Abstract
Peripheral nerve lesions are frequent occurrences in both human and animal patients, leading to important physiological and labor complications that affect the quality of life of those who suffer the injury. More severe injuries are often associated with poor nerve regeneration and inadequate functional recovery, even with early medical and surgical interventions. Peripheral nerve crush lesions are frequent and, therefore, an experimental lesion paradigm widely used in researches involving nerve injury models and therapies for its resolution. In recent years, many studies have focused on innovative approaches to peripheral nerve treatment after crush injuries with more or less success. This review addresses the theme of peripheral nerve injury, with a special focus on the axonotmesis lesion, its etiology, pathophysiological mechanisms, methods of functional evaluation and regenerative processes, therapeutic options and corresponding recent advances.
Public Interest Statement
Peripheral nerve injury is a common phenomenon and transversal to human and veterinary medicine. The causes of these occurrences are multiple, and may be traumatic or result from human interventions. Being able to manifest themselves with different degrees of severity, these nervous lesions are, in the majority, incapacitating from the physiological and labor point of view, and the treatments applied today are still far from being totally successful in their resolution. Axonotmesis, commonly known nerve crush injury, occurs frequently associated with compressive forces, fractures, joint displacements or hematomas. Being one of the most explored injuries in regenerative medicine, it is also the one in which the most advances have been achieved in recent years. The present article explores the characteristics of axonotmesis, the associated nerve changes as well as the therapeutic advances achieved in the last years for its resolution.
Competing Interests
The author declare no competing interest.
1. Introduction
Peripheral nerve injury (PNI) is a common occurrence both in humans and animals, leading to severe and long-term physiological and functional disabilities (Wojtkiewicz et al., Citation2015). Its clinical relevance is high, since PNI is much more frequent and undervalued than spinal cord injuries (Ronchi et al., Citation2009). The causes of PNI are multiple and distinct, and may include traumatic events or result from iatrogenic interventions, mostly medical or surgical (Antoniadis et al., Citation2014). Loss of motor, sensory or autonomic function in the denervated body segments is the main consequence associated with this type of injury and usually entails to severe functional deficits (Navarro, Citation2016).
It is known that the peripheral nervous system (PNS) presents a better reparative and regenerative capacity than the central nervous system (CNS), and this difference is based essentially on the characteristics of the functional environments in each one of the systems (Lutz & Barres, Citation2014), the age of the injured individual, the type of injury observed and the integrity of the neural cell body of the injured nerve (Faroni, Mobasseri, Kingham, & Reid, Citation2015). Nevertheless, ineffective functional recovery is common in the injured peripheral nerve, particularly due to phenomena of chronic axotomy, chronic Schwann cell denervation (Sulaiman & Gordon, Citation2013) or severe disruption of endoneurial tubes that prevent normal progression of the regenerative process (Burnett & Zager, Citation2004). Muscular denervation is most often secondary to the injury of the corresponding peripheral nerve and manifests mainly by neurogenic atrophy and structural fibrosis (Sulaiman & Gordon, Citation2013). The muscle tends to atrophy as the bare fibers shrink and lose their ability to expand (Krarup, Boeckstyns, Ibsen, Moldovan, & Archibald, Citation2016).
The range of possibilities in terms of severity and outcomes after PNI is broad and depends on the type of injury. The degree of recovery may vary between low or null in more severe injuries (Kemp, Cederna, & Midha, Citation2017) like neurotmesis (total disruption of the axons and its surrounding layers) and good recovery levels in neuropraxia (compression or mild crush injury with Schwann cell sheath affection, maintaining the integrity and continuity of the axons and connective tissue) or axonotmesis lesions (loss of integrity of the axon and myelin sheath, with maintenance of the outer layers of connective tissue and anatomical shape of the nerve) (Dahlin & Wiberg, Citation2017). The best outcomes are observed in lesions with lower severity or with rapid intervention, but even in these cases, the prolonged denervation of the nerve segments distal to the lesion site leads to low recovery rates and, sometimes, chronic and lifelong disabilities (Rochkind & Nevo, Citation2014).
PNI can occur isolated or be associated with CNS lesions like brain injuries. These situations make it difficult to identify and classify the peripheral problem due, for example, to the cognitive changes identified in the patient and to the priority given to life-sustaining measures. In these cases, and while the peripheral nerve injury is not identified, timely therapeutic intervention can not be achieved and the consequences will be more severe than that observed in a rapid intervention (Mete, Atalay, Yemişçi, Karataş, & Turhan, Citation2007). In cases of superimposition of PNI with CNS trauma, the initial clinical manifestations may be only flaccidness, areflexia, and decreased mobility of the corresponding limbs (Robinson, Citation2004). Since most peripheral nerves are composed of motor, sensory and autonomic neurons, an injury can cause changes in both the efferent (motor and autonomic) and afferent (sensory) components of the nerve (Menorca, Fussell, & Elfar, Citation2013). PNI promotes the loss of sensory input from the somatosensory system but also causes changes in neural circuits in the spinal cord, with later long-term changes in spinal somatosensory functions and development of neuropathic pain, allodynia, anesthesia, paresthesia, hypoesthesia, hyperesthesia, and pain in the areas supplied body segments (Fitzgerald & McKelvey, 2016; Houschyar et al., Citation2016). Motor deficits manifest mainly through phenomena of paresis or paralysis of the affected muscles, weakness and muscular atrophy (Lalkhen & Bhatia, Citation2011). Nerve crush injuries or axonotmesis can occur in several situations, including fractures, joint displacements, hematomas or extreme compressive forces (Algora, Chen, Seaber, Wong, & Urbaniak, Citation1996). When lesions occur in the hindlimb, the sciatic nerve is the most commonly affected due to compression of the nerve roots, femoral neck fractures, hip dysplasia or contusions (Kim, Murovic, Tiel, & Kline, Citation2004). In these cases of hindlimb injury, nerve involvement can occur either directly or indirectly. The occurrence of extensive laceration or complete transection of the nerve can be observed due to the presence of fractures and sharp bone fragments near the anatomical location of the nerve. The development of swelling in the soft tissues due to inflammation, severe infection or hemorrhage and the direct action provoked by the displacement of osseous fractures can lead to physical compression with development of secondary compressive neuropathy (Bigelow & Graves, Citation1952; Jacobson & Schrader, Citation1987). In men, sciatic neuropathy is a common occurrence with several etiologies, including compression with traumatic, ischemic or idiopathic origin (Feinberg & Sethi, Citation2006). Lesions of peripheral nerves in the upper limb are the most common in man, particularly those associated with traumatic events, (Castillo-Galván, Martínez-Ruiz, De la Garza-Castro, Elizondo-Omaña, & Guzmán-López, Citation2014; Ciaramitaro et al., Citation2010; Eftekhar-sadat, Babaei-Ghazani, Samadirad, & Mamaghany, Citation2017; Neal & Fields, Citation2010; Saadat, Eslami, & Rahimi-Movaghar, Citation2011), and the risk of injury to these nerves is increased due to their anatomical location. The most common risk factors include the superficial location of the nerves, their anatomical pathway in a large area exposed to possible trauma, and their passage through a narrow bony canal (Neal & Fields, Citation2010). A classic example of peripheral nerve injuries in the forelimb is the carpal tunnel syndrome in humans, in which compression and traction of the median nerve at the level of the carpal tunnel lead to a crush injury of this nerve (Aboonq, Citation2015). The crushing lesions in the facial nerve result from direct local impacts or from the transfer of energy from the surrounding skeletal and bony elements of the skull (L. N. Lee, Lyford-Pike, & Boahene, Citation2013). In these cases, the lesions may be caused by the external pressure applied to the nerve itself or by the isquemia that occurs when the crushing force exceeds the capillary perfusion pressure (Ozturk, Citation2015). The prognosis is highly dependent on the cause of the lesion, its extension and compression time.
The objective of this article is to address the PNI theme with a special focus on axonotmesis, revisiting relevant studies based on this type of lesion and reviewing the advances achieved in peripheral nerve regeneration after crush injury. In addition to a complete bibliographic review involving the classic and most recent knowledge regarding axonotmesis lesions, its mechanisms and pathophysiology, diagnosis, experimental studies and available therapies, the authors also present a table containing therapeutic trials used in studies of peripheral nerve regeneration after crush injury. In the same, a study considered relevant and exemplary of each pharmacological or surgical therapeutic approach was selected regardless of the nerve used or the year of publication. For the complete understanding of the same by the reader, the animal model used, the type of injury induced, the therapeutic approach selected, experimental groups defined, methods of diagnosis, characterization and observed outcomes are indicated in Table .
Table 1. Examples of scientific literature that explore the regeneration of peripheral nerves submitted to axonotmesis with different therapeutic approaches
1.1. Functional anatomy of the peripheral nerve
The peripheral nerve (Figure ) results from the fusion of two roots that extend from the spinal cord: the ventral root includes the motor neurons; the dorsal root includes the sensory neurons and its cell bodies are located in the dorsal root ganglion (Catala & Kubis, Citation2013). The cell bodies of motor neurons are found in the CNS, namely in the ventral horn of the spinal cord and in specific nuclei of the brainstem (Stifani, Citation2014). The long axons of sensory and motor neurons communicate with distant target organs (Grinsell & Keating, Citation2014).
Figure 1. Schematic representation of the peripheral nerve anatomy and structural overview of the PNS.
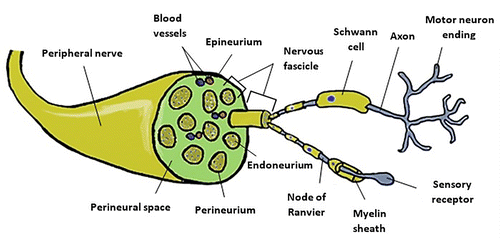
Each peripheral nerve is covered by three layers consisting essentially of connective tissue and which are histologically called stroma (Mills, Citation2007). The endoneurium directly coats each axon, and although it contains a thin network of capillaries and microvessels and an outstanding intrinsic elasticity, this layer guarantees little mechanical protection (Mizisin & Weerasuriya, Citation2011). A group of axons surrounded by endoneurium is called nervous fascicle, and each fascicle is covered by perineurium, a thin but dense connective layer. Stronger than the endoneurium, perineurium provides mechanical protection against tensile forces and supports the blood-nerve barrier and nerve hemostasis, protecting the endoneurial environment against sudden changes of concentration in the vascular and extracellular spaces (Weerasuriya & Mizisin, Citation2011). All fascicles of a nerve are included within the outermost coating layer, the epineurium, which, depending on the nerve in question and its dimensions, represents between 30% and 70% of the sectional area of the nerve trunk (Rigoard et al., Citation2009). The inner portion of the epineurium directly coats all fascicles and their perineurial coatings, contains blood vessels that irrigate and travel along the nerve and even small amounts of adipose tissue. The external layer coats the entire nerve, giving it mechanical protection and its anatomical shape (Grinsell & Keating, Citation2014). While the endoneurium has a longitudinal orientation, the perineurium and the epineurium are circumferentially disposed (Seddighi et al., Citation2016).
Within the endoneurium are myelinated or unmyelinated axons in close relation to the Schwann cells. These glial cells guarantee functional connections with the terminal organs such as muscle fibers or sensorial terminations by ensuring the saltatory propagation of action potentials along the axon (Said & Krarup, Citation2013). To the set formed by the axons and by the Schwann cells that surround it is given the histological denomination of parenchyma (Mills, Citation2007). The axon itself has a tubular shape. The axonal cytoskeleton has a microfibrillary structure consisting of three large groups of proteins: microfilaments, microtubules and intermediate filaments including neurofilaments. The function of the cytoskeleton is essentially to maintain the shape and participate in axonal growth (Rigoard et al., Citation2009), ensuring the transport of proteins and organelles between the cell body and the axon terminals (Josta & Casper, Citation2015). Some axons develop in close relationship with Schwann cell chains, each one associated with a single axon and responsible for producing their myelin sheaths. These sheets are essentially composed of several layers of Schwann cell membranes associated with secreted proteins. Between two myelin segments, also called internodes, there are demyelinated spaces called Ranvier nodes (Pereira, Lebrun-Julien, & Suter, Citation2012). The most abundant protein is P0, which mediates the cell-to-cell interactions and those between the neurons and myelin sheaths, being essential in the formation of the latter and in its maintenance (L. Zhao & Zheng, Citation2010).
Myelin helps insulate the axons of electrically charged atoms and molecules present in the fluids that surround the entire peripheral nervous system, but their main function is to specifically increase the rate at which neural electrical impulses propagate along the nerve fiber. In a demyelinated nerve fiber, the electrical impulse moves continuously in wave motions. In the myelinated fibers, the conduction is done through a saltatory propagation. The myelin depletes the capacitance and increases the electric resistance along the cell membrane in order to prevent the electric current from leaving the axon. This function is achieved through a heterogeneous distribution of voltage-dependent sodium channels along the myelinated fiber, arising at high density at the Ranvier nodes and at low density in the para and internodal regions (Saladin, Citationn.d.). In this way, as sodium losses to the extracellular fluid are reduced along the inner regions, a separation of electrical charges is maintained between intra- and extracellular fluid, allowing sodium to move along the axon more efficiently. Despite this, although sodium moves rapidly along the cell membrane, losses are unavoidable, and when the ion values are very low, there is an inability to open the charge-dependent sodium channels. Ranvier nodes, on the other hand, possess very high densities of easily excitable sodium channels when exposed to the ion present in the extracellular fluid, which is a sufficient amount for the channels to open and sodium to penetrate the axon and regenerate the action potential (Brady, Siegel, Albers, & Price, Citation2011). At these sites, the action potential is restored to values similar to those present at the beginning of the axon and travels rapidly along the myelinated axon, jumping between the nodes of Ranvier, with a propagation velocity that can reach 10–500 m/s in man (Schalow, Zäch, & Warzok, Citation1995; van Veen, Schellens, Stegeman, Schoonhoven, & Gabreëls-Festen, Citation1995). Smaller axons usually do not have myelin sheaths and the propagation of the electrical impulse is much slower. In these cases, the anatomical relationship between the axons and the corresponding Schwann cells is much more intimate and has direct influences on the phenomena of nerve regeneration (Said & Krarup, Citation2013).
The epineural circulation consists of networks of microvessels extending longitudinally along the epineurium and from which cross branches transverse through the perineurium to form a vascular plexus of capillaries at the level of the endoneurium. This vascular system is extremely fragile, and any change in the nerve can lead to reductions in blood supply to residual levels. The collagen coating layers ensure increased protection and less susceptibility to compression forces since the impact is not directly transmitted to the vessels (Gao, Weng, & Wang, Citation2013).
1.2. Peripheral nerve injury grading system
The regenerative success of the peripheral nerve is directly dependent on the severity of the injury. The adopting of grading systems related to the type of lesion has as the main objective establishing a direct relation between the microscopic changes observed and the clinical manifestations and functional prognosis.
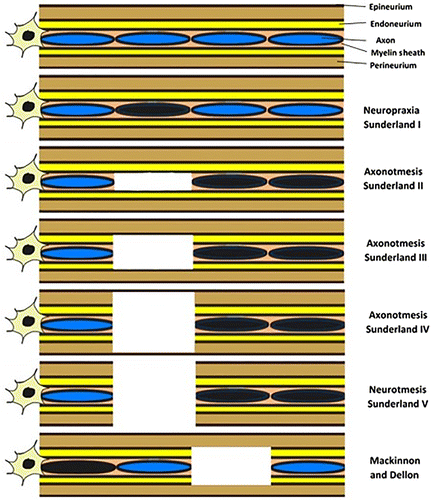
The first classification system was created in 1943 by Seddon (Figure ), who proposed the establishment of three categories of gravity dependent on the extent of damage to axons and coating tissues (Seddon, Citation1943). In this system, neuropraxia is the first degree and mildest PNI, with no loss of nerve continuity. The axons maintain their anatomical integrity but become dysfunctional. Typically, there are no Wallerian degeneration phenomena, although small foci of ischemia and segmental demyelination may lead to ion-induced conduction blockages and motor and sensory losses. Usually, motor fibers are more affected than sensory. Once the affected nerve fails to effectively transmit the electrical impulses, the corresponding body segments become temporarily paralyzed until remyelination occurs, although muscle atrophy rarely develops. Once the compressive force disappears, complete recovery occurs within days or weeks without the need for surgical intervention (Choi et al., Citation2016).
Axonotmesis, the second level of PNI and known as crush injuries, is characterized by a disruptive lesion of the axon and its myelin coating. The integrity of the outer connective tissue covers, namely the perineurium and the epineurium, remains, ensuring that the anatomical shape of the nerve is maintained. Once there is an effective injury of the axon, Wallerian degeneration occurs distally to the lesion site. However, since the integrity of collagen coats, whose function is also to guide and redirect the growth of axonal buds during the regeneration process, is maintained, the prognosis tends to be excellent and can be observed a good recovery rate depending on the degree of internal disorganization and the distance to the target organ (Burnett & Zager, Citation2004; Seddon, Citation1943).
The third level of injury, neurotmesis, is characterized by a complete disconnection between the two segments of the injured nerve, affecting the axons, disrupting and distorting all coating layers. Common causes for this type of injury include penetrating injuries, pulling forces and injection of toxic substances. In this case, there is a total functional loss and the recovery without surgical intervention or another alternative therapeutic method is practically non-existent since the exuberant scarring phenomena. The loss of the collagen coatings and their guiding function in the axonal regrowth prevent the normal regenerative sequence (Campbell, Citation2008; Seddon, Citation1943).
In 1951 Sunderland proposed a new classification system (Figure ) consisting of 5 categories related to the severity of the injury observed (Sunderland, Citation1951). First degree is equivalent to neuropraxia of Seddon’s classification system and the second, third and fourth degrees of injury correspond to subdivisions of axonotmesis. In the second degree of injury, axonal disruption occurs but there is preservation of the endoneurial structure, maintaining the fascicular alignment and the integrity of the perineurium and epineurium. Lesions of the endoneurium may be partial, and the prognosis depends on the degree of conservation of this layer. In the third degree, the axons, myelin sheaths, and endoneurium are disrupted, but the fascicular alignment and integrity of the outer layers of collagen are maintained. Recovery may occur over several months with conservative treatment or with surgical interventions to release entrapment sites, with or without performing neurolysis. In the fourth degree, all layers except the epineurium are disrupted. The occurrence of haemorrhage within the nerve and the presence of fibrous tissue associated with fascicular discontinuity imprison and hampers the growth of new axonal buds, promoting the formation of neuromas-in-continuity. Finally, the fifth category concerns to a complete transection of the nerve, including the epineurium, and the formation of end-bulb neuromas is common. In these latter two degrees, recovery without surgical intervention or another type of therapy is almost impossible (Campbell, Citation2008; Chhabra, Ahlawat, Belzberg, & Andreseik, Citation2014; Choi et al., Citation2016).
Finally, in 1988 Mackinnon and Dellon introduced a sixth-degree injury (Figure ) to the grading scheme of Sunderland, corresponding to the occurrence of mixed injuries (Mackinnon & Dellon, Citation1988). This last degree considers situations in which the same nerve can suffer distinct types of lesions with different severities throughout its extension and cross section. In cases of penetrating trauma and fractures near peripheral nerves, this may be the most common type of injury. The level of recovery and the need for intervention depends, therefore, on the type of lesions and their degree, also conditioning the type of treatment to be instituted (Chhabra et al., Citation2014).
2. Axonotmesis: The crush injury
2.1. Etiology
The mechanisms associated with PNI in general can be divided into three categories: mechanical or traumatic, vascular or ischemic and chemical or neurotoxic (Brull, Hadzic, Reina, & Barrington, Citation2015), and crushing injuries are particularly associated with traumatic and vascular events. The mechanical processes are associated to an acute trauma of high aggressiveness with a blunt object that does not result in a complete transection of the nerve (Zochodne & Levy, Citation2005) and to iatrogenic interventions like application of surgical clamps or anaesthesia administrations in the perineural or intraneural space (Hogan, Citation2008).
Nerve compression may lead to focal demyelination with ischemia and, if prolonged, to blockages in nerve conductivity and neuropeptide production (neuropraxia) or cause total crushing with axonal disruption (axonotmesis) (Burnett & Zager, Citation2004). Stretching lesions generally occur secondary to intense and exaggerated exercise, joint dislocations (Ozturk, Citation2015) and to fractures at the extremities where intimate contact of the peripheral nerves with the bones is established. Peripheral nerves have a remarkable intrinsic elasticity due to the collagen content of the endoneurium, but if the applied force exceeds the elasticity threshold, nerve avulsion and different degrees of injury (axonotmese or neurotmese) can occur (Hainline, Citation2014). Even injuries resulting from perforating objects can result in complete transection, crushing in some segments of the nerve and maintenance of its macroscopic continuity (Uzun et al., Citation2006). Ballistic lesions are particular cases combining both transection and crushing injuries originating from shock waves that propagate through the tissues upon penetration of the bullet. Even if the bullet does not reach the nerve itself, it suffers the effects of tearing and compression when it passes through the surrounding tissues (Shimon Rochkind, Citation2015).
Vascular changes occurring simultaneously to the nerve injury may trigger ischemic phenomena, obliteration of the vasa nervorum-derived arteries, and haemorrhage within the nerve sheaths (Lim et al., Citation2015). Sufficiently aggressive traumas may lead to changes in epineural vessel permeability and, in more severe cases, lesions in the endoneurial vessels with occurrence of oedema and intrafascicular haemorrhages secondary to nerve injury, despite the protective effect of the collagen coating on nerve vascular plexuses. These changes not only promote a hypoxic environment that contributes to the manifestations of neuropathic pain but also the haemorrhage and oedema itself can promote axonal compression, crushing and disruption (Gao et al., Citation2013; Lim et al., Citation2015).
The neurotoxicity associated with the use of anaesthetics for peripheral nerve blockages or other drugs is a common and well described occurrence. Intraneural injection is associated with loss of sensitivity, pain, causalgia, and the incidence, duration and associated sequelae are varied (Farber et al., Citation2013). The lesions associated with the injection are multifactorial and depend on the type and size of the needle, site and angle of insertion, pressure applied during administration and, of course, type and dose of drug administered (toxicity) (Kobayashi et al., Citation1997; Whitlock et al., Citation2010). The site of administration (extraneural, intraneural, interfascicular or intrafascicular) affects the degree of toxicity. Thus, the same substance administered under different conditions (different portions of the nerve) can have different toxic effects and lead to different degrees of injury (Cheng et al., Citation2016). The direct chemical effects originate in the toxicity of the solutions when in contact with the nerve or with the adjacent tissues, provoking acute inflammatory reactions and subsequent chronic fibrosis involving the nerve (Farber et al., Citation2013). Administrations of large quantities and under pressure can lead to crushing lesions and neurotmesis prior to the inflammatory reaction.
2.2. Pathophysiology and mechanisms of neural recovery
The crushing lesions may give rise to different degrees of neural lesions. Constituting mainly axonotmesis, may also be part of different degrees of injury of the Sunderland or Dellon and MacKinnon scheme (Kurtoglu et al., Citation2005). The crushing lesions are, however, tendentially less severe than those of nerve transection, since the basement membranes of Schwann cells covering the fascicles and nerve fibers are anatomically intact and guarantee that Schwann cells can serve as guide in axonal regeneration (Zimmerman & Granger, Citation1994). In the induced lesions, myelin and axonal degeneration were observed at the lesion site one week after its induction. In lesions with distal location in the peripheral nerve, after 3 weeks, most of the axons are already regenerated and remyelinated and the functional recovery is complete after 4 to 5 weeks (Lundborg, Citation2000). In more proximal lesions as well as in the nerve plexuses, the recovery times are much longer, around 1 to 2 years, since the rate of regeneration and reinnervation is about 1–4 mm per day (Saliba, Saliba, Pugh, Chhabra, & Diduch, Citation2009).
The nerve damage that occurs after the crushing injury originates both in the external pressure directly applied on the nerve, in the mechanical deformation resulting from the redistribution of the tissues from the compressed zones to the zones without compression and in the ischemic phenomena resulting from forces that exceed the capillary perfusion pressure. The combination of these types of forces will result in lesions of greater severity and worse prognosis (Algora et al., Citation1996; Li et al., Citation1996; Lundborg, Citation1988). After induction of a crushing force with a significant duration, a circulatory arrest occurs (Kobayashi et al., Citation1997). In addition, local ischemia instituted by the pressure promotes biochemical reactions due to microvascular endothelial lesions. The peripheral nerve responds to the crushing injury through an inflammatory reaction that promotes an increase in local vascular permeability and a subsequent intraneural oedema. The occurrence of endoneurial edema greatly alters the microenvironment of the nerve by increasing local pressure, thereby decreasing blood flow and altering the concentration of electrolytes at the endoneurium. If the circulation is not a quickly restored, the ischemic phenomena promote the establishment of Wallerian degeneration in the axon (Kurtoglu et al., Citation2005). Lesions observed in the nerve after its release are greatly influenced by reperfusion, which in turn appears to be mediated by reactive oxygen species (Kobayashi et al., Citation1997) and lactic acidosis (Cheng et al., Citation2016). Reperfusion lesions are complex and involve multiple pathways and molecules, including membrane lipids, enzymes, and specific receptors. Lesions originating from oxygen free radicals can be reduced by the effect of cellular antioxidants as superoxide dismutase (SOD) or catalase (CAT) which has the ability to eliminate excess of O2 (Lim et al., Citation1986; Zimmerman & Granger, Citation1994), ascorbic acid or α-lipoic acid (α-LA) and other antioxidants that protects the nerve against those types of lesions and promotes recovery (Shokouhi et al., Citation2005). Malondialdehyde (MDA) act as an indication of lipid peroxidation phenomena, and is generally increased in cases of oxidation after reperfusion lesions (Senoglu et al., Citation2009).
When denervation of an end-organ occurs, its reinnervation may develop through two mechanisms: collateral branching of the intact axons or regeneration of those injured (Aguayo, Peyronnard, & Bray, Citation1973). In lesions in which 20 to 30% of axons have been injured, collateral branching is the most common recovery mechanism. This phenomenon begins within the first four days after the injury and can last for three to six months (Zochodne & Levy, Citation2005). There are always a greater number of axons that sprout than those that eventually establish connections and reinnervate the target organs. The axons that fail their pathway are those that do not receive neurotrophic factors from the target organ and inevitably end up degenerating (Menorca et al., Citation2013). In lesions in which there is an affection of more than 90% of the axons within the nerve, like in crush injuries, axonal regeneration is the main recovery mechanism (Lunn, Brown, & Perry, Citation1990). For recovery to be effective and complete, it is necessary that three sequential phases occur: Wallerian degeneration, axonal regeneration and functional reinnervation of the end-organ. Failures in each one of these phases are the main causes of poor prognosis and outcomes observed in PNI.
Before the regenerative process begins, a series of degenerative phenomena are required, which act as a direct prelude to regeneration (Burnett & Zager, Citation2004). Thus, the success of nerve regeneration depends not only on the severity of the established lesion but also on the efficacy of the subsequent degenerative process, being a rapid and efficient inflammatory response a fundamental parameter (Gaudet, Popovich, & Ramer, Citation2011). The term Wallerian degeneration can be used to characterize the calcium dependent phenomena that occur in both PNS and CNS after a traumatic injury, although there are differences between the two systems with respect to the cells involved (Schwann cells and macrophages in PNS and oligodendrocytes and microglia in the CNS) and to the outcome (greater efficacy in the removal of myelin in the PNS). A sufficiently intense traumatic force promotes abrupt tissue damage at the injury site where the physical impact occurs. These structural changes at the level of the axons or their bi-phospholipidic layer unchain a cascade of events of programmed cell death that will not be interrupted if the intervention is not fast enough. The nerve segment distal to the lesion site undergoes a set of cellular alterations characteristic of Wallerian degeneration, even though they are not the direct targets of the lesion. Nevertheless, the sequence of degenerative events proliferates both proximally and distally (Menorca et al., Citation2013).
Once lesion and physical separation occur between the two axonal segments (proximal and distal to the lesion), the distal portion initiates the degenerative phenomenon due to loss of communication with the cell body and related metabolic resources. The proximal segment suffers reactive swelling after the injury, but the lesions associated with retrograde degradation are generally minimal (Hall, Citation2005). The axon in the distal segment degenerates through a process of swelling and subsequent proteolytic and autolytic granulation that takes place over 3 to 4 days (Hall, Citation2005). The first event involves an influx of calcium into Schwann cells and into axonal axoplasm due to the sudden interruption of oxygen supply (Smith & Hall, Citation1988). This influx of calcium in the axon causes activation of the protease calpain (Touma, Kato, Fukui, & Koike, Citation2007), is essential for the formation of growth cones (Chierzi, Ratto, Verma, & Fawcett, Citation2005) and for axonal outgrowth (Widerberg, Bergman, Danielsen, Lundborg, & Dahlin, Citation1997). During this period a mechanism called chromatolysis occurs (Moon, Citation2018). This event involves severe morphological changes in the neuron, with occurrence of changes in aggregation, organization and localization of Nissl bodies, cisternae present in the rough endoplasmic reticulum, replete with ribosomes and whose functions are related to the production of proteins for the functional machinery of the neuron (Johnson & Sears, Citation2013). In the chromatolysis, there is fragmentation of the aggregates of rough endoplasmic reticulum, often accompanied by degranulation and loss of ribosomes from rough endoplasmic reticulum, observing in electron microscopy the presence of zones without Nissl bodies. The disaggregation of poly and monoribosomes also occurs, and both these and endoplasmic reticulum fragments can be found within autophagic vacuoles. The cell body itself undergoes severe morphological changes with membrane changes and movement of the nucleus to an eccentric position, a direct consequence of loss of protein synthesis in cell (Johnson & Sears, Citation2013; Moon, Citation2018). Although in the initial descriptions the chromatolysis was considered a catastrophic event for the cell, later studies allow to identify its reversible nature during successful axonal regeneration phenomena (Gersh & Bodian, Citation1943). Not only has it been described that the chromatolysis is essential and allows the occurrence of axonal regeneration, as it was proposed that it is a catabolic process that nevertheless does not overlap with the anabolic processes of RNA and protein production that develops in neurons capable of an effective regeneration and that does not progress to irreversible apoptosis. Thus, some neurons may exhibit transient chromatolysis; sufficiently damaged neurons may not recover and remain chromatolytic and not return to normal levels of protein synthesis, progressing to atrophy or apoptosis; and some still manage to protect the protein synthesis machinery from catabolic events and promote regeneration (Matthews & Raisman, Citation1972; Moon, Citation2018). Myelin degeneration occurs by two mechanisms: not only do Schwann cells promote a breakdown process of their own myelin, but also attracts the hematogenous macrophages to perform phagocytosis of the resulting debris and lipidic droplets released into the medium. In turn, Schwann cells can also perform autophagy, assisting the macrophages in the phagocytosis of their own myelin debris in later stages of the lesion (Gomez-Sanchez et al., Citation2015). The axonal telodendria also disintegrate (De Lahunta, Glass, & Kent, Citation2009). All these debris attract phagocytic cells (macrophages and neutrophils assisted by the Schwann cells themselves) whose function is to phagocyte and destroy these products from cellular degeneration (Dubovy, Klusakova, & Hradilova Svizenska, Citation2014). Activation of immune cells residing in the peripheral nerves and the exuberant attraction of immune cells to the lesion site (neutrophils, lymphocytes, mast cells, macrophages) often aggravates Wallerian degeneration, inhibiting normal repair and regeneration of the peripheral nerve (Koeppen, Citation2004).
Schwann cells, which in the terminal phase of degeneration are reduced to their nucleus and cytoplasmic organelles, initiate a rapid phase of mitotic proliferation, partly due to calcium influx (Svenningsen & Kanje, Citation1998), synthesising a new extracellular matrix and forming a column of cells called Bands of Büngner whose function is to provide a pathway to guide the growth of the axons to the target organ (Napoli et al., Citation2012). Schwann cells also produce growth factors that, in addition to those produced by the target organs, stimulate the growth of new axonal buds from the proximal axonal segment still intact (Verge, Gratto, Karchewski, & Richardson, Citation1996). Among these factors are nerve growth factors (NGF), a set of neurotrophic factors capable of protecting sympathetic, sensory and cholinergic nerves, promoting the development and differentiation of nerve cells, increasing the number of sensory and sympathetic ganglia, and promoting nerve growth in the PNS (Ma et al., Citation2014). At the same time, Schwann cells produce interleukins that not only stimulate the proliferation of new Schwann cells but also promote proliferation and organization of axonal buds and fibroblastic cells (Verge et al., Citation1996). The absence of Schwann cells at the nerve injury site (or their destruction during the process) can significantly decrease the percentage of axons that regenerate and reinnervate the target organs (Kuffler, Citation2015).
Approximately 2 (Burnett & Zager, Citation2004) to 7 days after injury, and once all debris has been removed, the regenerative process begins in the proximal segment and extends to the distal one. A high number of axonal buds emanate from the last Ranvier node of the proximal segment and extend into the distal segment, guided by the Bands of Büngner (Deumens et al., Citation2010; Grinsell & Keating, Citation2014) and by the growth factors secreted (Mudo et al., Citation1993). During this step, different proteases are released from the growth cone to aid the progression of regenerating axons through the neighbouring tissue. Theoretically, the greater the number of axons in regeneration and the axonal buds that reach the distal segment, the greater the extent of neurological recovery (Madison, Archibald, & Brushart, Citation1996). But in practice, it is known that most of these regenerating axon buds can not extend to the distal tubules of the basement membrane. Of the various axonal extensions, only a few contacts the receptor at the distal ends and some are trapped in the haemorrhagic and fibrous tissue surrounding the lesion site. Therefore, is important that occurs an abrading of the remaining axonal buds, thus avoiding that they continue to grow disorganized and origin neuromas (De Lahunta et al., Citation2009). In summary, a high number of axonal buds does not guarantee the establishment of multiple effective and functional connections with the distal nerves or muscle fibers, and even if this happens, the way axonal buds can use their potential functional reserve and allow functional recovery of the different motor units in each situation remains unclear and even unexpected (An et al., Citation2015).
After nerve damage, Schwann cells contribute to the creation of a permissive environment that allows for axonal regeneration. At the same time, during Wallerian degeneration, there is a complete disruption of the axon-Schwann cell communication which is established during normal development. At this stage the Schwann cell undergoes a redifferentiation, with changes in genetic expression, and promotes the remyelination of regenerated axons, depending on the stimulation from molecules such as neuregulin (Stassart et al., Citation2013). As the axons regenerate, interactions with the Schwann cells are also re-established. This, of course, guarantees the remyelination of the axons and the restoration of the physiological function of the nerve fibers. Although regeneration and remyelination take place synergistically, remyelinated axons usually present a thinner myelin sheath and lower internodal lengths when compared to the correspondent axonal prior to the lesion, significantly reducing the velocity of the nerve impulse (Sherman & Brophy, Citation2005). These suboptimal results may be caused by inefficient stimulation of Schwann cells, by inhibitory factors, or by poor response of the damaged Schwann cells to myelination-inducing factors (Stassart et al., Citation2013). Several components are involved in the phenomenon of remyelination, among them the aforementioned neuregulin, matrix metallopeptidases and Insulin-like growth factor (IGF-1) (Svennigsen & Dahlin, Citation2013).
When low crushing loads are applied, the degree of nerve injury and the rate of functional recovery are directly dependent on the duration of the damaging force application. While crushing durations of about 10 min promote mild edema, diffuse axonal degeneration, and short-term paralysis in the affected limb, those in the range of 2 to 6 h are associated with total or subtotal axonal degeneration and long-term paralysis. In the latter case, the phenomena of ischemia, reperfusion and dysfunction of the blood-nerve barrier in the microcirculation have a profound influence on the severity of the nerve injury (Schmelzer, Zochodne, & Low, Citation1989).
Without any type of therapeutic intervention, PNI is rarely followed by total functional recovery. The regenerative process can be hampered by many factors, namely the formation of scar tissue within and around the nerve and the establishment of adhesions between the nerve and the surrounding tissues (Varitimidis, Riano, Vardakas, & Sotereanos, Citation2000). These new compounds are essentially deposits of collagen that not only cause painful traction and neuropathic pain during muscle contractions but also interfere with normal axonal regeneration, leading to low functional recovery (Zuijdendorp et al., Citation2008). The Schwann cells themselves not only produce growth factors but also an extracellular matrix which, in a given extension, can inhibit axonal regeneration processes (Chen & Brushart, Citation1998). Traditionally, surgeons attempt to mitigate this uncontrolled formation of collagen scars by approaching the corresponding nerve fascicles (Holmes & Young, Citation1942) but even with this intervention effective recovery in all patients is not guaranteed. In general, it can be affirmed that the regenerative success in the peripheral nerve after injury depends on the balance between the regeneration of Schwann cells and the formation of scar tissue (Kaplan et al., Citation2011).
Collateral branching and regrowth of axons to incorrect muscles is also a frequent cause of poor functional recovery, and proper reinnervation of the neuromuscular junctions is a limiting factor in the functional recovery after PNI (Guntinas-Lichius et al., Citation2005). Another great difficulty is that, in the peripheral nerve, the sensory and motor neurons are mixed and it is necessary to establish correct connections between them and the respective target organs so that the reinnervation occurs correctly. Often, axons with previous motor function undergo misdirection for sensory organs and vice versa. These occurrences and reinnervation of incorrect organs lead to serious changes at the level of the somatosensory cortex where the interpretation of peripheral signals is reversed (Rosen et al., Citation2012; Taylor, Anastakis, & Davis, Citation2009). Moreover, when reinnervation occurs inefficiently, there is a decrease in the cortical representation of this body segment in the corresponding cerebral hemisphere. Thus, adjacent ipsilateral regions and corresponding contralateral regions undergo overgrowth to compensate these deficits. Consequently, the interplay of stimuli becomes unpredictable (Li et al., Citation2011). Nonetheless, muscle reinnervation is more effective than cutaneous and glandular reinnervation. The production of growth factors increases significantly in the affected muscle after injury, but no such significant increase is observed in the skin (Hsieh et al., Citation2013). Motor neurons are thought to be able to recognize levels of trophic support in their terminal branches and axonal buds grow toward the site where growth factors are most abundant (Campenot, Citation1982). Most of the growth factors that are important for regeneration and remyelination are also essential for effective reinnervation of the target organ (Svennigsen & Dahlin, Citation2013).
2.3. Diagnosis and clinical manifestations
The diagnosis and precise location of the peripheral nerve lesions are based on the clinical history and the physical and neurological exams of the injured individual (Lee, Singh, Nazarian, & Ratliff, Citation2011). Although the regeneration of crushed nerves is usually spontaneous, the associated morbidity can be variable and depends on the cause of the injury, the moment it occurred and its extent. In the lesions of nerves located on the limbs, functional dysfunctions may range from paraesthesia and partial motor weakness to complete sensory loss and paralysis. Without therapeutic interventions and with longer recovery periods, these lesions may result in complete denervation and atrophy of the corresponding muscles (Ozturk, Citation2015). If the crushing injury occurs at the level of the facial nerve, facial weakness, muscular asymmetry, ocular changes, feeding and swallowing difficulties, changes in facial expressions and in vibrissa movements can be observed (Lee et al., Citation2013). In the case of the hypoglossal nerve, since it innervates the muscles of the tongue and presents an important motor function in the maxillofacial region, its lesions can cause changes in the tongue movements as difficulties to feeding and swallowing (Zhang & Tu, Citation2005). Similarly, injury to any peripheral nerve will manifest with important functional changes in their innervated regions and organs.
2.4. Experimental models
2.4.1. In Vitro models
In vitro research on peripheral nerve regeneration is still extremely limited due to the anatomical and structural complexity of these organs whose in vitro reproduction is very difficult and rarely adequately achieved (Geuna, Citation2015). Although some neuronal and glial cell lines have been proposed to replace or complement preclinical studies in vivo, their potential is very limited and insufficient to mimic nerve regeneration (Cirillo et al., Citation2014). It was thought that these limitations could be overcome using organized cultures to mimic the 3D disposition of the nerve and the organization of neuronal and glial cells. Nevertheless, maintenance of these cultures is technically complex and clinical translation has not yet been successfully achieved (Siddique, Vyas, Thakor, & Brushart, Citation2014). Thus, as long as no more effective, less expensive and technically less complex in vitro models are developed, in vivo models of peripheral nerve regeneration are still essential.
2.4.2. In vivo models
2.4.2.1. Animal model
Although in most biomedical applications rat and mouse are the most commonly used species, rat is the main animal model for axonotmesis. The anatomy of the rat is well studied and characterized (Greene, Citation1955) and there are morphological similarities between its peripheral nerves and those of humans. Other advantages of its use include the large relative dimensions of the rat nerves that allow reducing the complexity of the microsurgical procedures (Tos et al., Citation2008), the possibility of standardizing and comparing functional tests and also the resilience of this species when compared with mice (Tos et al., Citation2009), although in terms of dimensions and connective tissue density, they have differences with human nerves (Mackinnon, Hudson, & Hunter, Citation1985; Ronchi et al., Citation2009). The mouse is important in studies with specific objectives where the availability of genetically modified animals is required (Tos et al., Citation2008). The major disadvantage in both species of rodents is their extremely high neuroregenerative capacity, which makes it difficult to assess the efficacy of the therapeutic methods used (Myckatyn & Mackinnon, Citation2004).
Although smaller animal models represent the first choice in peripheral nerve regeneration, the use of larger models in preclinical studies has obvious advantages since the regenerative process in these species is similar to that seen in humans (CitationFullarton, Lenihan, Myles, & Glasby, 2000). Rabbits are particularly important in studies which include instruments and devices whose dimensions are too large for the sciatic nerve size of the rat or mouse, maintaining the advantages of working with rodents (Gao, You, et al., Citation2013). Other species of mammals used include, mini-pig (Uranus et al., Citation2013), guinea pig (Rao, Kotwal, Farooque, & Dinda, Citation2001), dog (Xue et al., Citation2012) and cat (Sufan et al., Citation2001), particularly because of their larger body dimensions. Primates were also used in preclinical studies because of the similarities between these species and the human. For ethical and legal reasons, the use of dogs, cats and primates has been progressively reduced (Geuna, Citation2015).
Among the larger models, the sheep model is considered one of the most relevant for clinical studies prior to translation for humans (Diogo et al., Citation2017). One of the great advantages of using this model is the dimensional similarity between sheep’s and men’s nerves, in both hindlimb and forelimb (Jeans, Gilchrist, & Healy, Citation2007), besides the rate of axonal regeneration is also identical to that observed in man (Lawson & Glasby, Citation1995) and the peripheral nerves of the sheep are polyfascicular and histomorphologically identical to those of man (Strasberg et al., Citation1996). The equivalence of ages between the sheep and the man is also well established, and it is known that a sheep about one year old corresponds to a young-adult human (CitationFullarton et al., 2000). This knowledge is important because it allows us to use the age of the model as an important variable to consider in the results of the studies carried out. From the technical point of view, sheep are easily available, easy to maintain and handle, relatively cheap and do not raise ethical issues in scientific and civil society (CitationFullarton, Myles, Lenihan, Hems, & Glasb, 2001). The median and facial nerves are the most explored in this model, allowing translational applications in areas such as maxillofacial, hand and finger surgery (Diogo et al., Citation2017). Unexpectedly, there are few published studies regarding to the sciatic nerve (Meuli-Simmen et al., Citation1997). Also, the majority of studies in nerve injury resort to the neurotmesis (Meuli-Simmen et al., Citation1997) model and not to the axonotmesis model (CitationFullarton, Lenihan, Myles, & Glasby, 2002). Despite the clear advantages of its use, the studies of peripheral nerve regeneration performed in this model are few and only started to be explored recently. There is a great opportunity to explore this model in studies of peripheral nerve regeneration, particularly in poorly explored nerve such as the sciatic and in lesions with little representation in the literature, namely axonotmesis for which there is still no well-described lesion model in the sheep model.
Some non-mammalian species have also been used in studies of peripheral nerve regeneration, but the phylogenetic distance with humans makes them more important for evolutionary understandings (Blanco, Rosado, Padilla, & Del Cueto, Citation1999).
2.4.2.2. Nerve model
Most studies in animal models are based on the approach to the sciatic nerve and its terminal branches, (Pavić, Pavić, Tvrdeić, Tot, & Heffer, Citation2011), mainly because this is the largest peripheral nerve (Ronchi et al., Citation2009) and due to the high number of functional and behavioural tests available for this nerve (Nichols et al., Citation2005), especially in rat model. The high number of data available in the literature on the sciatic nerve as an axonotmetic model also allows an efficient comparison of the results obtained in the current and previous studies. In the case of the mouse, the reduced dimensions of their nerves require advanced microsurgical techniques, which constitute a significant limitation in the performance of epineural repairs without injury to axonal tissue per se. Still, recent worldwide advances in reconstructive surgery, the discovery of common immunomarkers in both rat and mouse axons and the availability of several colonies of genetically engineered mice are positive indicators of the potential of this species in PNI and axonotmesis studies (Tos et al., Citation2008).
In addition to the sciatic nerve, other nerves of the hindlimb were explored in regeneration studies after PNI, namely the femoral (Robinson & Madison, Citation2009), tibial (Apel et al., Citation2009) and peroneal nerves (Alluin et al., Citation2009). Due to their reduced size in rodent models, these nerves are usually used in larger animal models.
In recent years, the forelimb nerves have gained importance in studies of peripheral nerve regeneration (Wang, Spinner, Sorenson, & Windebank, Citation2008). The median nerve has attracted attention due to the availability of easier and reliable behavioural tests when compared with sciatic nerve tests (Lee et al., Citation2007). In the sheep model, as indicated, the median nerve is precisely the most explored nerve in the literature (Diogo et al., Citation2017). One of the great advantages of using the nerves of the forelimb is the small interference with animal welfare, especially in the rodent models, since the hindlimb is more important in terms of locomotion and environmental exploration. On the other hand, the results obtained in studies of the forelimb are more easily translated due to the importance that surgeries in the nerves of the hand have in human medicine. The movements of the hands and fingers are precise and complex, and these features are common in rodents and humans. Another advantage of the median nerve is that, unlike the sciatic nerve, which normally consists of a single fascicle at its origin which is divided into a variable number of fascicles in its distal portions, this median nerve usually presents a single fascicle through all its extension, which facilitates the quantitative morphological analysis. The major disadvantage concerns to the reduced dimensions of the nerves of the forelimb, which require advanced microsurgical techniques, particularly in the mouse model (Tos et al., Citation2008).
Several non-limb nerves have already been considered and explored in regenerative studies. Among these, the facial (Hadlock et al., Citation2008), hypoglossal (Gonzalez-Forero, Portillo, Sunico, & Moreno-Lopez, Citation2004), inferior alveolar (Atsumi et al., Citation2000), mental (Li et al., Citation2012) (in the head), and vagus (Bregeon et al., Citation2007) and cavernous (Ding et al., Citation2009) (autonomic nerves) are indicated. Of this group of non-limb nerves, the only nerve for which a suitable functional test is available is the facial nerve (Hadlock et al., Citation2008).
2.4.3. Experimental lesion paradigm
Axonotmesis is one of the experimental lesion paradigms referring to nerve regeneration studies, along with neurotmesis. This is strictly related to the clinical manifestations observed in the patient, with the great difference that the crushing lesions in the rat do not lead to the formation of neuromas, unlike in humans (Tos et al., Citation2009). Experimental axonotmesis is induced through a crush injury in which an interruption of the nerve fibers is promoted, with maintenance of the connective tissue lining around the nerve (Sarikcioglu et al., Citation2007) and thus maintaining the anatomical continuity of the nervous trunk. Therefore, the injured axons have a guiding pathway guaranteed by the layers of connective tissue and Wallerian degeneration occurs in the distal segment, mimicking the real clinical situation. With the maintenance of the macroscopic anatomical integrity of the nerve trunk, there is no need to perform an epineural repair (Geuna, Citation2015).
Despite the consolidated use of animal models in studies of PNI by crushing, it has not yet been possible to establish a perfect and standardized method regarding to the crushing method used, duration of force application, instrument used, lesion size, magnitude and reproducibility (Ronchi et al., Citation2009). Several surgical models of crush injury induction have already been developed and tested, including those using surgical instruments, suture knots with specific strands and even the application of crushing forces through needles and flat surfaces (Bain, Mackinnon, & Hunter, Citation1989; De Koning, Brakkee, & Gispen, Citation1986). Among the first instruments available for the induction of crushing injury were simple or haemostatic forceps, but since none of them allows precise quantification of applied force, it made it very difficult to perform a correlational evaluation between induced injury and subsequent recovery. The application of tourniquets to induce injury was also used, although this method, being quantitative, was indirect (Chen et al., Citation1992). The development of a compression box emerged as a viable method of inducing crushing injuries, allowing to control the magnitude of the injury through pressure and duration of application. This device was effective for larger models such as the rabbit, but not for the rat whose body and nerve dimensions are too small (Rydevik & Lundborg, Citation1977). Latter, a specific device created specifically for rats allowed to induce quantitatively controlled lesions in this model (Chen et al., Citation1992).
In 2001, a method was established based on the use of a clamp in which the force, the pressure exerted and duration of the compression are standardized and reproducible (Beer, Steurer, & Meyer, Citation2001). This method has been successfully applied in different models of sciatic nerve (Varejao, Cabrita, et al., Citation2004) and median nerve crush injuries (Ronchi et al., Citation2009). The clamp is manufactured and commercially available from the Institute of Industrial Electronic and Material Sciences, University of Technology, Vienna, Austria. Being equipped with three different springs (41/43/49) and two washers, it can be used in different combinations to apply different forces depending on the manufacturer’s table and the need for the experience.
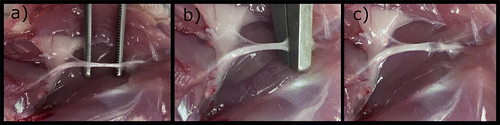
The induction of crushing lesions in the sciatic nerve of the rat is the most commonly performed. The animals should always be operated under anaesthesia and with appropriate analgesia. In lateral decubitus, and after complete trichotomy and local asepsis, access to the sciatic nerve is done through a careful dissection of the gluteal muscles. Once appropriate haemostasis has been established, soft tissue retractors can be applied to expand the surgical field, thereby helping to expose the sciatic nerve and its most important branches (posterior tibial and peroneal nerve). Dissection of the sciatic nerve is not difficult, once used magnifying glasses or surgical microscopes, the nerve being manipulated with microsurgical instruments and avoiding unnecessary trauma to the nerve. The crushing lesion may then be induced according to the intended method, followed by the application of drug therapy or materials, depending on the case. Immediately after acute compression, the crushed area appears flattened, with preservation of the nerve continuity (Figure ) (Tos et al., Citation2009). A 9/0 or 10/0 nylon suture can be performed to the epineurium, distally or proximally to the lesion site, so that it can later be determined whether there are differences in these distances over time due the regenerative process. Then, the gluteal muscles can be sutured alone or in combination with the skin. Usually, the contralateral limb is not operated and can be used as a control in the tests performed. Intervened animals should be observed daily to monitor the wound healing process or, where applicable, to determine nerve function (Ozturk, Citation2015).
Figure 3. Steps for the induction of an axonotmesis lesion in rat’s sciatic nerve: (a) Isolation of the sciatic nerve from neighboring tissues; (b) induction of injury (material used, time and force of application depends on the selected protocol); (c) anatomical flattening of the sciatic nerve after injury.
The lesions applied on other nerves follow the general lines described for the sciatic nerve, with the appropriate anatomical adaptations. For the facial nerve, the surgical access is made through an incision in the infra-auricular region, and the trunk of the nerve should be identified emerging anteriorly to the digastric muscle (Hadlock, Heaton, Cheney, & Mackinnon, Citation2005). To the hypoglossal nerve, access is made through a vertical incision in the submaxillary region so as to expose the nerve below the digastric tendon (Fan, Wang, Wang, Lan, & Tu, Citation2015). In the sheep, access to the median nerve must also be accompanied by particular care. Once anesthesia and analgesia have been established, the animal should be placed in lateral decubitus and the anterior limb properly trichotomized and the conditions of a special should be guaranteed. Cutaneous incision and muscular dissection should be careful due to the presence of large sensitive structures such as accessory cephalic vein, radial artery and vein. After exposing the muscle bellies, the tendon sheath of the tendinous junction of the flexor carpi radialis muscle can be released to facilitate access and allow tendon cutting. Once dissection of the muscles is completed, and with the aid of retractors, the median nerve can be exposed and subjected to a crushing injury with the desired instrument, force and duration. The fascia and subcutaneous tissue may then be re-approximated with absorbable 3/0 or 4/0 sutures and the skin sutured with absorbable 3/0 sutures (Ozturk, Uygur, & Lukaszuk, Citation2015).
The axonotmesis lesion paradigm presents clear advantages when compared to neurotmesis. From the ethical point of view, and considering that in the case of crushing lesions, nerve regeneration is faster, the functional impairment is transient and the discomfort for the animal is comparatively smaller, axonotmesis raises fewer questions than neurotmesis (Geuna, Citation2015). The lesion is technologically easier to induce, facilitating the task for operators with little experience in microsurgery. In addition, there are no significant differences in postoperative outcomes between the different animals, contrary to what happens in neurotmesis (Ronchi et al., Citation2009; Varejao, Cabrita, et al., Citation2004). Thus, this procedure is suitable for sequential and interrelated studies in which reproducibility is essential, facilitating the identification of changes not only in the tissue but also at cellular and molecular level (Chen et al., Citation1992) and still making this a great experimental model to study the time-related regenerative changes (Sta, Cappaert, Ramekers, Baas, & Wadman, Citation2014). Finally, changes identified in nerve regeneration after crush injury should be used as a pre-clinical end-point predictor of the efficacy of applied therapeutic agents. The main disadvantage is the fastness of regenerative phenomena under basal conditions, that is, when there is no therapeutic intervention, which makes it difficult to identify differences between experimental groups (Tos et al., Citation2009).
2.4.4. Methods of evaluation of axonal regeneration and functional assessment
2.4.4.1. Retrograde labelling
The techniques of retrograde labelling using fluorescent dyes are a good method for marking and studying the nerve pathways, allowing to analyse the connections between the peripheral nerve and the spinal cord or dorsal ganglion and to differentiate between the motor and sensory neurons (Hayashi et al., Citation2007). Different dyes can be applied to assess the neuronal population both before and after the injury, thus enabling neuronal death, regenerative success or misdirection phenomena to be monitored (Kemp et al., Citation2017). The dyes can be applied at various locations within the nerve and at different distances from the lesion site. Although it is a precise and specific method to evaluate axonal regeneration after injury, its application should consider some specific care. When different dyes are applied in a simultaneous manner, it is necessary to anticipate possible interactions between them that hinder the analysis. In addition, some dyes may be toxic after prolonged use. Finally, a healthy nerve should always be used as a positive control to guarantee a correct interpretation of the pathways observed (de Ruiter, Spinner, Verhaagen, & Malessy, Citation2014; Kemp et al., Citation2017).
2.4.4.2. Histology and Histomorphometry
Histological evaluation is used by most authors to quantify the number and dimensions of nerve fibers in regeneration and the thickness of the corresponding myelin sheaths after PNI. This analysis is an essential step in studies of peripheral nerve regeneration and should be used in a complementary way to functional, electrophysiological and molecular assessment methods. Classically, histological evaluation would be strictly descriptive, but currently the approach to the tissues is much more complex and it is possible to perform morphometric and quantitative analyses of the histological sections under study (Carriel, Garzon, Alaminos, & Cornelissen, Citation2014). The quantitative analysis allows the identification of intact and regenerated axons as well as inflammatory reaction and fibrosis within the nerve (important in crushing lesions) and outside it (perineural adhesions), besides the formation of neuromas; with the morphometric evaluation it is possible to determine the number of cells in a specific region of the nerve, the diameter of the cells and the proportion of area occupied by regenerating tissue compared to the injured tissue (Raimondo et al., Citation2009). In cases where the study involves the use of biomaterials, the histological evaluation also allows to determine the degree of degradation of the material used, to identify the presence of foreign body reactions and the formation of granulomas.
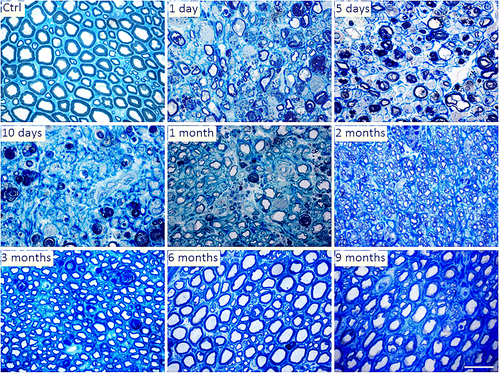
The most commonly used method in the histologic evaluation of the peripheral nerve is toluidine blue staining semithin sections (Figure ), which allows the identification of most myelinated axons and a good delineation of myelin sheaths. It is also the ideal method to perform a morphometric analysis, allowing to determine parameters such as number and density of nerve fibers, the diameter of fibers and axons, cross-section and perimeter of fibers and axons, thickness of myelin sheaths and different ratios between the axon diameters, myelin sheaths and fibers (Bozkurt et al., Citation2012; Mills, Citation2007). The ultrathin sections are also used to evaluate ultrastructural changes and regenerative phenomena in axons and myelin through transmission electron microscopy. It also allows to perform morphometric evaluations (Hirano, Citation2005). Regardless of the methods used from among those available, histological evaluation of the nerve requires additional experience and knowledge from the operator. It is important to know the topographic anatomy of the nerves and how the histological appearance varies in the different sites of the same nerve, but also in the same place between different animals. These variations are particularly important when trying to determine the number of dimensions of myelinated fibers, and the use of randomized protocols and biased counting and measurement methods is essential to avoid the presence of bias in the histomorphometric evaluation (Carriel et al., Citation2014).
Figure 4. Representative light micrographs of toluidine blue-stained semi-thin cross sections of control and crushed median nerves at different time points in the rat animal model. Bar: 20 μm.
The immunohistochemistry and immunofluorescent methods allow the identification of a high number of specific proteins in the tissue sections under study. Several available antibodies allow the recognition of neuronal cell-related proteins, surface and intracellular markers, cytoskeleton proteins, extracellular matrix proteins and growth factors related to the degree of peripheral nerve regeneration after PNI (Raimondo et al., Citation2009). Evidence the presence of axonal regrowth phenomena is the most important regeneration indicator. Neurotubes and neurofilaments can be easily identified by immunohistochemistry aimed at identifying components of the neuronal cytoskeleton (Huang et al., Citation2012). Proteins associated with newly formed axons, such as GAP-43, can be identified by similar methods (Carriel, Garzón, Campos, Cornelissen, & Alaminos, Citation2017). Schwann cells have a crucial importance during the regeneration of the peripheral nerve, but their identification by conventional histological methods is difficult. The use of antibodies to recognize glial fibrillar acid protein and S-100 proteins related to these glial cells facilitates this recognition. Identification of Schwann cells by immunostaining simultaneously to a regeneration pattern is considered a positive indicator of nerve regeneration (Carriel et al., Citation2014). The extracellular matrix produced by the Schwann cells plays a key role in guiding the new axons in the regenerative process. Laminin is an important component of the extracellular matrix of nerve fibers and its identification is a valid parameter in determining the degree of nerve regeneration (Chernousov, Yu, Chen, Carey, & Strickland, Citation2008). Other matrix components, such as the collagen fibers, may also be identified by common histochemical methods. Finally, myelin can also be identified by immunohistochemistry and immunofluorescence methods using antibodies that specifically recognize their basic proteins (Carriel et al., Citation2011).
Histological and histochemical methods can be used to evaluate axonal regeneration, but a direct relationship between axonal regeneration and effective functional recovery is not always established, and an appropriate level of axonal regeneration associated with a low functional outcome can be observed. In addition, the histological evaluation tends to hamper the evaluation of the nerve regeneration due to the phenomena of mismatch, separation, protruding, straddling, or kinking observed between proximal and distal axons. Additionally, even with the use of high-resolution optical microscopy, myelinated fibers less than 2 μm in diameter will not be easily detectable, underestimating the count (Ronchi et al., Citation2014).
Histomorphometric and histological studies can also be extended to the muscles directly innervated by the nerves in question. Studying the contractile force of one or more muscles innervated by a specific nerve is an assessment method also commonly used (Yu & Bellamkonda, Citation2003), as well as the determination of wet muscle weight. In cases of crush injury, wet muscle weights return to values almost identical to cases without injury in the space of two months (Vleggeert-Lankamp, Citation2007).
2.4.4.3. Electrophysiological assessment
The electrophysiological evaluation of the nerve after injury is an indirect method of predicting nerve regeneration, close to the determination of motor and sensory function. In this way, this method of evaluation can be applied in situations where, due to the complexity and material required, the direct functional tests cannot be used. The electrophysiological assessment can be carried out both for efferent and afferent components (Navarro & Udina, Citation2009) and after a crush injury, 8 weeks is the amount of time needed to the electrophysiological parameters to return to baseline levels (Bridge et al., Citation1994). Since in the rat model the motor recovery is the main goal of preclinical studies, evoked compound muscle action potential (CMAP) after electrical stimulation of the proximal and distal segments of the injury site is the most commonly used electrophysiological method (Nijhuis et al., Citation2013) through the application of electrodes in the muscle of interest, nerves could being tested in the hindlimb, forelimb or even testing the facial nerve (Navarro & Udina, Citation2009). The CMPA allows identifying action potentials of close muscle fibers from stimulation of the corresponding supplying motor nerve. In health animals, the CMAP presents values between 18 and 25 mV and in axonotmetic lesions in the sciatic nerve, with the use CMAP, that is possible to identify signs of reinnervation 3–4 weeks after the injury (Kemp et al., Citation2017).
Regarding the sensory component, the somatosensory evoked potential is the standard method. Electrophysiological assessment methods tend to overestimate the degree of nerve recovery since they reveal the functional potential of the PNS rather than its true functional capacity at the time (Toros et al., Citation2009).
2.4.4.4. Behavioural analysis
After any nerve damage, the main clinical objective is a high degree of functional recovery. In any therapeutic intervention, the goal is a complete recovery or a significant functional improvement, a reduction in the duration of the therapy instituted and a reduction in the economic losses for the patient and for the society.
Regarding the motor function recovery of the sciatic nerve, the most commonly used test is the walking tract analysis to determine the Sciatic Functional Index (SFI). Created in 1982 (de Medinaceli, Freed, & Wyatt, Citation1982), SFI is a quantitative and non-invasive method useful in assessing functional recovery of the hindlimb through recording and observation of the footprint, considering the relationship between the toes, the foot and the hindlimb as a whole. This index is applied in the determination of functional recovery in compression, stretching, crushing lesions, in the use of grafts and conduit and even in neurorrhaphy (Algora et al., Citation1996). Although it remains the most popular test among researchers of peripheral nerve regeneration, and even with subsequent modifications (Bain et al., Citation1989), its validity has already been questioned (Varejao, Cabrita, et al., Citation2004). The main limitations of the method are the frequent development of flexion contracture, autotomy phenomena and smearing of the paws and dragging of the tail during the footprint record (Dinh, Hazel, Palispis, Suryadevara, & Gupta, Citation2009). The BBB scale, a method traditionally applied in studies of spinal cord regeneration after injury, may also be applied in PNI studies. This test is based on the assessment of specific functional behaviours such as limb movement and paw placement during gait, assigning on a scale from 0 to 21 the value of 0 when there is no ability to perform voluntary movements and 21 when movements are considered normal (Dinh et al., Citation2009). The results observed in the SFI and BBB methods are tendentially correlated, and this correlation is particularly evident in the crush injury models. Since the BBB scale is based on the observation of the body as a whole during the evaluation of the animal, unlike the walking track analysis that is based on the observation of the footprint without taking into account how the movements are performed, the scale can be a more sensitive tool to analyse lesions by axonotmesis (Dinh et al., Citation2009). Another alternative method that can be used to evaluate the sciatic nerve after injury is the extensive postural thrust (Thalhammer et al., Citation1995), i.e. the force in grams that the animal manages to apply with injured and healthy limbs on a digital balance. It is a method that requires the complex activation of the plantarflexor muscle groups (soleus and gastrocnemius) and whose results are tendentially correlated with those obtained by SFI (Varejao, Melo-Pinto, Meek, Filipe, & Bulas-Cruz, Citation2004).
Currently, and with the availability of better image capture systems, it is possible to use computerized systems for more precise and useful gait analysis (Bozkurt et al., Citation2008). The kinematic evaluations are the set of analyses related to joint movements without considering the type of forces applied to them. The set of evaluations used to the sciatic nerve, which are predominantly performed using videographic recording and subsequent observation, involve the determination of gait-stance duration, evaluation of ankle kinematics and of toe out angle during the gait (Varejao, Cabrita, et al., Citation2004; Varejao, Melo-Pinto, et al., Citation2004). Its main disadvantage is the technical complexity and the material required for its realization.
Since rat, such as humans, present prehensile forelimbs, the functional tests used for the median nerve are those that stimulate and evaluate this function. One example is the grasping test, in which the animal is suspended over a dynamometer so that it can grasp it with the injured limb, being then raised. The dynamometer will assess the maximum weight the animal can bear and lift before releasing it (Jager, Ronchi, Vaegter, & Geuna, Citation2014).
Finally, the functional assessment of the facial nerve involves the determination of the movements of the whiskers (vibrassal test) and eyelids, both spontaneous and from controlled electrical stimuli. These movements can either be observed directly or recorded on video and identified using infrared and laser detectors, not only identifying the movements but also comparing them bilaterally (Heaton et al., Citation2008). After a facial nerve crush injury, the return to normal whiskers movements takes about 25 days (Hadlock et al., Citation2005).
Related to sensory function recovery of the sciatic nerve, different tests are also available. The withdrawal reflex latency test, associated with the use of a heating plate, electrical stimulation, mechanical stimulation or by pricking with a needle, is the most commonly used to determine the integrity of the nociceptive function (Wong, Kanagasabapathy, Bakar, Phan, & Sabaratnam, Citation2015). The stimulus is applied directly to the hindpaw’s plantar surface and withdrawal reflex, i.e. the time the animal takes to flex the limb, is measured in seconds. The better the animal’s sensitive function, the faster the limb is withdrawn from the source of painful stimuli (Boisse, Spencer, Mouihate, Vergnolle, & Pittman, Citation2005). Another commonly applied test is the von Frey test which allows to study the mechanisms of cutaneous stimulation-induced sensory input and measure the phenomenon of mechanical allodynia, characterized as a painful withdrawal responds to a previously non-painful stimulus. The test is performed by applying mechanical force with the von Frey filaments to the plantar surface of the rat’s hindpaw. During the sensory tests, it is important to avoid stimulating the medial aspect of the foot plantar region, since this zone is enervated by the saphenous nerve, direct branching of the femoral nerve, and not by the sciatic nerve and its ramifications (Kemp et al., Citation2017).
2.4.4.5. In vivo imaging
The use of ultrasonography (US) in the evaluation of PNI, particularly with improvements in image resolution and availability as observed in recent years, significantly increased. US evaluation of the nerve can determine if there is continuity after injury, gap length (if present), presence, location and dimensions of neuromas, presence of other lesions associated with nerve injury (tendons, muscles), degree of scarification and the state of adjacent and nerve-related tissues such as blood vessels or bones. Thus, US allows the diagnosis of cases of axonotmesis or neurotmesis in situations in which neurophysiological evaluation is not effective (Zeidenberg, Burks, Jose, Subhawong, & Levi, Citation2015). High resolution ultrasound (HRU), with superior image quality, allows the identification of all nerve branches in the limb under study, thus providing a clear identification of total or partial transections, nerve lacerations, epineural hematomas, formation of neuromas and the need to perform surgical interventions (Toros et al., Citation2009; Zhu, Liu, Li, Shao, & Hu, Citation2011). Regarding other diagnostic methods, the US allows a dynamic evaluation, continuous along the body, of the nerves under study, to observe and identify small abnormalities that would not be visualized by other methods, presents practically no contraindications for the patient and is widely available. The use of equipment and probes with reduced dimensions allows their use in smaller animal models.
Magnetic resonance imaging (MRI) is a good option to evaluate the extent of PNI. Providing high-resolution images using T2-weighted images in combination with fat and flow suppression, allows the representation and evaluation of the anatomy of the peripheral nerve in detail. Despite this, and due to the associated costs and the time required for the exams, it is not always a viable option for all research centres. Its use in smaller animal models is reduced (Chhabra & Carrino, Citation2015).
2.5. Advances in axonotmesis: Brief review
After years of studies devoted to the induction of crushing injuries in the peripheral nerve, it is now known that the rate of recovery is directly related to the initial load applied and distance to the cell body, being the speed of recovery dependent on the physical and pharmacological agents used. Despite the intrinsic capacity of the PNS to regenerate after injury, in cases of severe compression the results are mostly unsatisfactory. Current treatments are mainly based on surgical intervention (Faroni et al., Citation2015), although conservative and pharmacological treatments are also being explored in the literature.
2.5.1. Conservative treatments
Physical exercise contributes beneficially to regenerative phenomena after PNI since it induces the synthesis of growth factors, promotes synaptic elimination (Teodori et al., Citation2011) and accelerates the regrowth and regeneration of the peripheral axons. During exercise, Schwann cells themselves produce and release neurotrophic factors. Among the growth factors identified and released during exercise are NGF and brain derived neurotrophic factor (BDNF) (de Ruiter et al., Citation2014; English, Wilhelm, & Sabatier, Citation2011). In addition, physical exercise can relieve neuropathic pain by promoting the release of endogenous opioids (Stagg et al., Citation2011). These benefits are achieved through aerobic exercise (e.g. use of treadmills and swimming) (Boeltz et al., Citation2013; Teodori et al., Citation2011) but resistive exercise seems to have fewer benefits in stimulating the regenerative process and in relieving neuropathic pain (Antunes et al., Citation2016). The simultaneous use of physical exercise with other therapeutic techniques such as electrotherapy or phototherapy seems to result in even better prognosis in the peripheral nerve lesions (Rosa Junior et al., Citation2016).
Electrostimulation (ES) was proposed as a therapeutic method for the repair of peripheral nerve lesions in a clinical setting, and its experimental application was widely explored. The application of ES at low intensity in the proximal nerve stump promotes nerve regeneration (Kim, Han, Shin, Lee, & Choi, Citation2011; Lal et al., Citation2008) by increasing the expression of BDNF (Al-Majed, Neumann, Brushart, & Gordon, Citation2000), which promotes the proliferation of Schwann cells and the formation of myelin during the regenerative process (Wan, Xia, & Ding, Citation2010; Zhang, Xin, Tong, & Tong, Citation2013). In addition, ES also promotes expression of genes associated with growth factors (Geremia, Gordon, Brushart, Al-Majed, & Verge, Citation2007) and neutrophine signalling (W. English, Schwartz, Meador, Sabatier, & Mulligan, Citation2007). Several studies have shown that ES at low intensity (20 Hz or less) for 30 min to 1 h facilitates and promotes nerve regeneration and that the timing of ES application affects the effectiveness of the regenerative process, indicating that early interventions are essential for recovery (Alrashdan et al., Citation2010; Zhang et al., Citation2013). The application of brief pulses of suprathreshold ES also stimulates the phenomena of remyelination and functional recovery, improving the regenerative velocity of the motor and sensorial neurons (Zhang et al., Citation2013). ES can also be combined with surgical techniques, making it even more attractive to surgeons (Willand, Nguyen, Borschel, & Gordon, Citation2016). Despite this, there are also studies that contraindicate the use of ES in PNI. The application of high-frequency transcutaneous electrical nerve stimulation seems to delay nerve regeneration, functional recovery, and exacerbate atrophic muscle events after crushing lesions in the rat model (Baptista et al., Citation2008; Gigo-Benato et al., Citation2010), even delaying the mechanisms of nerve reinnervation and inducing muscle dysfunction and hypoexcitability after crush injuries (Pinheiro-Dardis, Erbereli, Gigo-Benato, Castro, & Russo, Citation2017). Thus, the benefits of its use remain controversial and require more research.
Stimulation of the injured nerve by an electromagnetic field is a viable non-invasive technique for stimulation of regeneration after PNI (Hei et al., Citation2016). The application of the electromagnetic field stimulates the regeneration of the peripheral nervous system, promoting functional recovery and increasing the number and diameter of the regenerated axons (Patel & Poo, Citation1982). Although not fully understood, its effect is thought to be the stimulation of NGF activity, decreased inflammatory cytokine activity and altered membrane gated channel activity (Hei et al., Citation2016). In addition, when used in conjunction with mesenchymal stem cells, it stimulates the differentiation of these into neuron-like cells by affecting the cell cycle (Sun et al., Citation2009). The effect of these fields has a specific biological window, and interference in survival, propagation, and regeneration of the peripheral nerve depends on the amplitude (0.3 to 300 mT), time (10 min/day to 24 h/day) and frequency (2 Hz to 2000 Hz) of exposure (Rusovan, Kanje, & Mild, Citation1992; Song et al., Citation2014). Protocols involving low frequencies and short exposure times (50 Hz, 1H) at 1 mT of amplitude seem to have good effects on the nerve after axonotmesis, being suitable for application in clinical situations and avoiding long periods of exposure and associated costs (Hei et al., Citation2016).
Ultrasounds are primarily used for diagnosis, but low-intensity ultrasound (LIU) can also be used as a therapeutic option, including in the stimulation of peripheral nerve regeneration after PNI (Raso, Barbieri, Mazzer, & Fasan, Citation2005). The mechanisms by which LIU induce a positive biological response are not fully understood, but are thought to be related to the interaction between the thermal and mechanical effects of ultrasound that cause intercellular material movements, stimulate membrane diffusion movements, accelerate the metabolism and blood circulation and improve tissue nutrition. In addition, it is believed that the ultrasound also stimulates the release of BDNF (Ni et al., Citation2017).
Phototherapy promotes and accelerates the regrowth and axonal regeneration after axonotmesis, particularly with low power radiation. Among the observed effects are reduced scar tissue formation, fewer degenerative phenomena in the motor neurons of the spinal cord, and a significant increase in axonal growth and myelination (Rochkind, Citation2009). The effect of the laser on the nerve promotes the synthesis of ATP and cell proliferation, increasing the available energy in the axons, the expression of neurotrophic factors and promoting an increase of axonal budding and nerve regeneration (Rosa Junior et al., Citation2016).
2.5.2. Pharmacological treatments
Wherever possible, pharmacological treatment should be preferred over surgical intervention. Various pharmacological agents have been used and described as effective in stimulating or improving nerve regeneration after crush injury.
The prevention of exuberant scar tissue is an important aspect in studies of peripheral nerve regeneration. The two major approaches used to evade these phenomena are the surgical creation of barriers between the injured nerve and surrounding tissues and the use of pharmacological agents such as steroid hormones to prevent the inflammatory response. The use of corticosteroids in axonotmesis models has always been involved in doubts due to the results obtained: although the animals treated with these therapies seem to be associated with higher recovery rates (Khan, Faruqi, & Ansari, Citation2014), the differences between treated and untreated animals do not always show statistically significant differences (CitationGalloway, Jensen, Dailey, Thompson, & Shelton, 2000) and the associated use of corticosteroids and other drugs shows little efficacy of the first group in reversing the harmful effects (Capadia, Shetty, Khambati, & Ghate, Citation2010). They act by selectively inhibiting fibroblast growth and the migration and phagocytic action of granulocytes (Mukudai et al., Citation2015). In any case, the use of dexamethasone, a glucocorticoid commonly applied to reduce edema and the effects of neural inflammation, appears to promote and accelerate nerve recovery after induction of crush injury, elevating the expression of proteins associated with a high activity of growth cones during regenerative processes (GAP-43), controlling inflammatory cell invasion at the lesion site and avoiding the harmful effects of the exuberant innate immune response (Feng & Yuan, Citation2015). Also methylprednisolone has been intensely investigated because of its neuroprotective properties, and the administration of high doses have the capacity to inhibit the action of oxygen radicals and lipid peroxidation, promoting axonal regeneration and inhibiting exuberant scar formation (Nachemson, Lundborg, Myrhage, & Rank, Citation1985). The use of corticosteroids locally appears to be more effective than its systemic application (Suslu, Altun, Erdivanli, & Turan, Citation2012) but its rapid degradation and absorption limits its effectiveness. Continuous systemic administration does not overcome these problems since the secondary metabolic effects are severe (Mukudai et al., Citation2015). Sustained release of drugs in the form of microspheres with the use of specific membranes seems to guarantee the best balance between the desired regenerative effects and undesirable side effects (Li, Li, Cao, Luo, & Lian, Citation2016).
Other alternative agents to control the exuberant inflammatory reaction following peripheral nerve crush injury include the use of gabapentin. Traditionally used as an anticonvulsant, it not only has an analgesic effect in the control of allodynia and neuropathic pain, but also modulates the inflammatory response, promoting the removal of debris during the degenerative phase and attenuating the exuberant effects of the inflammatory response and axonal remyelination during the regenerative phase (Camara et al., Citation2015, Citation2013). The use of phytoestrogens such as genistein withdrawn from soybean has similar effects, performing neuroprotective functions, reducing allodynia and neuropathic pain, having an anti-inflammatory effect by reducing the levels of cytokines associated with the peripheral nerve subjected to axonotmesis and promoting an increase of the expression of the myelin basic proteins (MBP) and GAP-43 proteins associated with remyelination and axonal regeneration respectively (Ozbek et al., Citation2016).
B vitamins have the ability to attenuate degenerative processes in the peripheral nerve, either alone or in combination (Jolivalt et al., Citation2009). Vitamin B12, or cobalamin, is essential for various metabolic processes, and its decrease promotes deficiencies of methionine, the amino acid involved in the synthesis of phospholipids and myelin. In addition, cobalamin has antioxidant properties. Its positive effects in cases of peripheral nerve axonotmesis have already been demonstrated (CitationGan et al., 2014; Hobbenaghi et al., Citation2016). promoting morphological and functional recovery. In addition to its direct metabolic effects, it is thought that the vitamin B12 can also act upregulating genes related to various growth factors (CitationGan et al., 2014). Its mechanisms of action in this context need further investigation.
The use of androgens has also been weighted to stimulate regeneration after PNI. The use of thyroid hormones reduces nerve recovery time after injury (Fargo, Alexander, Tanzer, Poletti, & Jones, Citation2008) and increases the expression of microtubule destabilizing factor (SCG10) (Voria et al., Citation2006), although the use of this type of substances still needs further studies.
Since oxidative stress is a mechanism associated with nerve damage but not its main component, the early application of antioxidant therapies for neuroprotection in cases of axonotmesis may have a concomitant effect on the stimulation of nerve regeneration, although they should not be used as single therapy (Hajimoradi, Fazilati, Gharib-Naseri, & Sarkaki, Citation2015). The use of antioxidants such as alpha-lipoic acid has protective effects in cases of crushing injury, stimulating an increase in endogenous antioxidants (SOD and CAT) and a decrease in lipid peroxidation indicators like MDA when applied prior to induction of the lesion (Senoglu et al., Citation2009). Zofenopril, an angiotensin converting enzyme, has a sulfhydryl group and therefore potential antioxidant activity (Subissi, Evangelista, & Giachetti, Citation1999). Its use after an induced crushing injury is associated with better functional recovery and more effective axonal regeneration (Kalender et al., Citation2009).
Trapidil is a vasorelaxant that decreases nerve damage associated with ischemia by decreasing vasospasm after crushing lesions, attenuating myelin separation and endoneurial and mitochondrial swelling (Kurtoglu et al., Citation2005).
Nimodipine, an L-type voltage-gated calcium channel antagonist traditionally used as a vasodilator, demonstrates positive effects in promoting axonal regeneration after crushing injury, (Strauss, Romstock, Fahlbusch, Rampp, & Scheller, Citation2006) stimulating improvements in neuromuscular function, accelerating muscle reinnervation (Nishimoto, Kumai, Minoda, & Yumoto, Citation2012), increasing the survival of motor neurons, (Mattsson, Aldskogius, & Svensson, Citation1999) and generally ensuring a functional recovery and axonal regrowth after nervous crushing (X.-s. Zheng, Ying, Yuan, & Li, Citation2015). Although its mechanisms of action are not yet fully understood, nimodipine is thought to act through stimulating remyelination by Schwann cells, decreasing the local inflammatory reaction (Li, Hu, Liu, Bao, & An, Citation2009) and increasing the expression of calcium-binding S-100b proteins that play an important role in the regulation of intracellular calcium and actively participate in the growth of neurites and in neuronal survival (Gonzalez-Martinez, Perez-Pinera, Diaz-Esnal, & Vega, Citation2003; Zheng et al., Citation2015).
Melatonin presents as a hormone with enormous potential to be used as a therapy in both axonotmesis and other types of PNI. The use of melanin in different studies demonstrated its ability to stimulate proliferation of Schwann cells both in vivo and in vitro and to improve nerve regeneration after PNI. The number of motor end plates observed on nerves after injury is higher in animals treated with melatonin when compared to animals treated with a simple saline solution (Chang et al., Citation2014). Further, melatonin treatment ensures better preservation of myelin sheaths after crushing or neurotmesis lesions, decreases levels of lipid peroxidation, and increases SOD, CAT and glutathione peroxidase activity (Kaya et al., Citation2013). All the beneficial effects of melatonin treatment in controlling the formation of exuberant scars and neuromas and in the stimulation of functional recovery of injured nerves make this hormone particularly attractive for clinical use, even though that some studies indicate potential toxic effects of its use, namely being able to interact negatively with the microtubules of the axons, promoting their disorganization, morphological alterations and potential disintegration (Piezzi & Cavicchia, Citation1981). Finally, melatonin is able to reduce not only the level of cell death by apoptosis in the CNS (Reiter, Citation1998) but also the death of motoneurons in the rat sciatic nerve after PNI (Rogerio et al., Citation2002). Essentially, more studies are needed to determine the balance between its benefits in peripheral nerve regeneration when compared to its potent nefarious effects.
The potentials effects of ES are probably related to the reestablishment of nerve conductivity at the level of the injured nerves. Thus, pharmacological restitution of nerve conductivity may also have beneficial effects. The use of 4-aminopyridine (4-AP), a potassium channel blocker, was considered as potential therapy in this subject. Early use of 4-AP after the induced crush injury allowed a better and faster motor and behavioural recovery, improved nerve conduction velocity, increased the sectional area of regenerated axons and correspondent myelin sheath thickness and increased P0 protein levels. In addition, there were no increased manifestations of neuropathic pain but rather a rapid restitution of the normal response to painful and thermal stimuli (Tseng et al., Citation2016). 4-AP allows the electrical impulses to be conducted even in demyelinated axons by blocking the K + channels, allowing the K + ions to leak from the axons and restoring the depolarization levels essential for the propagation of the action potentials (Hayes et al., Citation1994).
Sildenafil citrate is an effective inhibitor of phosphodiesterase-5 (PDE-5), and in addition to promoting protection in situations of ischemia and reperfusion in the rat model (Das, Maulik, Das, Kadowitz, & Bivalacqua, Citation2001), it also reduces flap necrosis by increasing the secretion of growth factors and ensuring a better structural organization of the nerve after regeneration. Simultaneously, the use of this drug seems to promote a better and faster return to the physical performance of the injured animal (Korkmaz et al., Citation2016).
The use of aspirin, which was already largely consolidated, has gained importance in recent years due to the evidence of its neuroprotective effect on CNS, namely protecting dopaminergic cells against neurotoxicity and promoting oligodendrogenesis and remyelination in cases of white matter lesions (Chen et al., Citation2014). Also its use in the SNP seems to have positive effects on axonal regeneration, promoting an increase in the number of surviving motoneurons after crushing injury, promoting the reaching of the distal stumps by the outgrowing nerve fibers, resulting in larger diameter of the myelin sheaths in the regenerated axons and improved functional recovery in treated animals (Cui et al., Citation2015). Although its mechanism of action has not yet been fully clarified, taking into account its effect on the attenuation of demyelination and promoting an increase in the thickness of the newly formed myelin sheaths (Chen et al., Citation2014), it is reasonable to infer that it acts directly on the cell Schwann, probably promoting their survival or their action after the nerve injury.
Lithium chloride, a glycogen synthase kinase 3β inhibitor used as a mood stabilizer, is also studied because of its neuroprotective, antiapoptotic and anti-inflammatory effects. This molecule seems to have special potential in the treatment of nerve lesions where axon injury and demyelination occur simultaneously, a situation in which axonotmesis is included. Its administration after nerve crush lesions stimulates the expression of myelin genes, promoting remyelination and effective functional recovery (Makoukji et al., Citation2012).
Natural products, although still used reluctantly, appear to have beneficial effects on nerve regeneration after injury, and can improve motor performance, sensory function and nerve conductivity. Although the list of available products is broad and its pharmacological targets are not yet understood, it is known that its mechanisms of action include activation of antiapoptotic signalling pathways, control of inflammatory cytokines and overexpression of growth factors (Araujo-Filho et al., Citation2016). For example, the ABPP, polypeptides extracted from Achyranthes bidentata Blume, presents regenerative effects identical to cobalamin in terms of SFI, nerve conductivity and morphometric analysis after crush injury (Yuan et al., Citation2010); curcumin and propolis promote better recovery rates after sciatic nerve crush injury than methylprednisolone (Yuce et al., Citation2015) and Ginsenoside Rg1, an active compound extracted from ginseng, promotes better axonal regeneration and functional recovery than mecobalamin (Ma, Li, Tian, & Lei, Citation2010). The list of natural substances and their beneficial effects in promoting regeneration after axonotmesis is extensive, corroborating the efficacy of these products in the treatment of different diseases. However, even with the evidence that these compounds may act on different pharmacological targets and accelerate nerve regeneration, additional successful evidences are required to determine the relationship between them and PNI models and to establish these products as valid therapeutic options in clinical practice (Araujo-Filho et al., Citation2016).
2.5.3. Cell-based therapies
Cell-based therapies have been a potential alternative for the treatment of different lesions and neurological problems, including PNI. Diverse types of cells have already been considered in this type of therapies (Figure ). Although theoretically able to differentiate into distinct types of cells and tissues, embryonic stem cells have been progressively discarded because of the ethical problems associated with their use, making adult stem cells the best candidates (Orbay, Uysal, Hyakusoku, & Mizuno, Citation2012). Schwann cells, also considered and studied in this field, present several limitations in terms of clinical application, with donor site morbidity, difficulties in the expansion and complexity of the application phases. In addition, the number of Schwann cells decrease with aging (Hood, Levene, & Levi, Citation2009) Studies and preclinical application of mesenchymal stem cells (MSCs) have undergone a major advance in recent years, mainly due to their ability to differentiate into the types of cells needed at each site, the secretion of growth factors essential to the regenerative process and its capacity to modulate and attenuate the local and systemic inflammatory reactions (Bhangra, Busuttil, Phillips, & Rahim, Citation2016) MSCs have the advantage of could be obtained from virtually all tissues in the donor organism, including adipose tissue, bone marrow, dental pulp, umbilical cord (both umbilical cord blood and Wharton’s jelly), foetal blood, circulatory system, hair follicle, lungs, synovium, skeletal muscle and olfactory mucosa (Caceres, Peress, Rameshwar, & Fernandez-Moure, Citation2016; Ge et al., Citation2016; Kobolak, Dinnyes, Memic, Khademhosseini, & Mobasheri, Citation2016) The list, however, is continuously expanding. When applied in regenerative medicine, these cells have characteristics that make them excellent therapeutics adjuvants, namely easy expansion, ability to differentiate into different types of cells, tropism to injured sites, immunoprivileges and local and systemic immune modulation, trophic stimulation and modulation of functions of the tissues where they are or on which they act (Scatena, Eaton, Jackson, Lund, & Giachelli, Citation2017). When applied specifically to peripheral nerve regeneration, MSCs can secrete neurotrophic factors and create a propitious environment to the occurrence of neurogenesis and proliferation of Schwann cells at injury sites. Moreover, they themselves can follow a neurogenic differentiation or differentiate into Schwann cells, in addition to modulating the inflammatory response and Wallerian degeneration (Kingham et al., Citation2007). Once in the appropriate microenvironment, MSCs can produce and secrete various growth factors such as NGF, BDNF, glial-derived neurotrophic factors (GDNF), neurotrophins, endothelial and ciliary growth factors (Walsh & Midha, Citation2009). Remyelination regeneration of injured axons are also stimulated by the presence of MSCs, which those synthetizing and release many myelin proteins, such as myelin MBP or P0 proteins (Zhang et al., Citation2017).
Figure 5. Different types of MSCs in culture, (a) Human Dental Pulp MSCs (P2); (b) Equine Synovial Membrane MSCs (P4); (c) Equine Umbilical Cord MSCs (P1); (d) Rat Olfactory Mucosa MSCs (P4).
The application of the cells at the site of crush injury can be challenging, although there are several options available. The first option involves the suspension of the MSCs in a suitable medium and their microinjection at the lesion site. This process, however, can be traumatic both for the cells and for the intraneural architecture, leading to a defective cellular distribution (Pang et al., Citation2013). Another option involves the suspension of the cells in a fibril matrix and injection of this matrix around the injured site (Zhao et al., Citation2014). When associated with the use of biomaterials, in the crushed nerve wrapping processes, cells can be injected directly into the lumen of the wrap material, this being natural or synthetic (Jiang, Jones, & Jia, Citation2017). Despite its apparent potential, the mechanisms of action of MSCs are still not fully understood and cell stability, the risk of tumorigenesis and the risks of cell migration are still concerns. Individual application of MSCs at injury sites, although associated with improvements in outcomes, are still less effective than conventional surgical techniques. Its efficacy seems to be potentiated when used concomitantly with biomaterials and associated with growth factors (Jiang et al., Citation2017).
2.5.4. Growth factors
Neurotrophins are the set of molecules naturally present and expressed in the process of nerve regeneration. When released at the proximal nerve endings and distal to the lesion site, they have the ability to stimulate and guide axonal growth and differentiation. Several of these neurotrophic factors have already been isolated and applied in preclinical studies to stimulate axonal regeneration. These factors include superfamily growth factor beta, NGF, insulin-like growth factors, neurotrophins 3, 4, and 5, ciliary neurotrophic factor, neuregulin-1, BDNF, GDNF and platelet-rich plasma (Gaudin et al., Citation2016; Sariguney et al., Citation2008). NGF is one of the most studied factors. It is present in low concentrations in the healthy nerves, its expression increasing significantly in nerves after injury, playing essential functions in the survival of the neurons and in the growth of the new neurites. In addition to promoting the proliferation and differentiation of neurons, it is still capable of modulating the differentiation of the injured nerve (Namiki, Kojima, & Tator, Citation2000). Administration of NGF at injury sites promotes nerve repair and functional recovery (Petruska & Mendell, Citation2004). At the same time, the presence of these growth factors can reduce the expression of p38MAPK, a pathway that is activated early in PNS (Agthong, Kaewsema, & Chentanez, Citation2012) lesions and appears to be involved in cell death phenomena. Thus, early administration of exogenous NGF after crushing lesions has beneficial effects both through its protective and regenerative action over regeneration and through inhibition of p38MAPK expression and its effects on cell death (Fan et al., Citation2015). BDNF administration promotes faster functional recovery after crush injury in mice, stimulating neuronal intrinsic growth capacity and behavioural recovery (Zheng et al., Citation2016). IGF-1, a neuropeptide naturally produced in the brain and peripheral nerve after injury has a trophic effect on several tissues (nervous, connective and muscular), stimulating the development and growth of neurons and glial cells, allowing axonal outgrowth, acceleration of remyelination and neuronal survival (Bayrak et al., Citation2017; Nachemson, Lundborg, & Hansson, Citation1990).
When applied in solution at the lesion site, however, these factors rapidly diffuse into body fluids, requiring periodic administration associated with increased costs and undesirable side effects (Apfel et al., Citation1998). To overcome these limitations alternative methods for administering NGF to the nervous system have already been tested, namely drug delivery systems (Haller & Saltzman, Citation1998), encapsulated cell transplantation, biomaterials (Houschyar et al., Citation2016) and gene therapies (Esaki et al., Citation2011).
2.5.5. Gene therapies
Gene therapy, which has steadily grown in recent years, involves the insertion, alteration or removal of genes in certain cells to treat diseases. The most common embodiment is the insertion of genes at a specific genomic location so that the transduced cells continuously produce proteins of interest (Oliveira, Lopes, de Almeida, & Martinez, Citation2014). The use of viruses to introduce therapeutics genes is the most efficient method for the nervous system (RJ Mason, R Tannemaat, JA Malessy, & Verhaagen, 2011). Although it is a relatively recent and unexplored therapeutic technique, has already been successfully applied in different studies of peripheral nerve regeneration after axonotmesis. One study has explored the use of a recombinant replication-deficient adenoviral vectors to induce the release of NGF delivery by Schwann cells, promoting a sensory recovery in a nerve after crushing (Li et al., Citation2012). In another study, adenovirus was used to increase the expression of sciatic nerve growth factors after crushing injury, stimulating axonal protection, attenuating myelin degeneration, and promoting functional recovery (Tsai et al., Citation2010). Another technique involves transplantation of cells previously genetically modified in vitro to the injured tissue to be treated (Mason, Tannemaat, Malessy, & Verhaagen, Citation2011). One study addressed the transplantation of human amniotic fluid-derived mesenchymal stem cells genetically modified to the production of GDNF in the treatment of an ischiatic nerve after axonotmesis, thus promoting axonal regeneration and decreasing the phenomena of apoptosis (Cheng et al., Citation2010). Despite these promising results, gene therapy is still far from being fully effective and safe. First, although methods that do not use viruses are safer in gene transfer, viral methods are much more effectives. Genetic therapies are always subject to the occurrence of immune responses and systemic reactions, and the methods used to reduce these occurrences (filtration methods) are not entirely safe. Finally, the risks of targeting wrong cells and of mutagenesis by inserting exogenous DNA into the genome are issues concerning biosafety that must be taken into account (Oliveira et al., Citation2014).
2.5.6. Surgical interventions
In clinical practice, with no standard pharmacological treatment yet available, surgical interventions are necessary in all cases where spontaneous recovery is not observed. In any case, perform or not a surgical intervention is a complex decision: if surgery is performed too early, much of the potential for spontaneous recovery is lost. On the other hand, establishing that the observed lesion has the potential for spontaneous recovery is equally risky, since the confirmation is essentially clinical and the neuropraxic lesion can only be confirmed weeks to months after lesion establishment. After this period, and if no recovery has been observed, the opportunity to perform an effective surgical intervention has been lost (Birch et al., Citation2012). These factors should be considered also in the experimental surgical intervention and in the corresponding experimental model instituted.
In the case of neurotmesis lesions, in which there is a true nerve gap with anatomical separation of the proximal and distal segments to the lesion, the surgical options are varied and include epineural repairs (neurorrhaphy) (Dahlin, Citation2008), intranerve sutures (Lundborg, Citation1988), sutures between healthy nerves and injured nerves (neurotilization) (Simon, Spinner, Kline, & Kliot, Citation2016), the use of grafts (autografts or allografts) (Grand, Myckatyn, Mackinnon, & Hunter, Citation2002; Millesi, Citation2007) and nerve guidance conduits (NGC) (Safa & Buncke, Citation2016). In the case of crush injuries, since the epineurium and the anatomic shape of the nerve trunk are maintained, most of these surgical approaches are not effective. For axonotmesis, traditional surgical techniques included neurolysis and nerve decompression, often associated with poor outcomes. A specific surgical technique has been developed to ensure a protective barrier around the crushed nerve, reducing the formation of scar tissue and adhesions with surrounding tissues and supplying an optimal environment for nerve regeneration. The concept of wrapping the crushed nerve aims to wrap the lesion site with a material that decreases the degree of exuberant healing, neural fibrosis, changes in metabolism and leads to effective wound healing. Nerve wrapping can be performed using autologous or allogenic tissues and biological or synthetic materials (Ozturk, Uygur, & Siemionow, Citation2015) and can be applied as a single surgical therapy or combined with administration of pharmacological agents, growth factors or stem cells (Suzuki et al., Citation2017). During application of the wrap, and once the crush injury is performed, this is wrapped around the crushed site to cover the entire injured area. Loose sutures can be performed between the wrap and adjacent muscle fascia’s to prevent migration of the wrap (Ozturk et al., Citation2015).
The use of autologous tissues to wrap the site of the crushing lesion, such vascular grafts, dermofascial fat grafts or muscle flaps, allows to create a local environment that promotes axonal migration and regeneration without the problems related to the antigenic components (Leuzzi et al., Citation2014; Millesi, Citation2007). Allografts are usually obtained from cadavers, and although from the structural point of view they are as functional as autografts, it is necessary to establish an immunosuppressive therapy, usually for extended periods of time, to avoid rejection reactions on the receptor (Grand et al., Citation2002). These materials may undergo enzymatic treatment prior to application to become acellular, reducing the need for immunosuppressive treatments and increasing the likelihood of success. Even so, the probability of inflammatory reactions is high and can lead to the formation of scarring phenomena, which is precisely the opposite of the purpose of its use (Kehoe, Zhang, & Boyd, Citation2012). The use of sheaths consisting of absorbable acellular extracellular collagen matrices, derived from animals or humans (human amniotic membrane; intestinal submucosa swine—AxoGuardTM Nerve Protector; collagen type I—NeuraWrapTM/NeuroMendTM) can also be used as a material for wrapping the injured site (Gaudin et al., Citation2016). Its use allows to stimulate the neovascularization, to promote survival, growth, and regeneration of axons, guarantees the presence and action of several growth factors and cytokines and is associated with few or no immunogenic complications (Yi, Lee, Lee, Lee, & Yang, Citation2013). All natural materials tend to degrade in a non-toxic manner, although the speed of this process is faster (Bell & Haycock, Citation2012).
The use of synthetic material for wrapping the axonotmesis injury allows the creation of a separation barrier between the injury site and the surrounding tissues and to keep the neurotrophic and neurotropic factors secreted in the injured site (Kehoe et al., Citation2012), overcoming the disadvantages of the organic options (Safa & Buncke, Citation2016). The synthetic materials used must meet the established criteria for the safe use of biomaterials, being: (a) biocompatible with the tissue where it will be applied, with absence of local or systemic inflammatory reactions (Scatena et al., Citation2017); (b) biodegradable; (c) mechanically and architecturally stable during the regenerative process (Basu, Citation2017); (d) resistant to the application of sutures and to possible local inflammatory reactions (e) flexible and resistant in a balanced way, in order to avoid compression during the regeneration and to limit the accumulation of inflammatory and fibrous material (Belanger et al., Citation2016); (f) capable of preventing excessive loss of neurotrophic factors at the site of injury (Kehoe et al., Citation2012); (g) capable to provide a good pathway for the growth cone, reducing the phenomena of misdirection (Peng, Li, Chiu, & Wang, Citation2017); (h) semipermeable and with pores of adequate dimensions to allow the influx of oxygen and nutrients necessary for the regenerative process but to prevent the entry of inflammatory cells and the loss of growth factors (Meek & Coert, Citation2008); (i) technically appropriate, allowing good standards of production, sterilization, storage and handling (Kaur, Citation2017). Various materials can be used, to wrapping the crushed nerve, divided between absorbable and non-absorbable (SalubridgeTM) materials (Kehoe et al., Citation2012). The use of synthetic materials is associated with the risk of production of acidic materials during degradation, which can have a detrimental effect on the regenerative microenvironment and cellular activity (Bell & Haycock, Citation2012). In any situation, the ideal material is the one that can protect the nerve, prevent the formation of neuromas, decrease the degree of adhesion, promote a minimal inflammatory reaction and stimulate axonal regeneration (Xu, Varitimidis, Fisher, Tomaino, & Sotereanos, Citation2000). Mostly, the best results are obtained when the nerve is wrapped in autologous weavers (fat grafts or twenty grafts).
3. Conclusion and further directions
PNI are lesions commonly identified in clinical practice, with several underlying causes, severe functional and behavioural consequences, and often poor and far from desirable outcomes. Despite the intrinsic capacity of the peripheral nervous system to spontaneously regenerate after injury, the degree of regeneration and functional recovery is fundamentally dependent on the type of injury instituted, with remarkable recovery rates for lesions of lesser severity rarely and suboptimal outcomes for neurotmesis if there is no therapeutic intervention that supports and promotes the reparative nerve behaviour.
Peripheral nerve crushing lesions are among the most common lesions within the PNI and represent the limit between the lesions susceptible to spontaneous regeneration and those that require compulsory surgical intervention for regeneration to happen. Crush lesions usually occur associated with extreme compressive forces, fractures and hematomas that affect not only the nerve itself but also all neighbouring tissues, creating a complex healing environment that interacts and directly affects the regenerative nerve phenomena. The main barriers to normal and efficient nerve regeneration in these cases are the establishment of exuberant inflammatory reactions, the occurrence of adhesions between the nerve and surrounding tissues, reperfusion injuries, failure in the stimulation and guiding of axonal regrowth and reinnervation of the target organs, occurrences of axonal misdirecting and failures in remyelination. For each of these failure mechanisms it is necessary to establish a precise and oriented therapeutic tactics, and in this need lies the complexity of the treatment of PNI.
Axonotmesis is an experimental lesion paradigm that mimics in a controlled environment the occurrence of crushing lesions in the peripheral nerve, allowing to test different therapeutic approaches, alone or in an association, in the promotion of normal nerve regeneration. In the last decades, several therapeutic approaches have been applied in this type of lesion, from pharmacological and hormonal treatment to surgical interventions of neurolysis and decompression. More recently, the development of new branches of medicine such as gene therapies and cell based therapies associated with new surgical techniques such as wrapping have allowed great advances in the treatment of crush injuries. Despite this, the results obtained to date are still far from being the ideal. Despite the years of interventions and the number of published works, it has not yet been possible to establish a single and uniform lesion model that allows a comparison between works free from inter-experience and inter-operator variability. In addition, methods of determining axonal regeneration and functional assessment, being multiple, are often used without considering the correlation between them and the specific component of regeneration that each method tests. The ideal tests will be those that allow an accurate analysis of several outcome measures and the subsequent recovery after treatment. If a comprehensive analysis is not performed, different components of the regenerative process may be ignored in the final analysis, thus leading to erroneous conclusions that overestimate or underestimate the therapeutic potential of an intervention. For example, given that the main clinical objective in any intervention is, above all, a high degree of functional recovery, the number of publications in which these tests are not performed in detriment of other methods of determining axonal regeneration is still very high.
In conclusion, and even considering all the remarkable advances already made in the treatment of the peripheral nerves after injury and, specifically, submitted to axonotmesis lesions, it is necessary to continue the efforts to determine the ideal therapy for this type of lesion, establishing uniform injury models and appropriate functional and regenerative assessment methods adapted to each situation.
| Abbreviations | ||
| 4-AP | = | 4-aminopyridine |
| BDNF | = | Brain-derived neurotrophic factor |
| CAT | = | Catalase |
| CNS | = | Central nervous system |
| EGM | = | Electromyography |
| ES | = | Electrostimulation |
| GDNF | = | glial-derived neurotrophic factors |
| HRU | = | High resolution ultrasound |
| IGF-1 | = | Insulin-like growth factor |
| LIU | = | low-intensity ultrasound |
| MBP | = | myelin basic proteins |
| MeCbl | = | methylcobalamin |
| MRI | = | Magnetic resonance imaging |
| MSC’s | = | mesenchymal stem cells |
| NGF | = | Nerve Growth Factor |
| PDE-5 | = | Phosphodiesterase-5 |
| PNI | = | Peripheral nerve injury |
| PNS | = | Peripheral nervous system |
| SCG10 | = | Microtubule destabilizing factor |
| SFI | = | Sciatic functional index |
| SOD | = | Superoxide dismutase |
| US | = | Ultrasonography |
| α-LA | = | Lipoic acid |
Funding
This research was supported by Programa Operacional Regional do Norte (ON.2 – O Novo Norte), QREN, FEDER with the project “iBone Therapies: Terapias inovadoras para a regeneração óssea” [reference number NORTE-01-0247-FEDER-003262]; and by the program COMPETE – Programa Operacional Factores de Competitividade [Project number PEst-OE/AGR/UI0211/2011], [Project number PEst-C/EME/UI0285/2013] funding from FCT. This research was also supported by Programa Operacional Competitividade e Internacionalização (P2020), Fundos Europeus Estruturais e de Investimento (FEEI) and FCT with the project “BioMate – A novel bio-manufacturing system to produce bioactive scaffolds for tissue engineering” with reference PTDC/EMS-SIS/7032/2014 and by COMPETE 2020, from ANI – Projectos ID&T Empresas em Copromoção, Programas Operacionais POCI, by the project “insitu.Biomas - Reinvent biomanufacturing systems by using an usability approach for in situ clinic temporary implants fabrication” [reference number POCI-01-0247-FEDER-017771]. The research was also supported by the research project “BEPIM III – Microdispositivos médicos com capacidades osteintegradoras por micoPIM” [reference number POCI-01-0247-FEDER-017935], from Fundo Europeu de Desenvolvimento Regional (FEDER), by the Programa Operacional da Competitividade & Internacionalização. Ana Rita Caseiro (SFRH/BD/101174/2014) and Rui Damásio Alvites (SFRH/BD/116118/2016.) acknowledges FCT, for financial support.
Additional information
Notes on contributors
Ana Colette Maurício
All authors made substantial contributions for the conception and design of the present article, participating in its drafting, revising it critically for important intellectual content and giving final approval of the version to be submitted. Our research group has been dedicated to peripheral nerve regeneration, and more recently we have focused in the neuro-muscular regeneration, with several published papers for the past 12 years. We have been focused not only in the treatment of several nerve and muscular injuries, using different biomaterials for the construction of tube-guides and vehicles, but also using cell based therapies, mostly mesenchymal stem cells and their secretion products. Our approach for this area of research, is a longitudinal approach, where we dedicate to biomaterials development and characterization, cell-based therapies preparation, animal trials including functional and morphologic evaluation of the healing and regeneration process, in order to transfer this knowledge to the Human and Veterinary Medicine.
References
- Aboonq, M. S. (2015). Pathophysiology of carpal tunnel syndrome. Neurosciences (Riyadh), 20(1), 4–9.
- Agthong, S., Kaewsema, A., & Chentanez, V. (2012). Inhibition of p38 MAPK reduces loss of primary sensory neurons after nerve transection. Neurological Research, 34(7), 714–720. doi:10.1179/1743132812Y.0000000070
- Aguayo, A. J., Peyronnard, J. M., & Bray, G. M. (1973). A quantitative ultrastructural study of regeneration from isolated proximal stumps of transected unmyelinated nerves. Journal of Neuropathology & Experimental Neurology, 32(2), 256–270.10.1097/00005072-197304000-00006
- Al-Majed, A. A., Neumann, C. M., Brushart, T. M., & Gordon, T. (2000). Brief electrical stimulation promotes the speed and accuracy of motor axonal regeneration. Journal of Neuroscience, 20(7), 2602–2608.10.1523/JNEUROSCI.20-07-02602.2000
- Algora, J., Chen, L. E., Seaber, A. V., Wong, G. H., & Urbaniak, J. R. (1996). Functional effects of lymphotoxin on crushed peripheral nerve. Microsurgery, 17(3), 131–135. doi:10.1002/(SICI)1098-2752(1996)17:3<131::AID-MICR6>3.0.CO;2-P
- Alluin, O., Wittmann, C., Marqueste, T., Chabas, J. F., Garcia, S., Lavaut, M. N., … Decherchi, P. (2009). Functional recovery after peripheral nerve injury and implantation of a collagen guide. Biomaterials, 30(3), 363–373. doi:10.1016/j.biomaterials.2008.09.043
- Alrashdan, M. S., Park, J. C., Sung, M. A., Yoo, S. B., Jahng, J. W., Lee, T. H., … Lee, J. H. (2010). Thirty minutes of low intensity electrical stimulation promotes nerve regeneration after sciatic nerve crush injury in a rat model. Acta Neurologica Belgica, 110(2), 168–179.
- An, S., Zhang, P., Peng, J., Deng, L., Wang, Z., Wang, Z., … Ha, N. (2015). Motor function recovery during peripheral nerve multiple regeneration. Journal of tissue engineering and regenerative medicine, 9(4), 415–423.10.1002/term.1833
- Antoniadis, G., Kretschmer, T., Pedro, M. T., Konig, R. W., Heinen, C. P., & Richter, H. P. (2014). Iatrogenic nerve injuries: Prevalence, diagnosis and treatment. Deutsches Ärzteblatt International, 111(16), 273–279. doi:10.3238/arztebl.2014.0273
- Antunes, J. S., Lovison, K., Karvat, J., Peretti, A. L., Vieira, L., Higuchi, G. H., … Bertolini, G. R. (2016). Nociceptive and neuronal evaluation of the sciatic nerve of wistar rats subjected to compression injury and treated with resistive exercise. Pain Research and Management, 2016, 6487160. doi:10.1155/2016/6487160
- Apel, P. J., Alton, T., Northam, C., Ma, J., Callahan, M., Sonntag, W. E., & Li, Z. (2009). How age impairs the response of the neuromuscular junction to nerve transection and repair: An experimental study in rats. Journal of Orthopaedic Research, 27(3), 385–393.10.1002/jor.20773
- Apfel, S. C., Kessler, J. A., Adornato, B. T., Litchy, W. J., Sanders, C., & Rask, C. A. (1998). Recombinant human nerve growth factor in the treatment of diabetic polyneuropathy. NGF Study Group. Neurology, 51(3), 695–702.
- Araujo-Filho, H. G., Quintans-Junior, L. J., Barreto, A. S., Almeida, J. R., Barreto, R. S., & Quintans, J. S. (2016). Neuroprotective effect of natural products on peripheral nerve degeneration: A systematic review. Neurochemical Research, 41(4), 647–658. doi:10.1007/s11064-015-1771-2
- Atsumi, Y., Imai, T., Matsumoto, K., Sakuda, M., Kurisu, K., & Wakisaka, S. (2000). Effects of neonatal injury of the inferior alveolar nerve on the development and regeneration of periodontal nerve fibers in the rat incisor. Brain Research, 871(2), 201–209.10.1016/S0006-8993(00)02446-X
- Bain, J. R., Mackinnon, S. E., & Hunter, D. A. (1989). Functional evaluation of complete sciatic, peroneal, and posterior tibial nerve lesions in the rat. Plastic and Reconstructive Surgery, 83(1), 129–138.10.1097/00006534-198901000-00024
- Baptista, A. F., Gomes, J. R., Oliveira, J. T., Santos, S. M., Vannier-Santos, M. A., & Martinez, A. M. (2008). High- and low-frequency transcutaneous electrical nerve stimulation delay sciatic nerve regeneration after crush lesion in the mouse. Journal of the Peripheral Nervous System, 13(1), 71–80. doi:10.1111/j.1529-8027.2008.00160.x
- Basu, B. (2017). Corrosion and degradation of implantable biomaterials. In Biomaterials for musculoskeletal regeneration (pp. 253–289). Singapore: Springer.10.1007/978-981-10-3059-8
- Bayrak, A. F., Olgun, Y., Ozbakan, A., Aktas, S., Kulan, C. A., Kamaci, G., … Olgun, L. (2017). The effect of insulin like growth factor-1 on recovery of facial nerve crush injury. Clinical and Experimental Otorhinolaryngology, 10(4), 296–302. doi:10.21053/ceo.2016.00997
- Beer, G. M., Steurer, J., & Meyer, V. E. (2001). Standardizing nerve crushes with a non-serrated clamp. Journal of Reconstructive Microsurgery, 17(7), 531–534. doi:10.1055/s-2001-17755
- Belanger, K., Dinis, T. M., Taourirt, S., Vidal, G., Kaplan, D. L., & Egles, C. (2016). Recent strategies in tissue engineering for guided peripheral nerve regeneration. Macromolecular Bioscience, 16(4), 472–481. doi:10.1002/mabi.201500367
- Bell, J. H. & Haycock, J. W. (2012). Next generation nerve guides: Materials, fabrication, growth factors, and cell delivery. Tissue Engineering. Part B, Reviews, 18(2), 116–128. doi:10.1089/ten.TEB.2011.0498
- Bhangra, K. S., Busuttil, F., Phillips, J. B., & Rahim, A. A. (2016). Using stem cells to grow artificial tissue for peripheral nerve repair. Stem Cells International, 2016, 7502178.
- Bigelow, N. H., & Graves, R. W. (1952). Peripheral-nerve lesions in hemorrhagic diseases. AMA Archives of Neurology & Psychiatry, 68(6), 819–830.10.1001/archneurpsyc.1952.02320240094010
- Birch, R., Misra, P., Stewart, M., Eardley, W. G., Ramasamy, A., Brown, K., … Dunn, R. (2012). Nerve injuries sustained during warfare. The Journal of Bone and Joint Surgery, 94(4), 529–535.10.1302/0301-620X.94B4.28488
- Blanco, R. E., Rosado, J., Padilla, J., & Del Cueto, C. (1999). Ultrastructural studies of dorsal root axons regenerating through adult frog optic and sciatic nerves. Microscopy Research and Technique, 46(4–5), 310–318.10.1002/(ISSN)1097-0029
- Boeltz, T., Ireland, M., Mathis, K., Nicolini, J., Poplavski, K., Rose, S. J., … English, A. W. (2013). Effects of treadmill training on functional recovery following peripheral nerve injury in rats. Journal of Neurophysiology, 109(11), 2645–2657. doi:10.1152/jn.00946.2012
- Boisse, L., Spencer, S. J., Mouihate, A., Vergnolle, N., & Pittman, Q. J. (2005). Neonatal immune challenge alters nociception in the adult rat. Pain, 119(1–3), 133–141. doi:10.1016/j.pain.2005.09.022
- Bozkurt, A., Lassner, F., O’Dey, D., Deumens, R., Bocker, A., Schwendt, T., … Pallua, N. (2012). The role of microstructured and interconnected pore channels in a collagen-based nerve guide on axonal regeneration in peripheral nerves. Biomaterials, 33(5), 1363–1375. doi:10.1016/j.biomaterials.2011.10.069
- Bozkurt, A., Tholl, S., Wehner, S., Tank, J., Cortese, M., mon O’Dey, D., … Gröger, A. (2008). Evaluation of functional nerve recovery with Visual-SSI – A novel computerized approach for the assessment of the static sciatic index (SSI). Journal of Neuroscience Methods, 170(1), 117–122.10.1016/j.jneumeth.2008.01.006
- Brady, S., Siegel, G., Albers, R. W., & Price, D. (2011). Basic neurochemistry: Principles of molecular, cellular, and medical neurobiology. Cambridge, MA: Academic Press.
- Bregeon, F., Alliez, J. R., Hery, G., Marqueste, T., Ravailhe, S., & Jammes, Y. (2007). Motor and sensory re-innervation of the lung and heart after re-anastomosis of the cervical vagus nerve in rats. The Journal of Physiology, 581(Pt 3), 1333–1340. doi:10.1113/jphysiol.2007.131326
- Bridge, P. M., Ball, D. J., Mackinnon, S. E., Nakao, Y., Brandt, K., Hunter, D. A., & Hertl, C. (1994). Nerve crush injuries–A model for axonotmesis. Experimental Neurology, 127(2), 284–290. doi:10.1006/exnr.1994.1104
- Brull, R., Hadzic, A., Reina, M. A., & Barrington, M. J. (2015). Pathophysiology and etiology of nerve injury following peripheral nerve blockade. Regional Anesthesia and Pain Medicine, 40(5), 479–490. doi:10.1097/AAP.0000000000000125
- Burnett, M. G., & Zager, E. L. (2004). Pathophysiology of peripheral nerve injury: A brief review. Neurosurgical Focus, 16(5), E1.10.3171/foc.2004.16.5.2
- Caceres, Z. B., Peress, L., Rameshwar, P., & Fernandez-Moure, J. S. (2016). Pathophysiology of peripheral nerve injury: A brief review. Neurosurgical Focus, 16(5), E1.
- Camara, C. C., Araujo, C. V., de Sousa, K. K. O., Brito, G. A. C., Vale, M. L., Raposo, R. D. S., … Oria, R. B. (2015). Gabapentin attenuates neuropathic pain and improves nerve myelination after chronic sciatic constriction in rats. Neuroscience Letters, 607, 52–58. doi:10.1016/j.neulet.2015.09.021
- Camara, C. C., Ramos, H. F., da Silva, A. P., Araujo, C. V., Gomes, A. S., Vale, M. L., … Oria, R. B. (2013). Oral gabapentin treatment accentuates nerve and peripheral inflammatory responses following experimental nerve constriction in Wistar rats. Neuroscience Letters, 556, 93–98. doi:10.1016/j.neulet.2013.10.010
- Campbell, W. W. (2008). Evaluation and management of peripheral nerve injury. Clinical Neurophysiology, 119(9), 1951–1965. doi:10.1016/j.clinph.2008.03.018
- Campenot, R. B. (1982). Development of sympathetic neurons in compartmentalized cultures: I. Local control of neurite growth by nerve growth factor. Developmental Biology, 93(1), 1–12.10.1016/0012-1606(82)90232-9
- Capadia, G. D., Shetty, V. P., Khambati, F. A., & Ghate, S. D. (2010). Effect of corticosteroid usage combined with multidrug therapy on nerve damage assessed using nerve conduction studies: A prospective cohort study of 365 untreated multibacillary leprosy patients. Journal of Clinical Neurophysiology, 27(1), 38–47.10.1097/WNP.0b013e3181cb426d
- Carriel, V., Garzon, I., Alaminos, M., & Cornelissen, M. (2014). Histological assessment in peripheral nerve tissue engineering. Neural Regeneration Research, 9(18), 1657–1660. doi:10.4103/1673-5374.141798
- Carriel, V., Garzón, I., Campos, A., Cornelissen, M., & Alaminos, M. (2017). Differential expression of GAP-43 and neurofilament during peripheral nerve regeneration through bio-artificial conduits. Journal of Tissue Engineering and Regenerative Medicine, 11(2), 553–563.10.1002/term.v11.2
- Carriel, V. S., Aneiros-Fernandez, J., Arias-Santiago, S., Garzon, I. J., Alaminos, M., & Campos, A. (2011). A novel histochemical method for a simultaneous staining of melanin and collagen fibers. Journal of Histochemistry & Cytochemistry, 59(3), 270–277. doi:10.1369/0022155410398001
- Castillo-Galván, M. L., Martínez-Ruiz, F. M., De la Garza-Castro, Ó., Elizondo-Omaña, R. E., & Guzmán-López, S. (2014). Study of peripheral nerve injury in trauma patients. Gaceta Medica de Mexico, 150(6), 519–523.
- Catala, M. & Kubis, N. (2013). Gross anatomy and development of the peripheral nervous system. Handbook of Clinical Neurology, 115, 29–41. doi:10.1016/B978-0-444-52902-2.00003-5
- Chang, H. M., Liu, C. H., Hsu, W. M., Chen, L. Y., Wang, H. P., Wu, T. H., … Liao, W. C. (2014). Proliferative effects of melatonin on Schwann cells: Implication for nerve regeneration following peripheral nerve injury. Journal of Pineal Research, 56(3), 322–332. doi:10.1111/jpi.12125
- Chen, J., Zuo, S., Wang, J., Huang, J., Zhang, X., Liu, Y., … Liu, Z. (2014). Aspirin promotes oligodendrocyte precursor cell proliferation and differentiation after white matter lesion. Frontiers in Aging Neuroscience, 6, 7. doi:10.3389/fnagi.2014.00007
- Chen, L. E., Seaber, A. V., Glisson, R. R., Davies, H., Murrell, G. A., Anthony, D. C., & Urbaniak, J. R. (1992). The functional recovery of peripheral nerves following defined acute crush injuries. Journal of Orthopaedic Research, 10(5), 657–664. doi:10.1002/jor.1100100508
- Chen, Y. G., & Brushart, T. M. (1998). The effect of denervated muscle and Schwann cells on axon collateral sprouting. Journal of Hand Surgery, 23(6), 1025–1033. doi:10.1016/S0363-5023(98)80010-5
- Cheng, F.-C., Tai, M.-H., Sheu, M.-L., Chen, C.-J., Yang, D.-Y., Su, H.-L., … Pan, H.-C. (2010). Enhancement of regeneration with glia cell line–derived neurotrophic factor–transduced human amniotic fluid mesenchymal stem cells after sciatic nerve crush injury [RETRACTED] Laboratory investigation. Journal of Neurosurgery, 112(4), 868–879.10.3171/2009.8.JNS09850
- Cheng, K. I., Wang, H. C., Wu, Y. C., Tseng, K. Y., Chuang, Y. T., Chou, C. W., … Lai, C. S. (2016). Sciatic nerve intrafascicular lidocaine injection-induced peripheral neuropathic pain: Alleviation by systemic minocycline administration. The Clinical Journal of Pain, 32(6), 513–521. doi:10.1097/AJP.0000000000000293
- Chernousov, M. A., Yu, W. M., Chen, Z. L., Carey, D. J., & Strickland, S. (2008). Regulation of Schwann cell function by the extracellular matrix. Glia, 56(14), 1498–1507. doi:10.1002/glia.20740
- Chhabra, A., Ahlawat, S., Belzberg, A., & Andreseik, G. (2014). Peripheral nerve injury grading simplified on MR neurography: As referenced to Seddon and Sunderland classifications. The Indian Journal of Radiology & Imaging, 24(3), 217.10.4103/0971-3026.137025
- Chhabra, A., & Carrino, J. (2015). Current MR neurography techniques and whole-body MR neurography. Paper presented at the Seminars in musculoskeletal radiology.
- Chierzi, S., Ratto, G. M., Verma, P., & Fawcett, J. W. (2005). The ability of axons to regenerate their growth cones depends on axonal type and age, and is regulated by calcium, cAMP and ERK. European Journal of Neuroscience, 21(8), 2051–2062.10.1111/ejn.2005.21.issue-8
- Choi, E. J., Choi, Y. M., Jang, E. J., Kim, J. Y., Kim, T. K., & Kim, K. H. (2016). Neural ablation and regeneration in pain practice. Korean Journal of Pain, 29(1), 3–11. doi:10.3344/kjp.2016.29.1.3.
- Ciaramitaro, P., Mondelli, M., Logullo, F., Grimaldi, S., Battiston, B., Sard, A., … Cocito, D. (2010). Traumatic peripheral nerve injuries: Epidemiological findings, neuropathic pain and quality of life in 158 patients. Journal of the Peripheral Nervous System, 15(2), 120–127.10.1111/(ISSN)1529-8027
- Cirillo, V., Clements, B. A., Guarino, V., Bushman, J., Kohn, J., & Ambrosio, L. (2014). A comparison of the performance of mono- and bi-component electrospun conduits in a rat sciatic model. Biomaterials, 35(32), 8970–8982. doi:10.1016/j.biomaterials.2014.07.010.
- Cui, Y., Li, J., Zhu, Y., Tang, H., He, X., & Xu, Y. (2015). The neuroprotective effects of aspirin following crush injury to rat sciatic nerve. International Journal of Clinical and Experimental Medicine, 8(10), 18185–18190.
- Dahlin, L. B. (2008). Techniques of peripheral nerve repair. Scandinavian Journal of Surgery, 97(4), 310–316. doi:10.1177/145749690809700407
- Dahlin, L. B., & Wiberg, M. (2017). Nerve injuries of the upper extremity and hand. EFORT Open Reviews, 2(5), 158–170.10.1302/2058-5241.2.160071
- Das, S., Maulik, N., Das, D., Kadowitz, P., & Bivalacqua, T. (2001). Cardioprotection with sildenafil, a selective inhibitor of cyclic 3’, 5’-monophosphate-specific phosphodiesterase 5. Drugs Under Experimental and Clinical Research, 28(6), 213–219.
- De Koning, P., Brakkee, J. H., & Gispen, W. H. (1986). Methods for producing a reproducible crush in the sciatic and tibial nerve of the rat and rapid and precise testing of return of sensory function. Beneficial effects of melanocortins. Journal of the Neurological Sciences, 74(2–3), 237–246.10.1016/0022-510X(86)90109-7
- De Lahunta, A., Glass, E., & Kent, M. (2009). Lower motor neuron: Spinal nerve, general somatic efferent system. Veterinary Neuroanatomy and Clinical Neurology, 3, 119–120.
- de Medinaceli, L., Freed, W. J., & Wyatt, R. J. (1982). An index of the functional condition of rat sciatic nerve based on measurements made from walking tracks. Experimental Neurology, 77(3), 634–643.10.1016/0014-4886(82)90234-5
- de Ruiter, G. C., Spinner, R. J., Verhaagen, J., & Malessy, M. J. (2014). Misdirection and guidance of regenerating axons after experimental nerve injury and repair: A review. Journal of Neurosurgery, 120(2), 493–501.10.3171/2013.8.JNS122300
- Deumens, R., Bozkurt, A., Meek, M. F., Marcus, M. A., Joosten, E. A., Weis, J., & Brook, G. A. (2010). Repairing injured peripheral nerves: Bridging the gap. Progress in Neurobiology, 92(3), 245–276. doi:10.1016/j.pneurobio.2010.10.002
- Ding, X. G., Li, S. W., Zheng, X. M., Hu, L. Q., Hu, W. L., & Luo, Y. (2009). The effect of platelet-rich plasma on cavernous nerve regeneration in a rat model. Asian Journal of Andrology, 11(2), 215–221. doi:10.1038/aja.2008.37
- Dinh, P., Hazel, A., Palispis, W., Suryadevara, S., & Gupta, R. (2009). Functional assessment after sciatic nerve injury in a rat model. Microsurgery, 29(8), 644–649. doi:10.1002/micr.20685
- Diogo, C. C., Camassa, J. A., Pereira, J. E., Costa, L. M. D., Filipe, V., Couto, P. A., … Varejão, A. S. (2017). The use of sheep as a model for studying peripheral nerve regeneration following nerve injury: Review of the literature. Neurological Research, 1–14.
- Dubovy, P., Klusakova, I., & Hradilova Svizenska, I. (2014). Inflammatory profiling of Schwann cells in contact with growing axons distal to nerve injury. BioMed Research International, 2014, 691041. doi:10.1155/2014/691041
- Eftekhar-sadat, B., Babaei-Ghazani, A., Samadirad, B., & Mamaghany, V. (2017). Traumatic cervical and upper limb peripheral nerve injuries in adults: Electrodiagnostic studies and patients symptoms. Hand, 13(12), 12.
- El Samad, A. A. A., Raafat, M. H., Shokry, Y., Zahra, F. A. A., & Abdellah, A. M. (2015). Histological study on the role of bone marrow-derived mesenchymal stem cells on the sciatic nerve and the gastrocnemius muscle in a model of sciatic nerve crush injury in albino rats. Egyptian Journal of Histology, 38(3), 438–451.10.1097/01.EHX.0000470653.67231.07
- English, A. W., Schwartz, G., Meador, W., Sabatier, M. J., & Mulligan, A. (2007). Electrical stimulation promotes peripheral axon regeneration by enhanced neuronal neurotrophin signaling. Developmental Neurobiology, 67(2), 158–172. doi:10.1002/dneu.20339
- English, A. W., Wilhelm, J. C., & Sabatier, M. J. (2011). Enhancing recovery from peripheral nerve injury using treadmill training. Annals of Anatomy-Anatomischer Anzeiger, 193(4), 354–361.10.1016/j.aanat.2011.02.013
- Esaki, S., Kitoh, J., Katsumi, S., Goshima, F., Kimura, H., Safwat, M., … Nakamura, T. (2011). Hepatocyte growth factor incorporated into herpes simplex virus vector accelerates facial nerve regeneration after crush injury. Gene Therapy, 18(11), 1063.10.1038/gt.2011.57
- Fan, L. Y., Wang, Z. C., Wang, P., Lan, Y. Y., & Tu, L. (2015). Exogenous nerve growth factor protects the hypoglossal nerve against crush injury. Neural Regeneration Research, 10(12), 1982–1988. doi:10.4103/1673-5374.172316
- Farber, S. J., Saheb-Al-Zamani, M., Zieske, L., Laurido-Soto, O., Bery, A., Hunter, D., … Mackinnon, S. E. (2013). Peripheral nerve injury after local anesthetic injection. Anesthesia & Analgesia, 117(3), 731–739. doi:10.1213/ANE.0b013e3182a00767
- Fargo, K. N., Alexander, T. D., Tanzer, L., Poletti, A., & Jones, K. J. (2008). Androgen regulates neuritin mRNA levels in an in vivo model of steroid-enhanced peripheral nerve regeneration. Journal of Neurotrauma, 25(5), 561–566. doi:10.1089/neu.2007.0466
- Faroni, A., Mobasseri, S. A., Kingham, P. J., & Reid, A. J. (2015). Peripheral nerve regeneration: Experimental strategies and future perspectives. Advanced Drug Delivery Reviews, 82–83, 160–167. doi:10.1016/j.addr.2014.11.010
- Feinberg, J., & Sethi, S. (2006). Sciatic neuropathy: Case report and discussion of the literature on postoperative sciatic neuropathy and sciatic nerve tumors. HSS Journal, 2(2), 181.10.1007/s11420-006-9018-z
- Feng, X., & Yuan, W. (2015). Dexamethasone enhanced functional recovery after sciatic nerve crush injury in rats. BioMed Research International, 2015, 627923. doi:10.1155/2015/627923
- Fitzgerald, M. & McKelvey, R. (2016). Nerve injury and neuropathic pain – A question of age. Experimental Neurology, 275(2), 296–302. doi:10.1016/j.expneurol.2015.07.013.
- Fullarton, A., Lenihan, D., Myles, L., & Glasby, M. (2000). Obstetric brachial plexus palsy: A large animal model for traction injury and its repair: Part 1: Age of the recipient. Journal of Hand Surgery, 25(1), 52–57.10.1054/jhsb.1999.0337
- Fullarton, A., Lenihan, D., Myles, L., & Glasby, M. (2002). Assessment of the method and timing of repair of a brachial plexus traction injury in an animal model for obstetric brachial plexus palsy. Journal of Hand Surgery, 27(1), 13–19.10.1054/JHSB.2001.0657
- Fullarton, A., Myles, L., Lenihan, D., Hems, T., & Glasb, M. (2001). Obstetric brachial plexus palsy: A comparison of the degree of recovery after repair of a C6 ventral root avulsion in newborn and adult sheep. British Journal of Plastic Surgery, 54(8), 697–704.10.1054/bjps.2001.3700
- Galloway, E. B., 3rd, Jensen, R. L., Dailey, A. T., Thompson, B. G., & Shelton, C. (2000). Role of topical steroids in reducing dysfunction after nerve injury. Laryngoscope, 110(11), 1907–1910. doi:10.1097/00005537-200011000-00026
- Gan, L., Qian, M., Shi, K., Chen, G., Gu, Y., Du, W., & Zhu, G. (2014). Restorative effect and mechanism of mecobalamin on sciatic nerve crush injury in mice. Neural Regeneration Research, 9(22), 1979–1984. doi:10.4103/1673-5374.145379
- Gao, H., You, Y., Zhang, G., Zhao, F., Sha, Z., & Shen, Y. (2013). The use of fiber-reinforced scaffolds cocultured with Schwann cells and vascular endothelial cells to repair rabbit sciatic nerve defect with vascularization. BioMed Research International, 2013.
- Gao, Y., Weng, C., & Wang, X. (2013). Changes in nerve microcirculation following peripheral nerve compression. Neural Regeneration Research, 8(11), 1041–1047. doi:10.3969/j.issn.1673-5374.2013.11.010
- Gaudet, A. D., Popovich, P. G., & Ramer, M. S. (2011). Wallerian degeneration: Gaining perspective on inflammatory events after peripheral nerve injury. Journal of Neuroinflammation, 8(1), 110. doi:10.1186/1742-2094-8-110
- Gaudin, R., Knipfer, C., Henningsen, A., Smeets, R., Heiland, M., & Hadlock, T. (2016). Approaches to peripheral nerve repair: Generations of biomaterial conduits yielding to replacing autologous nerve grafts in craniomaxillofacial surgery. BioMed Research International, 2016, 3856262. doi:10.1155/2016/3856262
- Ge, L., Jiang, M., Duan, D., Wang, Z., Qi, L., Teng, X., & Chen, P. (2016). Secretome of olfactory mucosa mesenchymal stem cell, a multiple potential stem cell. Stem Cells International, 2016.
- Geremia, N. M., Gordon, T., Brushart, T. M., Al-Majed, A. A., & Verge, V. M. (2007). Electrical stimulation promotes sensory neuron regeneration and growth-associated gene expression. Experimental Neurology, 205(2), 347–359. doi:10.1016/j.expneurol.2007.01.040
- Gersh, I., & Bodian, D. (1943). Some chemical mechanisms in chromatolysis. Journal of Cellular Physiology, 21(3), 253–279.10.1002/(ISSN)1553-0809
- Geuna, S. (2015). The sciatic nerve injury model in pre-clinical research. Journal of Neuroscience Methods, 243, 39–46. doi:10.1016/j.jneumeth.2015.01.021
- Gigo-Benato, D., Russo, T. L., Geuna, S., Domingues, N. R., Salvini, T. F., & Parizotto, N. A. (2010). Electrical stimulation impairs early functional recovery and accentuates skeletal muscle atrophy after sciatic nerve crush injury in rats. Muscle and Nerve, 41(5), 685–693. doi:10.1002/mus.21549
- Gomez-Sanchez, J. A., Carty, L., Iruarrizaga-Lejarreta, M., Palomo-Irigoyen, M., Varela-Rey, M., Griffith, M., & Aurrekoetxea, I. (2015). Schwann cell autophagy, myelinophagy, initiates myelin clearance from injured nerves. The Journal of Cell Biology, 210(1), 153–168.10.1083/jcb.201503019
- Gonzalez-Forero, D., Portillo, F., Sunico, C. R., & Moreno-Lopez, B. (2004). Nerve injury reduces responses of hypoglossal motoneurones to baseline and chemoreceptor-modulated inspiratory drive in the adult rat. The Journal of Physiology, 557(Pt 3), 991–1011. doi:10.1113/jphysiol.2003.059972
- Gonzalez-Martinez, T., Perez-Pinera, P., Diaz-Esnal, B., & Vega, J. A. (2003). S-100 proteins in the human peripheral nervous system. Microscopy Research and Technique, 60(6), 633–638. doi:10.1002/jemt.10304
- Grand, A. G., Myckatyn, T. M., Mackinnon, S. E., & Hunter, D. A. (2002). Axonal regeneration after cold preservation of nerve allografts and immunosuppression with tacrolimus in mice. Journal of Neurosurgery, 96(5), 924–932. doi:10.3171/jns.2002.96.5.0924
- Greene, E. C. (1955). Anatomy of the rat. Transactions of the American Philosophical Society, new ser., v. 27. New York, NY: Hafner Pub. Co.
- Grinsell, D., & Keating, C. P. (2014). Peripheral nerve reconstruction after injury: A review of clinical and experimental therapies. BioMed Research International, 2014, 698256. doi:10.1155/2014/698256
- Guntinas-Lichius, O., Irintchev, A., Streppel, M., Lenzen, M., Grosheva, M., Wewetzer, K., … Angelov, D. N. (2005). Factors limiting motor recovery after facial nerve transection in the rat: Combined structural and functional analyses. European Journal of Neuroscience, 21(2), 391–402. doi:10.1111/j.1460-9568.2005.03877.x
- Hadlock, T., Kowaleski, J., Lo, D., Bermejo, R., Zeigler, H. P., Mackinnon, S., & Heaton, J. T. (2008). Functional assessments of the rodent facial nerve: A synkinesis model. Laryngoscope, 118(10), 1744–1749. doi:10.1097/MLG.0b013e31817f5255
- Hadlock, T. A., Heaton, J., Cheney, M., & Mackinnon, S. E. (2005). Functional recovery after facial and sciatic nerve crush injury in the rat. Archives of Facial Plastic Surgery, 7(1), 17–20. doi:10.1001/archfaci.7.1.17
- Hainline, B. W. (2014). Peripheral nerve injury in sports. Continuum (Minneap Minn), 20(6 Sports Neurology), 1605–1628. doi:10.1212/01.CON.0000458971.86389.9c
- Hajimoradi, M., Fazilati, M., Gharib-Naseri, M. K., & Sarkaki, A. (2015). Gallic acid and exercise training improve motor function, nerve conduction velocity but not pain sense reflex after experimental sciatic nerve crush in male rats. Avicenna Journal of Phytomedicine, 5(4), 288.
- Hall, S. (2005). The response to injury in the peripheral nervous system. The Journal of Bone and Joint Surgery, 87(10), 1309–1319. doi:10.1302/0301-620X.87B10.16700
- Haller, M. F., & Saltzman, W. M. (1998). Nerve growth factor delivery systems. Journal of Controlled Release, 53(1–3), 1–6.10.1016/S0168-3659(97)00232-0
- Hayashi, A., Moradzadeh, A., Hunter, D. A., Kawamura, D. H., Puppala, V. K., Tung, T. H., … Myckatyn, T. M. (2007). Retrograde labeling in peripheral nerve research: It is not all black and white. Journal of Reconstructive Microsurgery, 23(07), 381–389.10.1055/s-2007-992344
- Hayes, K. C., Potter, P. J., Wolfe, D. L., Hsieh, J. T., Delaney, G. A., & Blight, A. R. (1994). 4-Aminopyridine-sensitive neurologic deficits in patients with spinal cord injury. Journal of Neurotrauma, 11(4), 433–446. doi:10.1089/neu.1994.11.433
- Heaton, J. T., Kowaleski, J. M., Bermejo, R., Zeigler, H. P., Ahlgren, D. J., & Hadlock, T. A. (2008). A system for studying facial nerve function in rats through simultaneous bilateral monitoring of eyelid and whisker movements. Journal of Neuroscience Methods, 171(2), 197–206.10.1016/j.jneumeth.2008.02.023
- Hei, W.-H., Byun, S.-H., Kim, J.-S., Kim, S., Seo, Y.-K., Park, J.-C., … Lee, J.-H. (2016). Effects of electromagnetic field (PEMF) exposure at different frequency and duration on the peripheral nerve regeneration: In vitro and in vivo study. International Journal of Neuroscience, 126(8), 739–748.
- Hirano, A. (2005). The role of electron microscopy in neuropathology. Acta Neuropathologica, 109(1), 115–123. doi:10.1007/s00401-004-0960-x
- Hobbenaghi, R., Javanbakht, J., Hosseini, E., Mohammadi, S., Rajabian, M., Moayeri, P., & Hassan, M. A. (2016). Neuropathological and neuroprotective features of vitamin B12 on the dorsal spinal ganglion of rats after the experimental crush of sciatic nerve: An experimental study (vol 8, pg 123, 2013). Diagnostic Pathology, 11.
- Hogan, Q. H. (2008). Pathophysiology of peripheral nerve injury during regional anesthesia. Regional Anesthesia and Pain Medicine, 33(5), 435–441. doi:10.1016/j.rapm.2008.03.002
- Holmes, W., & Young, J. Z. (1942). Nerve regeneration after immediate and delayed suture. J Anat, 77(Pt 1), 63–96.
- Hood, B., Levene, H. B., & Levi, A. D. (2009). Transplantation of autologous Schwann cells for the repair of segmental peripheral nerve defects. Neurosurgical Focus, 26(2), E4. doi:10.3171/FOC.2009.26.2.E4
- Houschyar, K. S., Momeni, A., Pyles, M. N., Cha, J. Y., Maan, Z. N., Duscher, D., … van Schoonhoven, J. (2016). The role of current techniques and concepts in peripheral nerve repair. Plastic Surgery International, 2016, 4175293. doi:10.1155/2016/4175293
- Hsieh, J. H., Lin, W. M., Chiang, H., Chang, L. Y., Wu, C. T., Pu, C. M., … Hsieh, S. T. (2013). Patterns of target tissue reinnervation and trophic factor expression after nerve grafting. Plastic and Reconstructive Surgery, 131(5), 989–1000. doi:10.1097/PRS.0b013e3182870445
- Huang, W., Begum, R., Barber, T., Ibba, V., Tee, N. C., Hussain, M., … Priestley, J. V. (2012). Regenerative potential of silk conduits in repair of peripheral nerve injury in adult rats. Biomaterials, 33(1), 59–71. doi:10.1016/j.biomaterials.2011.09.030
- Jacobson, A., & Schrader, S. (1987). Peripheral nerve injury associated with fracture or fracture-dislocation of the pelvis in dogs and cats: 34 cases (1978–1982). Journal of the American Veterinary Medical Association, 190(5), 569–572.
- Jager, S. B., Ronchi, G., Vaegter, C. B., & Geuna, S. (2014). The mouse median nerve experimental model in regenerative research. BioMed Research International, 2014, 701682. doi:10.1155/2014/701682
- Jeans, L., Gilchrist, T., & Healy, D. (2007). Peripheral nerve repair by means of a flexible biodegradable glass fibre wrap: a comparison with microsurgical epineurial repair. Journal of Plastic, Reconstructive & Aesthetic Surgery, 60(12), 1302–1308.10.1016/j.bjps.2006.06.014
- Jiang, L., Jones, S., & Jia, X. (2017). Stem cell transplantation for peripheral nerve regeneration: Current options and opportunities. International Journal of Molecular Sciences, 18(1), 94. doi:10.3390/ijms18010094
- Johnson, I. P., & Sears, T. (2013). Target-dependence of sensory neurons: An ultrastructural comparison of axotomised dorsal root ganglion neurons with allowed or denied reinnervation of peripheral targets. Neuroscience, 228, 163–178.10.1016/j.neuroscience.2012.10.015
- Jolivalt, C. G., Mizisin, L. M., Nelson, A., Cunha, J. M., Ramos, K. M., Bonke, D., & Calcutt, N. A. (2009). B vitamins alleviate indices of neuropathic pain in diabetic rats. European Journal of Pharmacology, 612(1–3), 41–47. doi:10.1016/j.ejphar.2009.04.028
- Josta, T., & Casper, C. (2015). The axonal cytoskeleton: From organization to function. Frontiers in Molecular Neuroscience, 8.
- Kalender, A. M., Dogan, A., Bakan, V., Yildiz, H., Gokalp, M. A., & Kalender, M. (2009). Effect of Zofenopril on regeneration of sciatic nerve crush injury in a rat model. Journal of Brachial Plexus and Peripheral Nerve Injury, 4(1), 6. doi:10.1186/1749-7221-4-6
- Kaplan, S., Piskin, A., Ayyildiz, M., Aktas, A., Koksal, B., Ulkay, M. B., … Geuna, S. (2011). The effect of melatonin and platelet gel on sciatic nerve repair: An electrophysiological and stereological study. Microsurgery, 31(4), 306–313. doi:10.1002/micr.20876
- Kaur, G. (2017). Biomaterials influencing human lives. In Bioactive glasses (pp. 1–20). Springer.10.1007/978-3-319-45716-1
- Kaya, Y., Sarıkcıoğlu, L., Aslan, M., Kencebay, C., Demir, N., Derin, N., … Yıldırım, F. B. (2013). Comparison of the beneficial effect of melatonin on recovery after cut and crush sciatic nerve injury: A combined study using functional, electrophysiological, biochemical, and electron microscopic analyses. Child’s Nervous System, 29(3), 389–401.10.1007/s00381-012-1936-0
- Kehoe, S., Zhang, X. F., & Boyd, D. (2012). FDA approved guidance conduits and wraps for peripheral nerve injury: A review of materials and efficacy. Injury, 43(5), 553–572. doi:10.1016/j.injury.2010.12.030
- Kemp, S. W., Cederna, P. S., & Midha, R. (2017). Comparative outcome measures in peripheral regeneration studies. Experimental Neurology, 287(Pt 3), 348–357. doi:10.1016/j.expneurol.2016.04.011
- Khan, A. A., Faruqi, N. A., & Ansari, M. S. (2014). Effects of hydrocortisone on the sciatic nerve crush injury in adult rat-a light microscopic study. Current Neurobiology, 5(1).
- Kim, D. H., Murovic, J. A., Tiel, R., & Kline, D. G. (2004). Management and outcomes in 353 surgically treated sciatic nerve lesions. Journal of Neurosurgery, 101(1), 8–17. doi:10.3171/jns.2004.101.1.0008
- Kim, J., Han, S. J., Shin, D. H., Lee, W. S., & Choi, J. Y. (2011). Subthreshold continuous electrical stimulation facilitates functional recovery of facial nerve after crush injury in rabbit. Muscle and Nerve, 43(2), 251–258. doi:10.1002/mus.21840
- Kingham, P. J., Kalbermatten, D. F., Mahay, D., Armstrong, S. J., Wiberg, M., & Terenghi, G. (2007). Adipose-derived stem cells differentiate into a Schwann cell phenotype and promote neurite outgrowth in vitro. Experimental Neurology, 207(2), 267–274.10.1016/j.expneurol.2007.06.029
- Kobayashi, J., Mackinnon, S. E., Langer, J. C., Hertl, M. C., Hunter, D. A., & Tarasidis, G. (1997). The effect of ammonium sulfate injection on peripheral nerve. Journal of Reconstructive Microsurgery, 13(6), 389–396. doi:10.1055/s-2007-1006418
- Kobolak, J., Dinnyes, A., Memic, A., Khademhosseini, A., & Mobasheri, A. (2016). Mesenchymal stem cells: Identification, phenotypic characterization, biological properties and potential for regenerative medicine through biomaterial micro-engineering of their niche. Methods, 99, 62–68.10.1016/j.ymeth.2015.09.016
- Koeppen, A. H. (2004). Wallerian degeneration: History and clinical significance. Journal of the Neurological Sciences, 220(1–2), 115–117. doi:10.1016/j.jns.2004.03.008
- Korkmaz, M. F., Parlakpinar, H., Ceylan, M. F., Ediz, L., Samdanci, E., Kekilli, E., & Sagir, M. (2016). The effect of sildenafil on recuperation from sciatic nerve injury in rats. Balkan Medical Journal, 33(2), 204–211. doi:10.5152/balkanmedj.2016.14701
- Krarup, C., Boeckstyns, M., Ibsen, A., Moldovan, M., & Archibald, S. (2016). Remodeling of motor units after nerve regeneration studied by quantitative electromyography. Clinical Neurophysiology, 127(2), 1675–1682. doi:10.1016/j.clinph.2015.08.008
- Kuffler, D. (2015). Promoting axon regeneration and neurological recovery following traumatic peripheral nerve injuries. International Journal of Neurorehabilitation, 2(148). doi:10.4172/2376-0281.10001
- Kurtoglu, Z., Ozturk, A. H., Bagdatoglu, C., Polat, G., Aktekin, M., Uzmansel, D., … Sargon, M. (2005). Effects of trapidil after crush injury in peripheral nerve. Acta Medica Okayama, 59(2), 37–44. doi:10.18926/AMO/31967
- Lal, D., Hetzler, L. T., Sharma, N., Wurster, R. D., Marzo, S. J., Jones, K. J., & Foecking, E. M. (2008). Electrical stimulation facilitates rat facial nerve recovery from a crush injury. Otolaryngology–Head and Neck Surgery, 139(1), 68–73. doi:10.1016/j.otohns.2008.04.030
- Lalkhen, A. G., & Bhatia, K. (2011). Perioperative peripheral nerve injuries. Continuing Education in Anaesthesia, Critical Care & Pain, 12(1), 38–42.
- Lawson, G., & Glasby, M. (1995). A comparison of immediate and delayed nerve repair using autologous freeze-thawed muscle grafts in a large animal model: the simple injury. Journal of Hand Surgery, 20(5), 663–700.10.1016/S0266-7681(05)80131-7
- Lee, F. C., Singh, H., Nazarian, L. N., & Ratliff, J. K. (2011). High-resolution ultrasonography in the diagnosis and intraoperative management of peripheral nerve lesions. Journal of Neurosurgery, 114(1), 206–211. doi:10.3171/2010.2.JNS091324
- Lee, J. M., Tos, P., Raimondo, S., Fornaro, M., Papalia, I., Geuna, S., & Giacobini-Robecchi, M. G. (2007). Lack of topographic specificity in nerve fiber regeneration of rat forelimb mixed nerves. Neuroscience, 144(3), 985–990. doi:10.1016/j.neuroscience.2006.11.001
- Lee, L. N., Lyford-Pike, S., & Boahene, K. D. (2013). Traumatic facial nerve injury. Otolaryngologic Clinics of North America, 46(5), 825–839. doi:10.1016/j.otc.2013.07.001
- Leuzzi, S., Armenio, A., Leone, L., De Santis, V., Di Turi, A., Annoscia, P., … Pascone, M. (2014). Repair of peripheral nerve with vein wrapping. Giornale di Chirurgia, 35(3–4), 101–106.
- Li, B. H., Kim, S. M., Yoo, S. B., Kim, M. J., Jahng, J. W., & Lee, J. H. (2012). Recombinant human nerve growth factor (rhNGF-beta) gene transfer promotes regeneration of crush-injured mental nerve in rats. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology, 113(3), e26–34. doi:10.1016/j.tripleo.2011.07.002
- Li, N., Downey, J. E., Bar-Shir, A., Gilad, A. A., Walczak, P., Kim, H., … Pelled, G. (2011). Optogenetic-guided cortical plasticity after nerve injury. Proceedings of the National Academy of Sciences of the United States of America, 108(21), 8838–8843. doi:10.1073/pnas.1100815108
- Li, Q., Li, T., Cao, X.-C., Luo, D.-Q., & Lian, K.-J. (2016). Methylprednisolone microsphere sustained-release membrane inhibits scar formation at the site of peripheral nerve lesion. Neural Regeneration Research, 11(5), 835.
- Li, Y., Bickel, K. D., Im, M. J., Hu, L., Dellon, A. L., Vander Kolk, C. A., & Manson, P. N. (1996). Effects of deferoxamine on ischemia/reperfusion injury after peripheral nerve compression. Annals of Plastic Surgery, 36(4), 365–369.10.1097/00000637-199604000-00007
- Li, Y., Hu, X., Liu, Y., Bao, Y., & An, L. (2009). Nimodipine protects dopaminergic neurons against inflammation-mediated degeneration through inhibition of microglial activation. Neuropharmacology, 56(3), 580–589.10.1016/j.neuropharm.2008.10.016
- Lim, K. H., Connolly, M., Rose, D., Siegman, F., Jacobowitz, I., Acinapura, A., & Cunningham, J. N. (1986). Prevention of reperfusion injury of the ischemic spinal cord: Use of recombinant superoxide dismutase. The Annals of Thoracic Surgery, 42(3), 282–286.10.1016/S0003-4975(10)62735-X
- Lim, T. K., Shi, X. Q., Johnson, J. M., Rone, M. B., Antel, J. P., David, S., & Zhang, J. (2015). Peripheral nerve injury induces persistent vascular dysfunction and endoneurial hypoxia, contributing to the genesis of neuropathic pain. Journal of Neuroscience, 35(8), 3346–3359. doi:10.1523/JNEUROSCI.4040-14.2015
- Lundborg, G. (1988). Nerve injury and repair. Edinburg: Churchill Livingstone.
- Lundborg, G. (2000). A 25-year perspective of peripheral nerve surgery: Evolving neuroscientific concepts and clinical significance. Journal of Hand Surgery, 25(3), 391–414. doi:10.1053/jhsu.2000.4165
- Lunn, E. R., Brown, M. C., & Perry, V. H. (1990). The pattern of axonal degeneration in the peripheral nervous system varies with different types of lesion. Neuroscience, 35(1), 157–165.10.1016/0306-4522(90)90130-V
- Lutz, A. B. & Barres, B. A. (2014). Contrasting the glial response to axon injury in the central and peripheral nervous systems. Developmental Cell, 28(1), 7–17.
- Ma, J., Li, W., Tian, R., & Lei, W. (2010). Ginsenoside Rg1 promotes peripheral nerve regeneration in rat model of nerve crush injury. Neuroscience Letters, 478(2), 66–71. doi:10.1016/j.neulet.2010.04.064
- Ma, K., Yan, N., Huang, Y., Cao, G., Deng, J., & Deng, Y. (2014). Effects of nerve growth factor on nerve regeneration after corneal nerve damage. International Journal of Clinical and Experimental Medicine, 7(11), 4584–4589.
- Mackinnon, S., & Dellon, A. (1988). Diagnosis of nerve injury. In Surgery of the peripheral nerve (pp. 74–79). New York, NY: Thieme.
- Mackinnon, S. E., Hudson, A. R., & Hunter, D. A. (1985). Histologic assessment of nerve regeneration in the rat. Plastic and Reconstructive Surgery, 75(3), 384–388.10.1097/00006534-198503000-00014
- Madison, R. D., Archibald, S. J., & Brushart, T. M. (1996). Reinnervation accuracy of the rat femoral nerve by motor and sensory neurons. Journal of Neuroscience, 16(18), 5698–5703.10.1523/JNEUROSCI.16-18-05698.1996
- Makoukji, J., Belle, M., Meffre, D., Stassart, R., Grenier, J., Shackleford, G., … Massaad, C. (2012). Lithium enhances remyelination of peripheral nerves. Proceedings of the National Academy of Sciences of the United States of America, 109(10), 3973–3978. doi:10.1073/pnas.1121367109
- Matthews, M. R., & Raisman, G. (1972). A light and electron microscopic study of the cellular response to axonal injury in the superior cervical ganglion of the rat. Proceedings of the Royal Society of London. Series B.
- Mattsson, P., Aldskogius, H., & Svensson, M. (1999). Nimodipine-induced improved survival rate of facial motor neurons following intracranial transection of the facial nerve in the adult rat. Journal of Neurosurgery, 90(4), 760–765. doi:10.3171/jns.1999.90.4.0760
- Meek, M. F., & Coert, J. H. (2008). US food and drug administration/conformit Europe-approved absorbable nerve conduits for clinical repair of peripheral and cranial nerves. Annals of Plastic Surgery, 60(1), 110–116. doi:10.1097/SAP.0b013e31804d441c
- Menorca, R. M., Fussell, T. S., & Elfar, J. C. (2013). Nerve physiology: Mechanisms of injury and recovery. Hand Clinics, 29(3), 317–330. doi:10.1016/j.hcl.2013.04.002
- Mete, G., Atalay, A., Yemişçi, O. U., Karataş, M., & Turhan, N. (2007). Unrecognized peripheral nerve lesions in a traumatic brain injury patient. Turkish Neurosurgery, 17(1), 45–47.
- Meuli-Simmen, C., Meuli, M., Yingling, C. D., Eiman, T., Timmel, G. B., Buncke, H. J., … Adzick, S. N. (1997). Midgestational sciatic nerve transection in fetal sheep results in absent nerve regeneration and neurogenic muscle atrophy. Plastic and Reconstructive Surgery, 99(2), 486–492.10.1097/00006534-199702000-00026
- Millesi, H. (2007). Bridging defects: Autologous nerve grafts. Acta Neurochirurgica Supplement, 100, 37–38.10.1007/978-3-211-72958-8
- Mills, S. E. (2007). Histology for pathologists. Philadelphia, PA.
- Mizisin, A. P., & Weerasuriya, A. (2011). Homeostatic regulation of the endoneurial microenvironment during development, aging and in response to trauma, disease and toxic insult. Acta Neuropathologica, 121(3), 291–312. doi:10.1007/s00401-010-0783-x
- Moon, L. (2018). Chromatolysis: Do injured axons regenerate poorly when ribonucleases fragment or degranulate rough endoplasmic reticulum, disaggregate polyribosomes, degrade monoribosomes and lyse RNA? Preprints 2018, 2018020111. doi: 10.20944/preprints201802.0111.v1
- Mudo, G., Persson, H., Timmusk, T., Funakoshi, H., Bindoni, M., & Belluardo, N. (1993). Increased expression of trkB and trkC messenger RNAs in the rat forebrain after focal mechanical injury. Neuroscience, 57(4), 901–912.10.1016/0306-4522(93)90036-F
- Mukudai, S., Matsuda, K. I., Nishio, T., Sugiyama, Y., Bando, H., Hirota, R., … Kawata, M. (2015). Differential responses to steroid hormones in fibroblasts from the vocal fold, trachea, and esophagus. Endocrinology, 156(3), 1000–1009. doi:10.1210/en.2014-1605
- Myckatyn, T. M., & Mackinnon, S. E. (2004). A review of research endeavors to optimize peripheral nerve reconstruction. Neurological Research, 26(2), 124–138. doi:10.1179/016164104225013743
- Nachemson, A. K., Lundborg, G., & Hansson, H. A. (1990). Insulin-like growth factor I promotes nerve regeneration: An experimental study on rat sciatic nerve. Growth Factors, 3(4), 309–314.10.3109/08977199009003673
- Nachemson, A. K., Lundborg, G., Myrhage, R., & Rank, F. (1985). Nerve regeneration and pharmacological suppression of the scar reaction at the suture site: An experimental study on the effect of estrogen-progesterone, methylprednisolone-acetate and cis-hydroxyproline in rat sciatic nerve. Scandinavian Journal of Plastic and Reconstructive Surgery, 19(3), 255–260.10.3109/02844318509074512
- Namiki, J., Kojima, A., & Tator, C. H. (2000). Effect of brain-derived neurotrophic factor, nerve growth factor, and neurotrophin-3 on functional recovery and regeneration after spinal cord injury in adult rats. Journal of Neurotrauma, 17(12), 1219–1231. doi:10.1089/neu.2000.17.1219
- Napoli, I., Noon, L. A., Ribeiro, S., Kerai, A. P., Parrinello, S., Rosenberg, L. H., & Lloyd, A. C. (2012). A central role for the ERK-signaling pathway in controlling Schwann cell plasticity and peripheral nerve regeneration in vivo. Neuron, 73(4), 729–742. doi:10.1016/j.neuron.2011.11.031
- Navarro, X. (2016). Functional evaluation of peripheral nerve regeneration and target reinnervation in animal models: A critical overview. European Journal of Neuroscience, 43(3), 271–286. doi:10.1111/ejn.13033
- Navarro, X., & Udina, E. (2009). Chapter 6: Methods and protocols in peripheral nerve regeneration experimental research: Part III-electrophysiological evaluation. International Review of Neurobiology, 87, 105–126. doi:10.1016/S0074-7742(09)87006-2
- Neal, S., & Fields, K. B. (2010). Peripheral nerve entrapment and injury in the upper extremity. American Family Physician Journa, 81(2), 147–155.
- Ni, X. J., Wang, X. D., Zhao, Y. H., Sun, H. L., Hu, Y. M., Yao, J., & Wang, Y. (2017). The effect of low-intensity ultrasound on brain-derived neurotropic factor expression in a rat sciatic nerve crushed injury model. Ultrasound in Medicine & Biology, 43(2), 461–468. doi:10.1016/j.ultrasmedbio.2016.09.017
- Nichols, C. M., Myckatyn, T. M., Rickman, S. R., Fox, I. K., Hadlock, T., & Mackinnon, S. E. (2005). Choosing the correct functional assay: A comprehensive assessment of functional tests in the rat. Behavioural Brain Research, 163(2), 143–158. doi:10.1016/j.bbr.2005.05.003
- Nijhuis, T. H., Bodar, C. W., van Neck, J. W., Walbeehm, E. T., Siemionow, M., Madajka, M., … Hovius, S. E. (2013). Natural conduits for bridging a 15-mm nerve defect: comparison of the vein supported by muscle and bone marrow stromal cells with a nerve autograft. J Plast Reconstr Aesthet Surg, 66(2), 251–259. doi:10.1016/j.bjps.2012.09.011.
- Nishimoto, K., Kumai, Y., Minoda, R., & Yumoto, E. (2012). Nimodipine accelerates reinnervation of denervated rat thyroarytenoid muscle following nerve-muscle pedicle implantation. Laryngoscope, 122(3), 606–613. doi:10.1002/lary.22487.
- Oliveira, J. T., Lopes, F. R. P., de Almeida, F. M., & Martinez, A. M. B. (2014). Gene therapy in rodents models of traumatic peripheral nerve injury. J Cell Sci Ther, 5(1).
- Orbay, H., Uysal, A. C., Hyakusoku, H., & Mizuno, H. (2012). Differentiated and undifferentiated adipose-derived stem cells improve function in rats with peripheral nerve gaps. Journal of Plastic, Reconstructive & Aesthetic Surgery, 65(5), 657–664. doi:10.1016/j.bjps.2011.11.035
- Ozbek, Z., Aydin, H. E., Koçman, A. E., Ozkara, E., Sahin, E., Bektur, E., … Bayçu, C. (2016). Neuroprotective effect of genistein in peripheral nerve injury. Turkish Neurosurgery, 27(5).
- Ozturk, C. (2015). Peripheral nerve surgery models sciatic nerve crush injury model. In Plastic and reconstructive surgery (pp. 513–517). Springer.
- Ozturk, C., Uygur, S., & Lukaszuk, M. (2015). Sheep as a large animal model for nerve regeneration studies. In Plastic and Reconstructive Surgery (pp. 507–511). Springer.
- Ozturk, C., Uygur, S., & Siemionow, M. Z. (2015). Peripheral nerve surgery models crush injury and epineural patch. In Plastic and reconstructive surgery (pp. 519–523). Springer.
- Pang, C. J., Tong, L., Ji, L. L., Wang, Z. Y., Zhang, X., Gao, H., … Tong, X. J. (2013). Synergistic effects of ultrashort wave and bone marrow stromal cells on nerve regeneration with acellular nerve allografts. Synapse, 67(10), 637–647. doi:10.1002/syn.21669
- Patel, N., & Poo, M. M. (1982). Orientation of neurite growth by extracellular electric fields. Journal of Neuroscience, 2(4), 483–496.10.1523/JNEUROSCI.02-04-00483.1982
- Pavić, R., Pavić, M. L., Tvrdeić, A., Tot, O. K., & Heffer, M. (2011). Rat sciatic nerve crush injury and recovery tracked by plantar test and immunohistochemistry analysis. Collegium Antropologicum, 35(1), 93–100.
- Peng, S. W., Li, C. W., Chiu, I. M., & Wang, G. J. (2017). Nerve guidance conduit with a hybrid structure of a PLGA microfibrous bundle wrapped in a micro/nanostructured membrane. International Journal of Nanomedicine, 12, 421–432. doi:10.2147/IJN.S122017
- Pereira, J. A., Lebrun-Julien, F., & Suter, U. (2012). Molecular mechanisms regulating myelination in the peripheral nervous system. Trends in Neurosciences, 35(2), 123–134. doi:10.1016/j.tins.2011.11.006
- Petruska, J. C., & Mendell, L. M. (2004). The many functions of nerve growth factor: Multiple actions on nociceptors. Neuroscience Letters, 361(1–3), 168–171. doi:10.1016/j.neulet.2003.12.012
- Piezzi, R. S., & Cavicchia, J. C. (1981). Effects of cold and melatonin on the microtubules of the toad sciatic nerve. The Anatomical Record, 200(1), 115–120. doi:10.1002/ar.1092000111
- Pinheiro-Dardis, C. M., Erbereli, B. T., Gigo-Benato, D., Castro, P., & Russo, T. L. (2017). Electrical stimulation delays reinnervation in denervated rat muscle. Muscle and Nerve, 56(6), E108–E118. doi:10.1002/mus.25589
- Raimondo, S., Fornaro, M., Di Scipio, F., Ronchi, G., Giacobini-Robecchi, M. G., & Geuna, S. (2009). Chapter 5: Methods and protocols in peripheral nerve regeneration experimental research: Part II-morphological techniques. International Review of Neurobiology, 87, 81–103. doi:10.1016/S0074-7742(09)87005-0
- Rao, P., Kotwal, P. P., Farooque, M., & Dinda, A. (2001). Muscle autografts in nerve gaps. Pattern of regeneration and myelination in various lengths of graft: An experimental study in guinea pigs. Journal of Orthopaedic Science, 6(6), 527–534.10.1007/s007760100008
- Raso, V. V. M., Barbieri, C. H., Mazzer, N., & Fasan, V. S. (2005). Can therapeutic ultrasound influence the regeneration of peripheral nerves? Journal of Neuroscience Methods, 142(2), 185–192.10.1016/j.jneumeth.2004.08.016
- Reiter, R. J. (1998). Oxidative damage in the central nervous system: Protection by melatonin. Progress in Neurobiology, 56(3), 359–384.10.1016/S0301-0082(98)00052-5
- Rigoard, P., Buffenoir, K., Wager, M., Bauche, S., Giot, J.-P., Robert, R., & Lapierre, F. (2009). Organisation anatomique et physiologique du nerf périphérique. Neuro-Chirurgie, 55, S3–S12.10.1016/j.neuchi.2008.03.009
- Mason, M. R., Tannemaat, M. R., Malessy, M. J., & Verhaagen, J. (2011). Gene therapy for the peripheral nervous system: A strategy to repair the injured nerve? Current Gene Therapy, 11(2), 75–89.10.2174/156652311794940764
- Robinson, G. A., & Madison, R. D. (2009). Influence of terminal nerve branch size on motor neuron regeneration accuracy. Experimental Neurology, 215(2), 228–235. doi:10.1016/j.expneurol.2008.10.002
- Robinson, L. R. (2004). Traumatic injury to peripheral nerves. Supplements to Clinical Neurophysiology, 57, 173–186.10.1016/S1567-424X(09)70355-1
- Rochkind, S. (2009). Phototherapy in peripheral nerve regeneration: From basic science to clinical study. Neurosurgical Focus, 26(2), E8. doi:10.3171/FOC.2009.26.2.E8
- Rochkind, S. (2015). Clinical aspects of ballistic peripheral nerve injury: Shrapnel versus gunshot. World Neurosurgery, 84(2), 597.10.1016/j.wneu.2015.05.038
- Rochkind, S., & Nevo, Z. (2014). Recovery of peripheral nerve with massive loss defect by tissue engineered guiding regenerative gel. BioMed Research International, 2014.
- Rogerio, F., de Souza Queiroz, L., Teixeira, S. A., Oliveira, A. L., de Nucci, G., & Langone, F. (2002). Neuroprotective action of melatonin on neonatal rat motoneurons after sciatic nerve transection. Brain Research, 926(1–2), 33–41.10.1016/S0006-8993(01)03286-3
- Ronchi, G., Jager, S. B., Vaegter, C. B., Raimondo, S., Giacobini-Robecchi, M. G., & Geuna, S. (2014). Discrepancies in quantitative assessment of normal and regenerated peripheral nerve fibers between light and electron microscopy. Journal of the Peripheral Nervous System, 19(3), 224–233.10.1111/jns.2014.19.issue-3
- Ronchi, G., Nicolino, S., Raimondo, S., Tos, P., Battiston, B., Papalia, I., … Geuna, S. (2009). Functional and morphological assessment of a standardized crush injury of the rat median nerve. Journal of Neuroscience Methods, 179(1), 51–57. doi:10.1016/j.jneumeth.2009.01.011
- Rosa Junior, G. M., Magalhães, R. M. G., Rosa, V. C., Bueno, C. R. D. S., Simionato, L. H., & Bortoluci, C. H. F. (2016). Effect of the laser therapy in association with swimming for a morphological nerve repair and functional recovery in rats submitted to sciatic axonotmesis. Fisioterapia e Pesquisa, 23(1), 12–20.
- Rosen, B., Chemnitz, A., Weibull, A., Andersson, G., Dahlin, L. B., & Bjorkman, A. (2012). Cerebral changes after injury to the median nerve: A long-term follow up. Journal of Plastic Surgery and Hand Surgery, 46(2), 106–112. doi:10.3109/2000656X.2011.653257
- Rusovan, A., Kanje, M., & Mild, K. H. (1992). The stimulatory effect of magnetic fields on regeneration of the rat sciatic nerve is frequency dependent. Experimental Neurology, 117(1), 81–84.10.1016/0014-4886(92)90113-5
- Rydevik, B., & Lundborg, G. (1977). Permeability of intraneural microvessels and perineurium following acute, graded experimental nerve compression. Scandinavian Journal of Plastic and Reconstructive Surgery, 11(3), 179–187.10.3109/02844317709025516
- Saadat, S., Eslami, V., & Rahimi-Movaghar, V. (2011). The incidence of peripheral nerve injury in trauma patients in Iran. Ulusal travma ve acil cerrahi dergisi = Turkish journal of trauma & emergency surgery: TJTES, 17(6), 539–544. doi: 10.5505/tjtes.2011.75735.
- Safa, B., & Buncke, G. (2016). Autograft substitutes: Conduits and processed nerve allografts. Hand Clinics, 32(2), 127–140. doi:10.1016/j.hcl.2015.12.012
- Said, G., & Krarup, C. (2013). Microscopic anatomy: Normal structure. Peripheral Nerve Disorders, 115, 7.
- Saladin, K. (n.d.). Anatomy & physiology: The unity of form and function (2nd ed.). New York, NY: McGraw-Hill.
- Saliba, S., Saliba, E. N., Pugh, K. F., Chhabra, A., & Diduch, D. (2009). Rehabilitation considerations of a brachial plexus injury with complete avulsion of c5 and c6 nerve roots in a college football player: A case study. Sports Health, 1(5), 370–375.10.1177/1941738109343544
- Sariguney, Y., Yavuzer, R., Elmas, C., Yenicesu, I., Bolay, H., & Atabay, K. (2008). Effect of platelet-rich plasma on peripheral nerve regeneration. Journal of Reconstructive Microsurgery, 24(3), 159–167. doi:10.1055/s-2008-1076752
- Sarikcioglu, L., Yaba, A., Tanriover, G., Demirtop, A., Demir, N., & Ozkan, O. (2007). Effect of severe crush injury on axonal regeneration: A functional and ultrastructural study. Journal of Reconstructive Microsurgery, 23(3), 143–149. doi:10.1055/s-2007-974649
- Scatena, M., Eaton, K. V., Jackson, M. F., Lund, S. A., & Giachelli, C. M. (2017). Macrophages: The bad, the ugly, and the good in the inflammatory response to biomaterials. In The immune response to implanted materials and devices (pp. 37–62). Springer.10.1007/978-3-319-45433-7
- Schalow, G., Zäch, G., & Warzok, R. (1995). Classification of human peripheral nerve fibre groups by conduction velocity and nerve fibre diameter is preserved following spinal cord lesion. Journal of the Autonomic Nervous System, 52(2–3), 125–150.10.1016/0165-1838(94)00153-B
- Schmelzer, J. D., Zochodne, D. W., & Low, P. A. (1989). Ischemic and reperfusion injury of rat peripheral nerve. Proceedings of the National Academy of Sciences of the United States of America, 86(5), 1639–1642.10.1073/pnas.86.5.1639
- Seddighi, A., Nikouei, A., Seddighi, A. S., Zali, A. R., Tabatabaei, S. M., Sheykhi, A. R., … Naeimian, S. (2016). Peripheral nerve injury: A review article. International Clinical Neuroscience Journal, 3(1), 1–6.
- Seddon, H. (1943). Three types of nerve injury. Brain, 66(4), 237–288.10.1093/brain/66.4.237
- Senoglu, M., Nacitarhan, V., Kurutas, E. B., Senoglu, N., Altun, I., Atli, Y., & Ozbag, D. (2009). Intraperitoneal alpha-lipoic acid to prevent neural damage after crush injury to the rat sciatic nerve. Journal of Brachial Plexus and Peripheral Nerve Injury, 4(1), 22. doi:10.1186/1749-7221-4-22
- Sherman, D. L., & Brophy, P. J. (2005). Mechanisms of axon ensheathment and myelin growth. Nature Reviews Neuroscience, 6(9), 683–690. doi:10.1038/nrn1743
- Shokouhi, G., Hadidchi, S., Ghorbanihaghjo, A., Rahbani-Noubar, M., Panahi, S., Forouzanfar, M., … Mesgari, M. (2005). Neuroprotective effect of ascorbic acid in experimental blunt sciatic nerve injury in rats. The Internet Journal of Nutrition and Wellness, 1(2), 1–5.
- Siddique, R., Vyas, A., Thakor, N., & Brushart, T. M. (2014). A two-compartment organotypic model of mammalian peripheral nerve repair. Journal of Neuroscience Methods, 232, 84–92. doi:10.1016/j.jneumeth.2014.05.005
- Simon, N. G., Spinner, R. J., Kline, D. G., & Kliot, M. (2016). Advances in the neurological and neurosurgical management of peripheral nerve trauma. Journal of Neurology, Neurosurgery, and Psychiatry, 87(2), 198–208. doi:10.1136/jnnp-2014-310175
- Smith, K. J., & Hall, S. M. (1988). Peripheral demyelination and remyelination initiated by the calcium-selective ionophore ionomycin: In vivo observations. Journal of the Neurological Sciences, 83(1), 37–53.10.1016/0022-510X(88)90018-4
- Song, M. Y., Yu, J. Z., Zhao, D. M., Wei, S., Liu, Y., Hu, Y. M., … Wu, H. (2014). The time-dependent manner of sinusoidal electromagnetic fields on rat bone marrow mesenchymal stem cells proliferation, differentiation, and mineralization. Cell Biochemistry and Biophysics, 69(1), 47–54. doi:10.1007/s12013-013-9764-8
- Sta, M., Cappaert, N. L., Ramekers, D., Baas, F., & Wadman, W. J. (2014). The functional and morphological characteristics of sciatic nerve degeneration and regeneration after crush injury in rats. Journal of Neuroscience Methods, 222, 189–198. doi:10.1016/j.jneumeth.2013.11.012
- Stagg, N. J., Mata, H. P., Ibrahim, M. M., Henriksen, E. J., Porreca, F., Vanderah, T. W., & Malan, T. P. (2011). Regular exercise reverses sensory hypersensitivity in a rat neuropathic pain modelrole of endogenous opioids. Anesthesiology: The Journal of the American Society of Anesthesiologists, 114(4), 940–948.10.1097/ALN.0b013e318210f880
- Stassart, R. M., Fledrich, R., Velanac, V., Brinkmann, B. G., Schwab, M. H., Meijer, D., … Nave, K. A. (2013). A role for Schwann cell-derived neuregulin-1 in remyelination. Nat Neurosci, 16(1), 48–54. doi:10.1038/nn.3281
- Stifani, N. (2014). Motor neurons and the generation of spinal motor neuron diversity. Frontiers in Cellular Neuroscience, 8, 293. doi:10.3389/fncel.2014.00293
- Strasberg, S. R., Mackinnon, S. E., Genden, E. M., Bain, J. R., Purcell, C. M., Hunter, D. A., & Hay, J. B. (1996). Long-segment nerve allograft regeneration in the sheep model: Experimental study and review of the literature. Journal of Reconstructive Microsurgery, 12(08), 529–537.10.1055/s-2007-1006625
- Strauss, C., Romstock, J., Fahlbusch, R., Rampp, S., & Scheller, C. (2006). Preservation of facial nerve function after postoperative vasoactive treatment in vestibular schwannoma surgery. Neurosurgery, 59(3), 577–584. doi:10.1227/01.NEU.0000230260.95477.0A
- Subissi, A., Evangelista, S., & Giachetti, A. (1999). Preclinical profile of zofenopril: An angiotensin converting enzyme inhibitor with peculiar cardioprotective properties. Cardiovascular Therapeutics, 17(2), 115–133.
- Sufan, W., Suzuki, Y., Tanihara, M., Ohnishi, K., Suzuki, K., Endo, K., & Nishimura, Y. (2001). Sciatic nerve regeneration through alginate with tubulation or nontubulation repair in cat. Journal of Neurotrauma, 18(3), 329–338.10.1089/08977150151070991
- Sulaiman, W., & Gordon, T. (2013). Neurobiology of peripheral nerve injury, regeneration, and functional recovery: From bench top research to bedside application. The Ochsner Journal, 13(1), 100–108.
- Sun, L. Y., Hsieh, D. K., Yu, T. C., Chiu, H. T., Lu, S. F., Luo, G. H., … Chiou, T. W. (2009). Effect of pulsed electromagnetic field on the proliferation and differentiation potential of human bone marrow mesenchymal stem cells. Bioelectromagnetics, 30(4), 251–260. doi:10.1002/bem.20472
- Sunderland, S. (1951). A classification of peripheral nerve injuries producing loss of function. Brain, 74(4), 491–516.10.1093/brain/74.4.491
- Suslu, H., Altun, M., Erdivanli, B., & Turan, S. H. (2012). Comparison of the effects of local and systemic dexamethasone on the rat traumatic sciatic nerve model. Turkish Neurosurgery, 23(5), 623–629.
- Suzuki, K., Tanaka, H., Ebara, M., Uto, K., Matsuoka, H., Nishimoto, S., … Yoshikawa, H. (2017). Electrospun nanofiber sheets incorporating methylcobalamin promote nerve regeneration and functional recovery in a rat sciatic nerve crush injury model. Acta Biomaterialia, 53, 250–259. doi:10.1016/j.actbio.2017.02.004
- Svennigsen, Å. F., & Dahlin, L. B. (2013). Repair of the peripheral nerve – Remyelination that works. Brain Sciences, 3(3), 1182–1197.10.3390/brainsci3031182
- Svenningsen, Å. F., & Kanje, M. (1998). Regulation of Schwann cell proliferation in cultured segments of the adult rat sciatic nerve. Journal of Neuroscience Research, 52(5), 530–537.10.1002/(ISSN)1097-4547
- Taylor, K. S., Anastakis, D. J., & Davis, K. D. (2009). Cutting your nerve changes your brain. Brain, 132(Pt 11), 3122–3133. doi:10.1093/brain/awp231
- Teodori, R. M., Betini, J., de Oliveira, L. S., Sobral, L. L., Takeda, S. Y., & de Lima Montebelo, M. I. (2011). Swimming exercise in the acute or late phase after sciatic nerve crush accelerates nerve regeneration. Neural Plasticity, 2011, 783901. doi:10.1155/2011/783901
- Thalhammer, J. G., Vladimirova, M., Bershadsky, B., & Strichartz, G. R. (1995). Neurologic evaluation of the rat during sciatic nerve block with lidocaine. Anesthesiology, 82(4), 1013–1025.10.1097/00000542-199504000-00026
- Toros, T., Karabay, N., Ozaksar, K., Sugun, T. S., Kayalar, M., & Bal, E. (2009). Evaluation of peripheral nerves of the upper limb with ultrasonography: A comparison of ultrasonographic examination and the intra-operative findings. The Journal of Bone and Joint Surgery, 91(6), 762–765. doi:10.1302/0301-620X.91B6.22284
- Tos, P., Ronchi, G., Nicolino, S., Audisio, C., Raimondo, S., Fornaro, M., … Geuna, S. (2008). Employment of the mouse median nerve model for the experimental assessment of peripheral nerve regeneration. Journal of Neuroscience Methods, 169(1), 119–127.10.1016/j.jneumeth.2007.11.030
- Tos, P., Ronchi, G., Papalia, I., Sallen, V., Legagneux, J., Geuna, S., & Giacobini-Robecchi, M. G. (2009). Chapter 4: Methods and protocols in peripheral nerve regeneration experimental research: Part I-experimental models. International Review of Neurobiology, 87, 47–79. doi:10.1016/S0074-7742(09)87004-9
- Touma, E., Kato, S., Fukui, K., & Koike, T. (2007). Calpain-mediated cleavage of collapsin response mediator protein(CRMP)-2 during neurite degeneration in mice. European Journal of Neuroscience, 26(12), 3368–3381. doi:10.1111/j.1460-9568.2007.05943.x
- Tsai, M. J., Pan, H. A., Liou, D. Y., Weng, C. F., Hoffer, B. J., & Cheng, H. (2010). Adenoviral gene transfer of bone morphogenetic protein-7 enhances functional recovery after sciatic nerve injury in rats. Gene Therapy, 17(10), 1214–1224. doi:10.1038/gt.2010.72
- Tseng, K. C., Li, H., Clark, A., Sundem, L., Zuscik, M., Noble, M., & Elfar, J. (2016). 4-Aminopyridine promotes functional recovery and remyelination in acute peripheral nerve injury. EMBO Molecular Medicine, 8(12), 1409–1420. doi:10.15252/emmm.201506035
- Uranus, S., Bretthauer, G., Nagele-Moser, D., Saliba, S., Tomasch, G., Rafolt, D., … Koch, H. (2013). New synthetic prosthesis for peripheral nerve injuries: An experimental pilot study. Surgical Innovation, 20(2), 171–175. doi:10.1177/1553350612458546
- Uzun, N., Tanriverdi, T., Savrun, F. K., Kiziltan, M. E., Sahin, R., Hanimoglu, H., & Hanci, M. (2006). Traumatic peripheral nerve injuries: Demographic and electrophysiologic findings of 802 patients from a developing country. Journal of Clinical Neuromuscular Disease, 7(3), 97–103. doi:10.1097/01.cnd.0000203641.38887.63
- van Veen, B. K., Schellens, R. L., Stegeman, D. F., Schoonhoven, R., & Gabreëls-Festen, A. A. (1995). Conduction velocity distributions compared to fiber size distributions in normal human sural nerve. Muscle & Nerve, 18(10), 1121–1127.10.1002/mus.v18:10
- Varejao, A. S., Cabrita, A. M., Meek, M. F., Bulas-Cruz, J., Melo-Pinto, P., Raimondo, S., … Giacobini-Robecchi, M. G. (2004). Functional and morphological assessment of a standardized rat sciatic nerve crush injury with a non-serrated clamp. Journal of Neurotrauma, 21(11), 1652–1670. doi:10.1089/neu.2004.21.1652
- Varejao, A. S., Melo-Pinto, P., Meek, M. F., Filipe, V. M., & Bulas-Cruz, J. (2004). Methods for the experimental functional assessment of rat sciatic nerve regeneration. Neurological Research, 26(2), 186–194. doi:10.1179/016164104225013833
- Varitimidis, S., Riano, F., Vardakas, D., & Sotereanos, D. (2000). Recurrent compressive neuropathy of the median nerve at the wrist: Treatment with autogenous saphenous vein wrapping. Journal of Hand Surgery, 25(3), 271–275.10.1054/jhsb.2000.0379
- Verge, V. M., Gratto, K. A., Karchewski, L. A., & Richardson, P. M. (1996). Neurotrophins and nerve injury in the adult. Philosophical Transactions of the Royal Society of London. Series B, 351(1338), 423–430. doi:10.1098/rstb.1996.0038
- Vleggeert-Lankamp, C. L. (2007, December). The role of evaluation methods in the assessment of peripheral nerve regeneration through synthetic conduits: A systematic review. Journal of Neurosurgery, 107(6), 1168–1189. doi:10.3171/JNS-07/12/1168
- Voria, I., Hauser, J., Axis, A., Schenker, M., Bichet, S., Kuntzer, T., … Barakat-Walter, I. (2006). Improved sciatic nerve regeneration by local thyroid hormone treatment in adult rat is accompanied by increased expression of SCG10. Experimental Neurology, 197(1), 258–267.10.1016/j.expneurol.2005.10.001
- Walsh, S., & Midha, R. (2009). Practical considerations concerning the use of stem cells for peripheral nerve repair. Neurosurgical Focus, 26(2), E2.10.3171/FOC.2009.26.2.E2
- Wan, L., Xia, R., & Ding, W. (2010). Short-term low-frequency electrical stimulation enhanced remyelination of injured peripheral nerves by inducing the promyelination effect of brain-derived neurotrophic factor on Schwann cell polarization. Journal of Neuroscience Research, 88(12), 2578–2587. doi:10.1002/jnr.22426
- Wang, H., Spinner, R. J., Sorenson, E. J., & Windebank, A. J. (2008). Measurement of forelimb function by digital video motion analysis in rat nerve transection models. Journal of the Peripheral Nervous System, 13(1), 92–102. doi:10.1111/j.1529-8027.2008.00162.x
- Weerasuriya, A. & Mizisin, A. P. (2011). The blood-nerve barrier: Structure and functional significance. Methods in Molecular Biology, 686, 149–173. doi:10.1007/978-1-60761-938-3_6
- Whitlock, E. L., Brenner, M. J., Fox, I. K., Moradzadeh, A., Hunter, D. A., & Mackinnon, S. E. (2010). Ropivacaine-induced peripheral nerve injection injury in the rodent model. Anesthesia & Analgesia, 111(1), 214–220. doi:10.1213/ANE.0b013e3181de574e
- Widerberg, A., Bergman, S., Danielsen, N., Lundborg, G., & Dahlin, L. B. (1997). Nerve injury induced by vibration: Prevention of the effect of a conditioning lesion by D600, a Ca2+ channel blocker. Occupational and Environmental Medicine, 54(5), 312–315.10.1136/oem.54.5.312
- Willand, M. P., Nguyen, M. A., Borschel, G. H., & Gordon, T. (2016). Electrical stimulation to promote peripheral nerve regeneration. Neurorehabilitation and Neural Repair, 30(5), 490–496. doi:10.1177/1545968315604399
- Wojtkiewicz, D. M., Saunders, J., Domeshek, L., Novak, C. B., Kaskutas, V., & Mackinnon, S. E. (2015). Social impact of peripheral nerve injuries. Hand (N Y), 10(2), 161–167. doi:10.1007/s11552-014-9692-0
- Wong, K.-H., Kanagasabapathy, G., Bakar, R., Phan, C.-W., & Sabaratnam, V. (2015). Restoration of sensory dysfunction following peripheral nerve injury by the polysaccharide from culinary and medicinal mushroom, Hericium erinaceus (Bull.: Fr.) Pers. through its neuroregenerative action. Food Science and Technology (Campinas), 35(4), 712–721.10.1590/1678-457X.6838
- Xu, J., Varitimidis, S. E., Fisher, K. J., Tomaino, M. M., & Sotereanos, D. G. (2000). The effect of wrapping scarred nerves with autogenous vein graft to treat recurrent chronic nerve compression. Journal of Hand Surgery, 25(1), 93–103. doi:10.1053/jhsu.2000.jhsu025a0093
- Xue, C., Hu, N., Gu, Y., Yang, Y., Liu, Y., Liu, J., … Gu, X. (2012). Joint use of a chitosan/PLGA scaffold and MSCs to bridge an extra large gap in dog sciatic nerve. Neurorehabilitation and Neural Repair, 26(1), 96–106.10.1177/1545968311420444
- Yi, J.-S., Lee, H.-J., Lee, H.-J., Lee, I.-W., & Yang, J.-H. (2013). Rat peripheral nerve regeneration using nerve guidance channel by porcine small intestinal submucosa. Journal of Korean Neurosurgical Society, 53(2), 65–71.10.3340/jkns.2013.53.2.65
- Yu, X., & Bellamkonda, R. V. (2003). Tissue-engineered scaffolds are effective alternatives to autografts for bridging peripheral nerve gaps. Tissue Eng, 9(3), 421–430. doi:10.1089/107632703322066606
- Yuan, Y., Shen, H., Yao, J., Hu, N., Ding, F., & Gu, X. (2010). The protective effects of achyranthes bidentata polypeptides in an experimental model of mouse sciatic nerve crush injury. Brain Research Bulletin, 81(1), 25–32. doi:10.1016/j.brainresbull.2009.07.013
- Yuce, S., Cemal Gokce, E., Iskdemir, A., Koc, E. R., Cemil, D. B., Gokce, A., & Sargon, M. F. (2015). An experimental comparison of the effects of propolis, curcumin, and methylprednisolone on crush injuries of the sciatic nerve. Annals of Plastic Surgery, 74(6), 684–692. doi:10.1097/SAP.0000000000000026
- Zeidenberg, J., Burks, S. S., Jose, J., Subhawong, T. K., & Levi, A. D. (2015). The utility of ultrasound in the assessment of traumatic peripheral nerve lesions: Report of 4 cases. Neurosurgical Focus, 39(3), E3. doi:10.3171/2015.6.FOCUS15214
- Zhang, C. & Tu, L. (2005). Nerve growth factor and its receptor and hypoglossal nerve injury. Kouqiang Yixue Yanjiu, 21, 470–471.
- Zhang, Q., Nguyen, P., Xu, Q., Park, W., Lee, S., Furuhashi, A., & Le, A. D. (2017). Neural progenitor-like cells induced from human gingiva-derived mesenchymal stem cells regulate myelination of schwann cells in rat sciatic nerve regeneration. Stem Cells Translational Medicine, 6(2), 458–470. doi:10.5966/sctm.2016-0177
- Zhang, X., Xin, N., Tong, L., & Tong, X. J. (2013). Electrical stimulation enhances peripheral nerve regeneration after crush injury in rats. Molecular Medicine Reports, 7(5), 1523–1527. doi:10.3892/mmr.2013.1395
- Zhao, L., & Zheng, Y. (2010). Correlation and toxicological significance between myelin protein zero and peripheral nerve disease. Wei sheng yan jiu/Journal of hygiene research, 39(5), 635–638.
- Zhao, Z., Wang, Y., Peng, J., Ren, Z., Zhang, L., Guo, Q., … Lu, S. (2014). Improvement in nerve regeneration through a decellularized nerve graft by supplementation with bone marrow stromal cells in fibrin. Cell Transplantation, 23(1), 97–110.10.3727/096368912X658845
- Zheng, J., Sun, J., Lu, X., Zhao, P., Li, K., & Li, L. (2016). BDNF promotes the axonal regrowth after sciatic nerve crush through intrinsic neuronal capability upregulation and distal portion protection. Neuroscience Letters, 621, 1–8.10.1016/j.neulet.2016.04.006
- Zheng, X.-S., Ying, T.-T., Yuan, Y., & Li, S.-T. (2015). Nimodipine-mediated re-myelination after facial nerve crush injury in rats. Journal of Clinical Neuroscience, 22(10), 1661–1668.
- Zhu, J., Liu, F., Li, D., Shao, J., & Hu, B. (2011). Preliminary study of the types of traumatic peripheral nerve injuries by ultrasound. European Radiology, 21(5), 1097–1101. doi:10.1007/s00330-010-1992-3
- Zimmerman, B. J. & Granger, D. N. (1994). Mechanisms of reperfusion injury. The American Journal of the Medical Sciences, 307(4), 284–292.10.1097/00000441-199404000-00009
- Zochodne, D. W., & Levy, D. (2005). Nitric oxide in damage, disease and repair of the peripheral nervous system. Cellular and Molecular Biology (Noisy-le-grand), 51(3), 255–267.
- Zuijdendorp, H. M., Smit, X., Blok, J. H., Caruelle, J. P., Barritault, D., Hovius, S. E., & van Neck, J. W. (2008). Significant reduction in neural adhesions after administration of the regenerating agent OTR4120, a synthetic glycosaminoglycan mimetic, after peripheral nerve injury in rats. Journal of Neurosurgery, 109(5), 967–973. doi:10.3171/JNS/2008/109/11/0967