Abstract
Aims: The aims of the present study were to monitor electromyography activity in the masticatory muscles of individuals with both hemiparesis and Temporomandibular Disorders and determine possible correlations with gender and time elapsed since the stroke event. Methods: A descriptive, cross-sectional study was conducted involving 50 individuals. Group 1 comprises individuals with complete hemiparesis and Group 2 comprises individuals with incomplete hemiparesis. The Research Diagnostic Criteria for Temporomandibular Disorders was used for the diagnosis of Temporomandibular Disorders. To capture the electromyography signal of the masseter and anterior temporal muscles bilateral was used a four-channel acquisition system. Results: There was a statistically significant difference between the right and left side was found in the electromyography signal to the jaw at rest, both in the group with complete hemiparesis and the group with incomplete hemiparesis. No statistically significant differences in the EMG signal were found between the right and left sides during isometric contraction or isotonic contraction. Conclusions: The hemiparesis alters electromyographic signals in the masticatory muscles with the mandible at rest. Negative correlations were found between the electromyography signal and both the male gender and time elapsed since the stroke, demonstrating less electrical activity with a longer time since the neurological event, especially among men.
Public Interest Statement
The stroke can promote several changes in the functioning of the joints. A joint that can be compromised is the Temporomandibular Joint that participates in important actions in the day-to-day. Any change in this joint can cause headaches, difficulty in chewing food hard, difficulty swallowing, yawning, and even cause postural changes, among other things.
To evaluate the activity of the muscles of mastication (Masseters and Temporal) helps to decrease complications in this joint. One of the goals of this study was to alert you that the after effects of a stroke can affect the temporomandibular joint, impairing important functions in the patients. These changes should be taken into account at the time when the objectives are therapeutic for the rehabilitation of this individual.
Competing interests
The authors declare no competing interest.
1. Introduction
Cerebrovascular diseases, including stroke, are the third major cause of death and the first major cause of physical and mental disability throughout the world (American Heart Association, Citation2005). Stroke is defined by the World Health Organization as a clinical syndrome consisting of rapidly developing clinical signs of focal (or global in case of coma) disturbance of cerebral function lasting more than 24 h or leading to death with no apparent cause other than a vascular origin (Hatano, Citation1976). Because of the consequent hemiparesis, the stroke survivors often have a residual musculoskeletal impairment experience, as well as sensory and cognitive alterations, which cause limitations in the performance of activities of daily living (Ayo et al., Citation1999). Any alteration on the face, caused by the hemiparesis, such as pain, weakness in the masticatory muscles, crackling, clenching teeth, and even in association with the preference masticatory prior, can lead to temporomandibular disorder (TMD) (Ahlberg et al., Citation2003; Ries & Bérzin, Citation2016), since temporomandibular disorder is considered a set of joint and muscular disorders in the orofacial region characterized mainly by pain, tinnitus, articular noises and irregular mandibular function (Klasser & Okeson, Citation2006).
Changes in orofacial musculoskeletal structures can be evaluated using electromyography (EMG), which is a noninvasive method that is easy to administer (Amorim et al., Citation2010; Grossi & Chaves, Citation2004). The use of EMG in studies on the function of the masticatory muscles can provide important physiological information on the functioning os this system and allows the analysis of muscle activity under different conditions (at rest, during maximum clenching and during chewing) and assists in the diagnosis and monitoring of TMD (Amorim et al., Citation2010).
Considering the need of the early identification of problems that predispose individuals with hemiparesis to abnormalities of the stomatognathic system, EMG can contribute to better understanding of masticatory muscle activity in stroke survivors as well as responses to specific treatment modalities.
The aims of the present study were to monitor EMG activity in the masticatory muscles of individuals with both hemiparesis and TMD and determine possible correlations with gender and time elapsed since the stroke event.
2. Materials and methods
Individuals with hemiparesis stemming from a stroke were recruited from the Physical Therapy Clinic of University Nove de Julho (São Paulo, Brazil). A descriptive, cross-sectional study was conducted involving 98 individuals with complete or incomplete hemiparesis with brachial or crural predominance. Individuals with cognitive impairment, dentofacial deformities, complete or partial edentulism, quadriparesis and those currently in dental treatment were excluded. Forty-eight individuals did not meet the eligibility criteria (15 had trunk impairment, 20 had complete or partial edentulism and 13 had cognitive impairment). Thus, 50 individuals were included in the study.
The sample was divided into two groups. Group 1 comprised individuals with complete hemiparesis (face, upper limb and lower limb affected on one side of the body). Group 2 comprised individuals with incomplete hemiparesis (only the upper and lower limb affected) and was considered the control group. Inter-group and intra-group comparisons were performed for bite force, TMD, gender, age, time elapsed since stroke, predominance and type of impairment.
2.1. Diagnosis of temporomandibular disorder
The Research Diagnostic Criteria for Temporomandibular Disorders (RDC/TMD) have been the most widely employed diagnostic protocol for TMD research since its publication in Dworkin and LeResche (Citation1992). This classification system was based on the biopsychosocial model of pain that included an Axis I physical assessment, using reliable and well-operationalized diagnostic criteria, and an Axis II assessment of psychosocial status and pain-related disability. The intent was to simultaneously provide a physical diagnosis and identify other relevant characteristics of the patient that could influence the expression and thus management of their TMD. Indeed, the longer the pain persists, the greater the potential for emergence and amplification of cognitive, psychosocial, and behavioral risk factors, with resultant enhanced pain sensitivity, greater likelihood of additional pain persistence, and reduced probability of success from standard treatments (Merskey, Citation2012).
The findings of the physical exam were compared to the descriptions on the questionnaire for the diagnosis of the specific group of TMD: muscle involvement (Group I), disk displacement (Group II) and joint involvement—arthralgia, arthritis and arthrosis (Group III). The diagnosis was non-hierarchical and a given participant could have more than one diagnosis. For such, the volunteer remained seated comfortably with hips flexed at 90°, feet supported on the floor and the mandible in a comfortable position with the teeth lightly in contact. Axis II contains 31 questions for the evaluation of psychosocial aspects of TMD (Kanayama, et al., Citation2001).
2.2. Electromyography
The EMG signals were captured using a four-channel acquisition system (EMG 432 C, EMG System do Brasil Ltda.) composed of a signal conditioning module, bipolar active electrodes, analog band pass filter of 20–500 Hz and a common mode rejection rate of 120 dB. The sampling frequency was 2 kHz, digitized using an analog-digital converter with 16 bits of resolution and the acquisition program was EMGLab (EMG System do Brasil Ltda.). The electrodes had a pre-amplification of 20x.
To capture the EMG signal of the right and left masseter and anterior temporal muscles, self-adhesive, disposable, Ag/AgCl surface electrodes (Meditrace®) measuring 10 mm in diameter were attached to the belly of the muscle in the region of greatest tone, determined during moderate clenching (Wang, Arima, Arendt-Nielsen, & Svensson, Citation2000). After cleaning the skin with 70% alcohol to diminish impedance, the electrodes were spaced at a distance of 20 mm center to center. A reference electrode was used on the left wrist in order to reduce noises arising from the difference between the reference points of the human body and the signal acquisition equipment.
EMG of the masticatory muscles was determined under three conditions: (I) at rest; (II) during mastication (isotonic contraction); and (III) during maximum voluntary clenching (MVC—isometric contraction). For such, the volunteer was seated comfortably on a chair with hands resting on the thighs. Three readings were made under each condition with a three-minute rest period between readings. In the rest position, each reading lasted 15 s. The volunteer then performed three five-second readings during MVC, clenching on folded Parafilm M® measuring 3 mm in thickness (15 × 35 mm) placed bilaterally between the molars. After a rest period, the volunteer was instructed to perform habitual bilateral chewing (isotonic contraction) at a rhythm determined using a metronome adjusted to 60 beats per minute; each reading lasted 15 s. Finally, MVC was performed for five seconds without the use of Parafilm M®.
2.3. Processing of electromyographic signals
EMG activity of the masseter and temporal muscles at rest, during isometric contraction and during isotonic contraction was analyzed based on the root mean square (RMS) of the amplitude of the signal (expressed in μV). The RMS was calculated for the entire 15 s reading with the muscles at rest and during isotonic contraction. During MVC, the first and last seconds of the crude EMG signal were discarded and the RMS was calculated using a 100 ms moving window with no overlap; the mean value of the windows was used for analysis. The RMS under each condition was normalized by the highest MVC value (μV/μV × 100: % MVC) and expressed as percentage of MVC. The normalization of the MVC signal was done using bilateral parafilm. The EMG signals were process using specific routines developed for the Matlab program, version 7.1 (The MathWorks Inc., Natick, Massachusetts, USA).
2.4. Statistical analysis
For characterization of the sample and distribution of the obtained scores, descriptive statistics were used, through measures of central tendency (mean) and dispersion (standard deviation). The non-parametric variables were summarized in median and interquartile range. The results were submitted to the Kolmogorov-Smirnov test and later to the Pearson or Spearman correlation coefficients, according to data distribution. The strength or magnitude of the relationship between variables was classified as weak (correlation coefficient between 0.1 and 0.3), moderate (between 0.4 and 0.6), and strong (between 0.7 and 0.9). For the statistical analysis, the Chi-square test and Fisher’s exact test were used to evaluate the categorical variables, to compare the means Student’s t-test and Analysis of Variance (ANOVA), complemented by the LSD test and the correlation between the continuous variables Was analyzed by the Pearson correlation test, adopting a level of significance of p < 0.05.
3. Results
Fifty individuals were analyzed (mean age: 60.8 years). The female gender accounted for 38% of the sample (n = 19) and the male gender accounted for 62% (n = 31). Mean time elapsed since the stroke was 24.3 months. A total of 52% (n = 26) had right-side hemiparesis, 48% (n = 24) had left-side hemiparesis, 58% (n = 29) had complete hemiparesis, 42% (n = 21) had incomplete hemiparesis, 52% (n = 26) had brachial predominance and 48% (n = 24) had crural predominance (Table and Figures –).
Table 1. Prevalence of temporomandibular disorder in overall sample
Figure 1. Comparison of EMG signal between right and left sides in individuals with complete or incomplete hemiparesis with mandible at rest.
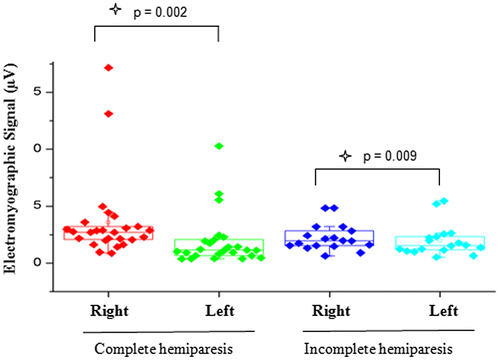
Figure 2. Comparison of EMG signal between right and left sides in individuals with complete or incomplete hemiparesis during MVC.
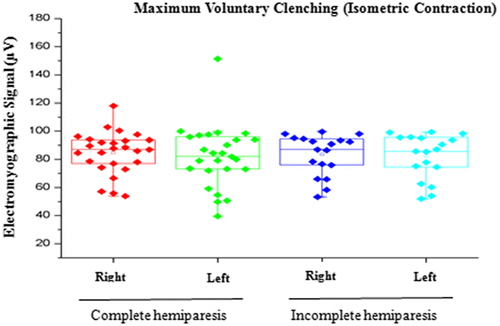
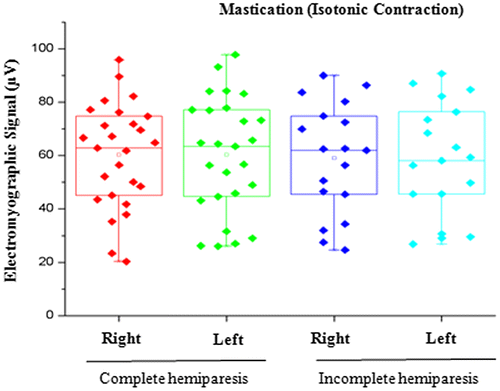
Figure 3. Comparison of EMG signal between right and left sides in individuals with complete or incomplete hemiparesis during isotonic contraction.
In relation to electromyography, a correlation was found between MVC and stroke time between those in subjects without TMD. That is, the longer the time elapsed since the minor stroke, the MVC was shown (Tables and ).
Table 2. Analysis of EMG signal and both gender and time since stroke (complete hemiparesis)
Table 3. Analysis of EMG signal and both gender and time since stroke (incomplete hemiparesis)
4. Discussion
In the present study, a statistically significant difference between the right and left side was found in the EMG signal with the mandible at rest in both the group with complete hemiparesis and the group with incomplete hemiparesis. This finding may be attributed to a possible change in the tone of the muscles studied due to hypertonia caused by a change in motor control, which is commonly found in individuals with injuries to the central nervous system (Freriks & Hermens, Citation2000). The same has been found to occur during reflex contractions, which is reported to be greater in individuals without paresis (Basmajian, Citation1998).
However, the physiological basis of the mandible in the rest position is one of the more controversial aspects of oral physiology, as the muscles may be contracted and demonstrate slight electrical activity. In some studies the authors evaluated EMG activity in the massetter and temporal muscles in women with and without TMD and concluded that those with TMD exhibited greater asymmetry between muscle pairs as well as hyperactivity of the masseter muscles (Amadio, Citation1996; Sgobbi De Faria & Berzin, Citation1998; Suvinen & Kemppainen, Citation2007).
In the present study, no statistically significant differences in the EMG signal were found between the right and left sides during isometric contraction (MVC) or isotonic contraction (mastication), These findings may be explained by the ability of the central nervous system to program itself in a process of neuronal and biomechanical organization, recruiting the adjacent muscles as a compensatory response to execute the functions of biting and chewing (Mercer & Sahrmann, Citation1999; Suvinen & Kemppainen, Citation2007; Wessberg, Epker, & Elliott, Citation1993; ).
A correlation was found between the EMG signal during MVC and time elapsed since the stroke among individuals without TMD, as MVC was weaker when the time elapsed since the stroke was longer. The literature describes hyperreflexia in stroke survivors, which is due to the loss of inhibition of the peripheral ring, thereby enhancing the stretching reflex response when a stimulus is given (Miles, Citation2007). This finding may be explained by the fact that motor impairment in patients with hemiparesis is not only caused by the primary neural injury, but also by the secondary deterioration of the contractile properties of the muscles, likely resulting in muscle fiber atrophy (van der Glas, van der Bilt, Abbink, Mason, & Cadden, Citation2007).
TMD does not necessarily affect electrical activity in the masticatory muscles. Changes in EMG signals may be considered either physiological or functional. However, Turcio, Garcia, Derogis, and Zuim (Citation2002) found that TMD causes a change in muscle activity, with a reduction in endurance, especially on the side not used during mastication.
Investigations involving EMG for patients with different neurological disorders have furnished additional information on such conditions (Tyler & Karst, Citation2004). However, it is necessary to take the due precautions. If a strict, standardized protocol is followed, EMG is an effective method for analyzing the stomatognathic system with good reliability that offers an additional reference during the clinical evaluation (Bevilaqua-Grosso, Monteiro-Pedro, Guirro, & Berzin, Citation2002; Bodéré, Téa, Giroux-Metges, & Woda, Citation2005; Ceneviz et al., Citation2006; Ferrario, Sforza, Tartaglia, & Dellavia, Citation2002; Landulpho, E Silva, E Silva, & Vitti, Citation2004; Landulpho et al., Citation2004; McGill & Cholewicki, Citation2001; Tartaglia, Lodetti, Paiva, Felicio, & Sforza, Citation2011; Tartaglia et al., Citation2008; Toft, Sinkjjer, & Espersen, Citation1999; Zupan, Stokic, Bohanec, Priebe, & Sherwood, Citation1998).
5. Conclusion
Based on the present findings, hemiparesis alters electromyographic signals in the masticatory muscles with the mandible at rest, which may be explained by the typical increase in muscle tone in patients with brain lesions. Moreover, negative correlations were found between the EMG signal and both the male gender and time elapsed since the stroke, demonstrating less electrical activity with a longer time since the neurological event, especially among men.
Further studies are needed to investigate the events involved in this neurological process.
Funding
This work was supported by the FAPESP [grant number 2013/04065-0].
Author’s contributions
Fernanda Cordeiro da Silva, Paulo Roberto da Costa Palácio, Gabriela Regina de Lima- Substantial contributions to conception and design, Acquisition of data, Drafting the article.
Fabiano Politti, Daniela de Fátima Teixeira da Silva, Alessandro Melo Deana - Analysis and interpretation of data.
Raquel Agnelli Mesquita-Ferrari, Kristianne Porta Santos Fernandes, Daniela Aparecida Biasotto-Gonzalez - Revising it critically for important intellectual content.
Andréa Oliver Gomes - Drafting the article and revising it critically for important intellectual content.
Sandra Kalil Bussadori- Substantial contributions to conception and design, Acquisition of data, Drafting the article, Revising it critically for important intellectual content and final approval of the version to be published.
Additional information
Notes on contributors
Sandra Kalil Bussadori
Fernanda Cordeiro da Silva, physiotherapist, PhD Sciences Rehabilitation, Nove de Julho University. Member of the research group, coordinated by Prof Dr Sandra Kalil Bussadori, with a focus on Assessment and Therapeutic Intervention of Disorders of the Systems Neuromuscular. Our line search is based on photobiomodulation, electromyographic evaluation in individuals with neurological dysfunctions.
References
- Ahlberg, J. P., Kovero, O. A., Hurmerinta, K. A., Zepa, I., Nissinen, M. J., & Könönen, M. H. (2003). Maximal bite force and its association with signs and symptoms of TMD, occlusion, and body mass index in a cohort of young adults. CRANIO®, 21(4), 248–252.10.1080/08869634.2003.11746258
- Amadio, A. C. (1996). Fundamentos biomecânicos para análise do movimento (pp. 9–58). São Paulo: Laboratório de Biomecânica EEFE-USP.
- American Heart Association. (2005). Heart disease and stroke statistics update.
- Amorim, C. F., Giannasi, L. C., Ferreira, L. M., Magini, M., Oliveira, C. S., de Oliveira, L. V., … Politti, F. (2010). Behavior analysis of electromyographic activity of the masseter muscle in sleep bruxers. Journal of Bodywork and Movement Therapies, 14(3), 234–238.10.1016/j.jbmt.2008.12.002
- Ayo, N. E., Wood-Dauphinee, S., Ahmed, S., Gordon, C., Higgins, J., McEwen, S., & Salbach, N. (1999). Disablement following stroke. Disability and rehabilitation, 21(5–6), 258–268.
- Basmajian, J. V. (1998). Muscle tone, fatigue and neural influences. In J. V. Basmajian (Ed.), Muscles alive (4th ed.). (pp. 1–11). Baltimore, MD: Williams & Wilkins.
- Bevilaqua-Grosso, D., Monteiro-Pedro, V., Guirro, R. R., & Berzin, F. (2002). A physiotherapeutic approach to craniomandibular disorders: A case report. Journal of Oral Rehabilitation, 29(3), 268–273.10.1046/j.1365-2842.2002.00832.x
- Bodéré, C., Téa, S. H., Giroux-Metges, M. A., & Woda, A. (2005). Activity of masticatory muscles in subjects with different orofacial pain conditions. Pain, 116(1), 33–41.10.1016/j.pain.2005.03.011
- Ceneviz, C., Mehta, N. R., Forgione, A., Sands, M. J., Abdallah, E. F., Lobo Lobo, S., & Mavroudi, S. (2006). The immediate effect of changing mandibular position on the EMG activity of the masseter, temporalis, sternocleidomastoid, and trapezius muscles. CRANIO®, 24(4), 237–244.10.1179/crn.2006.038
- Dworkin, S. F., & LeResche, L. (1992). Research diagnostic criteria for temporomandibular disorders: Review criteria, examinations and specifications, critique. J Craniomandib Disord., 6, 301–355. [PubMed: 1298767].
- Ferrario, V. F., Sforza, C., Tartaglia, G. M., & Dellavia, C. (2002). Immediate effect of a stabilization splint on masticatory muscle activity in temporomandibular disorder patients. Journal of Oral Rehabilitation, 29(9), 810–815.10.1046/j.1365-2842.2002.00927.x
- Freriks, B., & Hermens, H. (2000). European recommendations for surface electromyography, results of the SENIAM Project (CD-ROM). The Netherlands: Roessingh Research and Development.
- Grossi, D. B., & Chaves, T. C. (2004). Physiotherapeutic treatment for temporomandibular disorders (TMD). Braz J Oral Sci, 3(10), 492–497.
- Hatano, S. (1976). Experience from a multicentre stroke register: A preliminary report. Bulletin of the World Health Organisation, 54(5), 541–553.
- Kanayama, T., Minowa, K., Inoue, N., Yamaguchi, T., Yoshida, S., & Kawasaki, T. (2001). Comparison of phosphocreatine concentration in the human masseter and medial pterygoid muscles by 31P-CSI. Journal of Oral Rehabilitation, 28, 1075–1079.10.1046/j.1365-2842.2001.00777.x
- Klasser, G. D., & Okeson, J. P. (2006). The clinical usefulness of surface electromyography in the diagnosis and treatment of temporomandibular disorders. The Journal of the American Dental Association, 137(6), 763–771.10.14219/jada.archive.2006.0288
- Landulpho, A. B., E Silva, W. A. B., E Silva, F. A., & Vitti, M. (2004). Electromyographic evaluation of masseter and anterior temporalis muscles in patients with temporomandibular disorders following interocclusal appliance treatment. Journal of Oral Rehabilitation, 31(2), 95–98.10.1046/j.0305-182X.2003.01204.x
- McGill, S. M., & Cholewicki, J. (2001). Biomechanical basis for stability: An explanation to enhance clinical utility. Journal of Orthopaedic & Sports Physical Therapy, 31(2), 96–100.10.2519/jospt.2001.31.2.96
- Mercer, V. S., & Sahrmann, S. A. (1999). Postural synergies associated with a stepping task. Physical Therapy, 79(12), 1142–1152.
- Merskey, H. (2012). Introduction. In Giamberardino, M. A. & Jensen, T. S. (Ed.), Pain comorbidities: Understanding and treating the complex patient (pp. 1–20). Seattle, WA: IASP Press.
- Miles, T. S. (2007). Postural control of the human mandible. Archives of Oral Biology, 52(4), 347–352.10.1016/j.archoralbio.2006.12.017
- Ries, L. G. K., & Bérzin, F. (2016). Cervical pain in individuals with and without temporomandibular disorders. Brazilian Journal of Oral Sciences, 6(20), 1301–1307.
- Sgobbi De Faria, C. R. S., & Berzin, F. (1998). Electromyographic study of the temporal, masseter and suprahyoid muscles in the mandibular rest position. Journal of Oral Rehabilitation, 25(10), 776–780.
- Suvinen, T. I., & Kemppainen, P. (2007). Review of clinical EMG studies related to muscle and occlusal factors in healthy and TMD subjects. Journal of Oral Rehabilitation, 34(9), 631–644.10.1111/jor.2007.34.issue-9
- Tartaglia, G. M., Antonio Moreira Rodrigues da Silva, Moreira, Bottini, Stefano, Sforza, Chiarella, Ferrario, Virgilio F., & Ferrario, V. F. (2008). Masticatory muscle activity during maximum voluntary clench in different research diagnostic criteria for temporomandibular disorders (RDC/TMD) groups. Manual Therapy, 13(5), 434–440.10.1016/j.math.2007.05.011
- Tartaglia, G. M., Lodetti, G., Paiva, G., Felicio, C. M., & Sforza, C. (2011). Surface electromyographic assessment of patients with long lasting temporomandibular joint disorder pain. Journal of Electromyography and Kinesiology, 21(4), 659–664.10.1016/j.jelekin.2011.03.003
- Toft, E., Sinkjjer, T., & Espersen, G. T. (1999). Quantitation of the strech reflex: Tecnical procedures and clinical application. Acta Neurologica Scandinavica, 79, 384–390.
- Turcio, K. H. L., Garcia, A. R., Derogis, A. R., & Zuim, P. R. J. (2002). Electromyographic and electrovibratographic evaluation before and after TMJ treatment. Pós Grad Rev Odontol, 5(2), 36–43.
- Tyler, A. E., & Karst, G. M. (2004). Timing of muscle activity during reaching while standing: Systematic changes with target distance. Gait and Posture, 20(2), 126–133.10.1016/j.gaitpost.2003.07.001
- van der Glas, H. W., van der Bilt, A., Abbink, J. H., Mason, A. G., & Cadden, S. W. (2007). Functional roles of oral reflexes in chewing and biting: Phase-, task- and site-dependent reflex sensitivity. Archives of Oral Biology, 52(4), 365–369.10.1016/j.archoralbio.2006.10.022
- Wang, K., Arima, T., Arendt-Nielsen, L., & Svensson, P. (2000). EMG-force relationships are influenced by experimental jaw-muscle pain. Journal of Oral Rehabilitation, 27, 394–402.10.1046/j.1365-2842.2000.00617.x
- Wessberg, G. A., Epker, B. N., & Elliott, A. C. (1993). Comparison of mandibular rest positions induced by phonetics, transcutaneous electrical stimulation, and masticatory electromyography. Journal of Prosthetic Dentistry, 49(1), 100–105.
- Zupan, B., Stokic, D. S., Bohanec, M., Priebe, M. M., & Sherwood, A. M. (1998). Relating clinical and neurophysiological assessment of spasticity by machine learnig. Intemational Joumal of Medicallnformatics, 49, 243–251.
