two plants were purchased from local grocery stores in Tehran, Iran. They were subsequently identified by comparison with those raised in the Medical Plant Farm, Jahad Daneshgah, Islamic Republic of Iran. The plant names have been checked with www.theplantlist.org. A voucher specimen for each of them has been deposited in the Herbarium of the Department of Pharmacology, Jahad Daneshgah, Tehran, Iran. The aerial parts of the plants were used in the study. The extraction was performed with 100 mL of ethanol (Merck, Germany, 96%) in triplicates. The extract was then maintained in sealed dark vials at 4°C (Sarikurkcu, Targan, Ozer, & Tepe, Citation2017). Chemical profile of each species was provided as supplementary data. The phenolic content of each extract was measure by the Folin–Ciocalteu reagent assay (Azimi, Sharifan, & Ghiasi Tarzi, Citation2017). The total phenols were measured 5.5 ± 0.1 and 11.1 ± 0.4 mg GAEs/g extract for MC and ZM, respectively.
2.2. Enzyme inhibition activity
Enzyme inhibition assay was performed using Sun, Zheng, and Feng’s (Citation1998) method. Initially, a certain amount of human prostate was provided from Imam Khomeini hospital in Tehran, and then cut into very small pieces. A 10 mL of medium containing 20 mM sodium phosphate (Sigma-Aldrich, USA), 0.32M sucrose (Sigma-Aldrich, USA) and 1 mM ethylenediamine tetraacetic acid (EDTA; Sigma-Aldrich, USA) was mixed with the prostate pieces to yield a homogenate. Thereafter, centrifugation was carried out in duplicate at 4000 rpm for 15 min. The ultimate supernatant underwent Bradford method to gauge the concentration of enzyme (Bradford, Citation1976). The results indicated that the concentration of 5αRII was 372.38 μg/mL. Study reactions were prepared through mixing a 3 mL of nicotinamide adenine dinucleotide phosphate (NADPH; Sigma, USA, 22 μM), 1 mL of enzyme, 4 mL of Tris–HCl buffer (Sigma, USA, 0.5 M), 2 mL of testosterone (Sigma, Germany, 75 μM), and 2 mL of ethanolic extracts (1 mg/mL). All the samples were analyzed using spectrophotometer at 340 nm. The time interval between measurements was 10 min for a period of 30 min. Along with study reactions, an enzyme blank, a negative control (2 mL of testosterone added to the blank), and a positive control (2 mL of 200 nM finasteride added to the negative control) were considered to calculate antioxidant capacity of the extracts (Nahata & Dixit, Citation2014).
2.3. Participants
In light of 5α-reductase inhibitory activity of MC and ZM, their clinical effectiveness was investigated to not only validate the in vitro results, but also to assess their therapeutic potentials as an androgenic agent. In this randomized controlled trial with 3 months of intervention with the ethanolic extracts. The protocol for this treatment was given approval by the Ethical Committee for Medical Research affiliated to Shadara Caspian Company. Moreover, written informed consent was obtained from all patients. This trial has conformed to the CONSORT guidelines. As many as 60 subjects were enrolled between December 2016 and January 2017 based on the pattern of hair loss and trichoscopy assessment. This study included those participants who were at least 20 years (at the time of assessment); possessed an overall hairless extension of patches below 10 cm²; were currently involved occurrence in androgenetic alopecia (≤10 years) based on Hamilton criterion. The reason why this classification for the male pattern was used is because it was also employed in a cross-sectional study for Iranian population (Kheirabadi, Yazdani, & Golfam, Citation2013). If participants underwent antiandrogen therapy or any drug influencing alopecia (Aminosalicylic acid, Enalapril, Amphetamines, Etretinate, Bromocriptine, Levodopa, Captopril Lithium, Carbamazepine, Metoprolol, Cimetidine, Propanolol, Coumadin, Pyridostigmin Danazol, Trimethadione and valproate sodium), they were allowed to continue this regimen prior to randomization. Short message service text messaging and phone calls were daily utilized to enhance patient’s adherence. Allergy to chamomile or thyme, taking steroids or hormonal medication, any significant failure or serious dermatological and neurological complications in previous antiandrogen therapy, and participation in another androgenic-based intervention would exclude them from trial recruitment.
2.4. Study intervention
Following the assessment of participant’s eligibility, they were divided into 1:1:1 ratio to Groups A, B and C on random. The first two groups were respectively treated with the MC and ZM extracts. The last group served as the control to receive placebo. All the treatments were rubbed on the patches two times a day. Moreover, the extracts and placebo were the same in terms of color, odor, and size. All groups underwent treatment with 5% topical minoxidil twice a day, as well. Randomization was carried out using random number table. In this study, hairlessness was considered as the outcome measure. This scoring system describes each participant’s condition on the basis of the size of patches, total number of grown hair, and number of terminal hair. The study dermatologists who were blinded to group allocation were supposed to collect the data.
2.5. Statistical analysis
Data was described as mean ± standard deviation (SD). Given normal distribution, one-way ANOVA and t-test were performed to determine any significant difference at p-values <0.05 (SPSS 19.0, IBM Inc., USA). If the normality assumption was violated, the nonparametric Kruskal-Wallis ANOVA was used instead. All analyses in the randomized clinical trial were performed according to an intention-to-treat. A one tailed analysis was used to demonstrate the significant differences in hairlessness at p < 0.05.
3. Results
Two plants with vast applications in cuisine and traditional medicine by the Iranian were utilized to test their enzyme inhibitory activities. The ethanol-based extraction procedure resulted in extract yield of 3.42 and 3.70% for MC and ZM, respectively. Plant species were also examined for their inhibitory potentials on 5αRII (Figure ). On the whole, the inhibitory enzyme activity was on the notable rise during 30 min (p < 0.05). In the case of plant genera, an influential disparity was observed at 10 and 20 min (p = 0.01). That is, MC and ZM showed a different potency against 5αRII, with ZM being stronger. This test demonstrated the superiority of ZM at 30 min (15.01 ± 0.06 μg/mL). Having confidence in the antiandrogen activity of CM and ZM, 60 patients with androgenetic alopecia were enrolled upon inclusion criteria. The study groups were instructed to apply the distinct treatments twice a day for three months (Group A: CM + minoxidil; Group B: ZM + minoxidil; Group C: placebo + minoxidil). These patients were recruited from Imam Khomeini Hospital, Tehran Iran. As can be seen in Table , all patients were male with a mean age of 40.3 ± 12.5 years. A third of patients served as a control (Group C). It was revealed that the demographic characteristics of the participants were comparable across three groups (p > 0.05). Table summarized the results of between and within group comparisons of hairlessness. Overall, 3-month exposure to antiandrogen therapy with topical minoxidil or its combination with MC and ZM caused a significant difference in pre- and post-test values of patch size, terminal hair, and grown hair (p < 0.001). On the other hand, no significant difference was reported between the three groups at baseline (p > 0.05). Conversely, the treatment with the herbal extracts was associated with a notable disparity among the three groups in terms of patch size (p = 0.02) and terminal hair count (p = 0.00). However, the grown hair count did not differ significantly among them (p = 0.38). Paired comparisons indicated a marked improvement in the values of patch size in Group B when compared with the control (p = 0.01). Besides this, Group A presented an insignificant decrease in patch size (p > 0.05). Moreover, the ethanol extracts of MC and ZM substantially increased the number of terminal hair in Groups A and B in comparison with the control (p < 0.001) (Table ).
Figure 1. Enzyme inhibitory activity of the ethanol extracts at 10, 10, 30 min (μg/mL; mean ± SD).
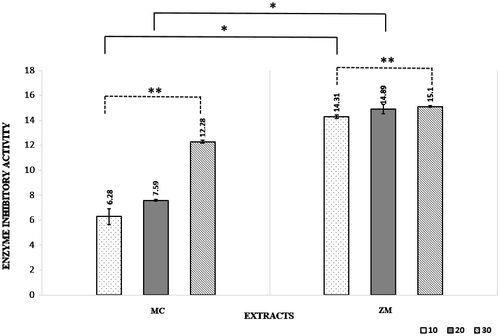
Table 1. Patient’s demographics at baseline
Table 2. The hairlessness scores at baseline and three months
Table 3. The difference of hairlessness among Groups A, B, and C at 3 months
4. Discussion
With focus on the pathophysiology of androgenetic alopecia, there have been two FDA-approved drugs (i.e. minoxidil and finasteride) commonly used by men. Despite the extant evidence in favor of their antiandrogenic protection against hair loss, a host of men have voiced concern on sexual dysfunction and feminization (Santos, Avci, & Hamblin, Citation2015). As a result, botanical extracts have garnered much attention due to their manageable or no side effects. For instance, Eclipta alba and Polygonum multiflorum are two plant species which were traditionally utilized for the treatment of baldness and hair loss in East Asia. Animal trials have shown that the methanol extract of E. alba as well as P. multiflorum extract in water can afford to promote hair growth through increases in anagen-phase hair follicle (Datta et al., Citation2009; Park, Zhang, & Park, Citation2011). In this regard, Zhang, Park, and Park (Citation2013) reported the antiandrogen properties of Thuja orientalis extract through its inhibitory action against 5α-R. In this study, considering the potent role of MC and ZM as antiandrogen agents in the experimental section, their combination with topical minoxidil was used as a treatment approach for androgenetic alopecia in the clinical context. The rational beyond using MC and MC arises from the contribution of phenolic content and numerous documentation of in vitro as well as in vivo studies highlighting the negative effects of phenolic compounds on the level of 5α-R (Hiipakka, Zhang, Dai, Dai, & Liao, Citation2002; Kumar, Chaiyasut, Rungseevijitprapa, & Suttajit, Citation2011; Kuroyanagi, Arakawa, Hirayama, & Hayashi, Citation1999; Lee, Chattopadhyay, Gong, Ahn, & Lee, Citation2003; Paris et al., Citation2002). In the ethanol extract of MC and ZM, the presence of phenolic compounds was substantiated by the Folin–Ciocalteu reagent assay. As the results demonstrated, ZM had a higher content of phenols than MC while the in vitro phase was determined the stronger inhibitory activity of ZM against 5α-R. This finding was also evident in the clinical trial where the patients in Group B were found with marked improvements in hairlessness.
The results of this investigation came up with other two outcomes; time had a statistically positive impact on the progress of enzyme inhibition, which was in broad agreement with Nahata and Dixit’s (Citation2014) findings. Also, antiandrogen therapy in all the study patients increased the number of grown hair and terminal hair, and yet decreased the patch size at months 1, 2, and 3 (data not shown). Therefore, the antiandrogen potential of MC and ZM was evidenced by the inhibition of 5α-R and promotion of hair growth in experimental and clinical phases, respectively; pairwise comparisons were indicative of remarkable synergism between the botanical extracts as well as topical minoxidil in Groups A and B at enhancing hair growth by patch size and terminal hair count. Although there was no statistically significant difference in the number of grown hair among the three groups, additive therapeutic improvements were obvious in the values of Groups A and B.
Strength of this study regards its design where the rigorous statistical analyses were performed on the experimental and clinical data to elucidate the interventional relationship between high antiandrogen activity and enhanced hair growth. Moreover, the results of the clinical trial were accomplished in an approximately long-term follow-up without any missing outcome data. When it comes to limitation, we cannot apply a dose-dependent treatment due to the participants’ gender. Accordingly, a dose-dependent study would be in the interest of the reader to add more information concerning the plants. In conclusion, this was the first randomized clinical trial aiming at the relationship between the antiandrogen activities of these botanical extracts naturally rich in phenols on the hair growth of patients with androgenetic alopecia in Iranian adults. This study indicated that MC and ZM possessed quite strong anti-androgenic potentials to inhibit 5α-R activity at each time point during the in vitro phase. Moreover, it demonstrated an interventional association of plant-based antiandrogen activity with androgenetic alopecia hairlessness.
Funding
The authors received no direct funding for this research.
Additional information
Notes on contributors
Anoosheh Sharifan
Anoosheh Sharifan is an associate professor whose main focus is the development of new products out of natural resources, such as probiotics, foods or herbs. Of her recent outstanding articles, “The interventional relationship between frequent fish consumption and depression symptoms in aging adults: a randomized controlled trial”, “The use of Pistacia khinjuk essential oil to modulate shelf-life and organoleptic traits of mechanically deboned chicken meat” or “Developing probiotic jelly desserts with Lactobacillus acidophilus” are mentioned.
References
- Andersson, S., Berman, D. M., Jenkins, E. P., & Russell, D. W. (1991). Deletion of steroid 5 alpha-reductase 2 gene in male pseudohermaphroditism. Nature, 354(6349), 159–161. doi:10.1038/354159a0
- Andersson, S., & Russell, D. W. (1990). Structural and biochemical properties of cloned and expressed human and rat steroid 5 alpha-reductases. Proceedings of the National Academy of Sciences of the United States of America, 87(10), 3640–3644.10.1073/pnas.87.10.3640
- Azimi, M., Sharifan, A., & Ghiasi Tarzi, B. (2017). The use of Pistacia khinjuk essential oil to modulate shelf-life and organoleptic traits of mechanically deboned chicken meat. Journal of Food Processing and Preservation, 41(2), e12814. doi:10.1111/jfpp.12814
- Bahney, J. L. B. (2009). Human hair root stimulator using emu oil to deliver specific therapeutic grade essential oils to the hair follicle. Google Patents.
- Bradford, M. M. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry, 72(1–2), 248–254.10.1016/0003-2697(76)90527-3
- Brawley, O. W. (2003). Hormonal prevention of prostate cancer. Urologic Oncology: Seminars and Original Investigations, 21(1), 67–72.10.1016/S1078-1439(03)00004-8
- Cranwell, W., & Sinclair, R. (2000/2016). Male androgenetic alopecia. In L. J. De Groot, G. Chrousos, K. Dungan, K. R. Feingold, A. Grossman, J. M. Hershman, … A. Vinik (Eds.), Endotext [Internet]. South Dartmouth, MA: MDText.com, Inc. Retrieved from https://www.ncbi.nlm.nih.gov/books/NBK278957/
- Dallob, A. L., Sadick, N. S., Unger, W., Lipert, S., Geissler, L. A., Gregoire, S. L., & Tanaka, W. K. (1994). The effect of finasteride, a 5 alpha-reductase inhibitor, on scalp skin testosterone and dihydrotestosterone concentrations in patients with male pattern baldness. Journal of Clinical Endocrinology and Metabolism, 79(3), 703–706. doi:10.1210/jcem.79.3.8077349
- Datta, K., Singh, A. T., Mukherjee, A., Bhat, B., Ramesh, B., & Burman, A. C. (2009). Eclipta alba extract with potential for hair growth promoting activity. Journal of Ethnopharmacology, 124(3), 450–456. doi:10.1016/j.jep.2009.05.023
- Duke, J. A. (2001). Handbook of medical herbs. Boca Raton, FL: CRC Press LLC.
- Geller, J. (1992). Nonsurgical treatment of prostatic hyperplasia. Cancer, 70(1 Suppl), 339–345.
- Gowda, T. N. V., Farooqi, A. A., Subbaiah, T., & Raju, B. (1991). Influence of plant density, nitrogen and phosphorus on growth, yield and essential oil content of chamomile (Matricaria chamomilla Linn.). Indian Perfumers, 35, 168–172.
- Gupta, G. S., & Gupta, N. L. (1972). Constituents of Zataria multiflora. Phytochemistry, 11, 455–456.10.1016/S0031-9422(00)90054-2
- Gupta, V., Mittal, P., Bansal, P., Khokra, S. L., & Kaushik, D. (2010). Pharmacological potential of Matricaria recutita-A review. International Journal of Pharmaceutical Sciences and Drug Research, 2(1), 12–16.
- Hajheydari, Z., & Golpour, M. (2007). Prevalence of skin disorders in Sari, 2003–2004. Journal of Mazandaran University of Medical Sciences, 17(57), 94–98.
- Hiipakka, R. A., Zhang, H.-Z., Dai, W., Dai, Q., & Liao, S. (2002). Structure–activity relationships for inhibition of human 5α-reductases by polyphenols. Biochemical Pharmacology, 63(6), 1165–1176.10.1016/S0006-2952(02)00848-1
- Hosseinimehr, S. J., Mahmoudzadeh, A., Ahmadi, A., Ashrafi, S. A., Shafaghati, N., & Hedayati, N. (2011). The radioprotective effect of Zataria multiflora against genotoxicity induced by gamma irradiation in human blood lymphocytes. Cancer Biotherapy & Radiopharmaceuticals, 26(3), 325–329. doi:10.1089/cbr.2010.0896
- Hosseinzadeh, H., Ramezani, M., & Salmani, G. (2000). Antinociceptive, anti-inflammatory and acute toxicity effects of Zataria multiflora Boiss extracts in mice and rats. Journal of Ethnopharmacology, 73(3), 379–385.10.1016/S0378-8741(00)00238-5
- Ivens, G. M. (1979). Stinking mayweed. New Zealand Journal of Agricultural Research, 138, 21–23.
- Jain, R., & De-Eknamkul, W. (2014). Potential targets in the discovery of new hair growth promoters for androgenic alopecia. Expert Opinion on Therapeutic Targets, 18(7), 787–806. doi:10.1517/14728222.2014.922956
- Kheirabadi, G. R., Yazdani, A., & Golfam, L. (2013). Comparison of Alopecia severity and blood level of testosterone in men suffering schizophrenia with control group. Advanced Biomedical Research, 2, 58. doi:10.4103/2277-9175.115801
- Krieg, M., Weisser, H., & Tunn, S. (1995). Potential activities of androgen metabolizing enzymes in human prostate. The Journal of Steroid Biochemistry and Molecular Biology, 53(1–6), 395–400.10.1016/0960-0760(95)00085-E
- Kumar, T., Chaiyasut, C., Rungseevijitprapa, W., & Suttajit, M. (2011). Screening of steroid 5-reductase inhibitory activity and total phenolic content of Thai plants. Journal of Medicinal Plants Research, 5(7), 1265–1271.
- Kuroyanagi, M., Arakawa, T., Hirayama, Y., & Hayashi, T. (1999). Antibacterial and antiandrogen flavonoids from Sophora flavescens. Journal of Natural Products, 62(12), 1595–1599. doi:10.1021/np990051d
- Labrie, F., Belanger, A., Simard, J., Labrie, C., & Dupont, A. (1993). Combination therapy for prostate cancer. Endocrine and biologic basis of its choice as new standard first-line therapy. Cancer, 71(3 Suppl), 1059-1067.
- Lee, H. J., Chattopadhyay, S., Gong, E.-Y., Ahn, R. S., & Lee, K. (2003). Antiandrogenic effects of bisphenol A and nonylphenol on the function of androgen receptor. Toxicological Sciences, 75(1), 40–46.10.1093/toxsci/kfg150
- Levy, M. A., Brandt, M., Sheedy, K. M., Holt, D. A., Heaslip, J. I., Trill, J. J., … Bergsma, D. J. (1995). Cloning, expression and functional characterization of type 1 and type 2 steroid 5 alpha-reductases from Cynomolgus monkey: Comparisons with human and rat isoenzymes. The Journal of Steroid Biochemistry and Molecular Biology, 52(4), 307–319.10.1016/0960-0760(94)00183-M
- Li, X., Chen, C., Singh, S. M., Labire, F., & Labire, F. (1995). The enzyme and inhibitors of 4-ene-3-oxosteroid 5 alpha-oxidoreductase. Steroids, 60(6), 430–441.10.1016/0039-128X(95)00021-H
- Lieberman, R. (2003). Evolving strategies for prostate cancer chemoprevention trials. World Journal of Urology, 21(1), 3–8. doi:10.1007/s00345-003-0317-4
- Mansouri, P., Mortazavi, M., Eslami, M., & Mazinani, M. (2005). Androgenetic alopecia and coronary artery disease in women. Dermatology Online Journal, 11(3), 2.
- McKay, D. L., & Blumberg, J. B. (2006). A review of the bioactivity and potential health benefits of chamomile tea (Matricaria recutita L.). Phytotherapy Research, 20(7), 519–530. doi:10.1002/ptr.1900
- Naghibi, F., Mosaddegh, M., Mohammadi Motamed, M., & Ghorbani, A. (2010). Labiatae family in folk medicine in Iran: From ethnobotany to pharmacology. Iranian Journal of Pharmaceutical Research, 2, 63–79.
- Nahata, A., & Dixit, V. K. (2014). Evaluation of 5α-reductase inhibitory activity of certain herbs useful as antiandrogens. Andrologia, 46(6), 592–601.10.1111/and.2014.46.issue-6
- Paris, F., Balaguer, P., Térouanne, B., Servant, N., Lacoste, C., Cravedi, J.-P., … Sultan, C. (2002). Phenylphenols, biphenols, bisphenol-A and 4-tert-octylphenol exhibit α and β estrogen activities and antiandrogen activity in reporter cell lines. Molecular and Cellular Endocrinology, 193(1), 43–49.10.1016/S0303-7207(02)00094-1
- Park, H. J., Zhang, N., & Park, D. K. (2011). Topical application of Polygonum multiflorum extract induces hair growth of resting hair follicles through upregulating Shh and beta-catenin expression in C57BL/6 mice. Journal of Ethnopharmacology, 135(2), 369–375. doi:10.1016/j.jep.2011.03.028
- Saleem, M., Nazli, R., Afza, N., Sami, A., & Ali, M. S. (2004). Biological significance of essential oil of Zataria multiflora boiss. Natural Product Research, 18(6), 493–497. doi:10.1080/14786410310001608064
- Sansone, G., & Reisner, R. M. (1971). Differential rates of conversion of testosterone to dihydrotestosterone in acne and in normal human skin–a possible pathogenic factor in acne. Journal of Investigative Dermatology, 56(5), 366–372.10.1111/1523-1747.ep12261252
- Santos, Z., Avci, P., & Hamblin, M. R. (2015). Drug discovery for alopecia: Gone today, hair tomorrow. Expert Opinion on Drug Discovery, 10(3), 269–292. doi:10.1517/17460441.2015.1009892
- Sarikurkcu, C., Targan, S., Ozer, M. S., & Tepe, B. (2017). Fatty acid composition, enzyme inhibitory and antioxidant activities of the ethanol extracts of selected wild edible plants consumed as vegetable in the Aegean region of Turkey. International Journal of Food Properties, 20(3), 560–572. doi:10.1080/10942912.2016.1168837
- Schilcher, H., & Kamille, D. (1987). Handbuch fur arzte: apotheker und andere naturwissenschaftler. Germany: Wissenschaft Verlagsgesellschaft.
- Sharma, K. H., & Jindal, A. (2014). Association between androgenetic alopecia and coronary artery disease in young male patients. International Journal of Trichology, 6(1), 5–7. doi:10.4103/0974-7753.136747
- Sharma, L., Dubey, A., Gupta, P. R., & Agrawal, A. (2013). Androgenetic alopecia and risk of coronary artery disease. Indian Dermatology Online Journal, 4(4), 283–287. doi:10.4103/2229-5178.120638
- Srivastava, J. K., Pandey, M., & Gupta, S. (2009). Chamomile, a novel and selective COX-2 inhibitor with anti-inflammatory activity. Life Sciences, 85(19–20), 663–669. doi:10.1016/j.lfs.2009.09.007
- Sun, Z. Y., Zheng, W. J., & Feng, J. (1998). A convenient and rapid method to study enzymatic kinetics of steroid 5a-reductase inhibitors. Indian Journal of Pharmacology, 30(4), 257–262.
- Tolino, A., Petrone, A., Sarnacchiaro, F., Cirillo, D., Ronsini, S., Lombardi, G., & Nappi, C. (1996). Finasteride in the treatment of hirsutism: New therapeutic perspectives. Fertility and Sterility, 66(1), 61–65.10.1016/S0015-0282(16)58388-5
- Trüeb, R. M. (2015). The difficult hair loss patient: Guide to successful management of alopecia. New York, NY: Springer.10.1007/978-3-319-19701-2
- Zhang, N. N., Park, D. K., & Park, H. J. (2013). Hair growth-promoting activity of hot water extract of Thuja orientalis. BMC Complementary and Alternative Medicine, 13, 53. doi:10.1186/1472-6882-13-9
- Zouboulis, C. C., Xia, L., Akamatsu, H., Seltmann, H., Fritsch, M., Hornemann, S., … Orfanos, C. E. (1998). The human sebocyte culture model provides new insights into development and management of seborrhoea and acne. Dermatology, 196(1), 21–31.10.1159/000017861
