Abstract
Objectives: Chromogranins (Cg) and secretogranins (Sg) are expressed by endocrine cells and may be important for the pathophysiology of ulcerative colitis (UC). We investigated dynamics of faecal granin expression in patients with UC during a period of 18 months, both during remission and relapse, and association to disease outcome the following 3 years.
Materials and methods: Secretoneurin (SN), Sg3, CgA and CgB were measured in three to seven serial faecal samples from UC patients who did not (n = 20) or did (n = 20) relapse during study time. All patients were in remission at baseline and disease characteristic were monitored during sampling and 3 years after the final sample.
Results: Faecal SN, Sg3, CgA and CgB levels showed no association to patient characteristic or disease history at baseline. Faecal granin levels showed low intra-patient variability and levels stayed constant during short and long intervals at remission, did not alter during or after clinical relapse and were not associated to medical therapy. In contrast, high inter-patient variability was detected and multivariate analysis revealed three distinct patient groups, where extensive disease was more common in patients with high levels of SN and CgA as compared to patients with low levels of all granins or patients with high levels of Sg3 and CgB. These patient subgroups did, however, not differ in patient characteristics, disease history or future disease course.
Conclusions: Faecal granin levels are stable over time but are unrelated to disease history, activity and outcome and are thus not valuable markers for disease activity in UC patients.
PUBLIC INTEREST STATEMENT
People with ulcerative colitis suffer from chronic inflammation of the large intestine and rectum. The disease is lifelong and alternates between periods of active disease (flare) and remission with a large individual variation in flare frequency and severity. To improve quality of life, patients and clinicians would benefit from knowing how disease outcome will develop. The purpose of this study was to explore if levels of granins in faecal samples from patients with ulcerative colitis could be used as biomarkers of disease severity. Granins are glycoproteins secreted by endocrine cells in the gut and we measured faecal levels of four granins in samples collected over time in patients who did or did not experience a flare. Results showed that granin levels are stable over time but are unrelated to disease history, activity and outcome and are thus not valuable as disease activity markers in patients with ulcerative colitis.
Competing Interest
No conflicts of interests were declared.
1. Introduction
The family of granins is composed of acidic, secretory glycoproteins that are mainly expressed by endocrine cells such as the enteroendocrine cells (EEC) of the gut. Members of the family include e.g. chromogranin A (CgA) and B (CgB) and secretogranin 2 (Sg2) and 3 (Sg3) (Bartolomucci et al., Citation2011). The granins have broad effects and function in granule biosynthesis and sorting/secretion of other proteins as well as being precursors of biologically active peptides involved in pathways including pain, inflammation, blood pressure, glucose balance and emotional behaviour (Bartolomucci et al., Citation2011). Regarding regulation of the immune system, it has been shown that the Sg2-derived peptide secretoneurin (SN) and the CgA-derived peptide catestatin are chemotactic towards monocytes (Egger et al., Citation2008; Reinisch et al., Citation1993), and that peptides derived from CgA, CgB and Sg2 have antimicrobial effects (Shooshtarizadeh et al., Citation2010). As a biomarker, circulating CgA is used for diagnosis and disease monitoring of neuroendocrine tumours (Baudin et al., Citation2001; Rossi, Garcia-Hernandez, & Meyer et al., Citation2015; Welin et al., Citation2009).
Ulcerative colitis (UC) is a chronic inflammatory bowel disease (IBD) characterized by mucosal inflammation of the colon. The disease course in IBD, from mild to aggressive with rare to frequent relapses, differs between patients and the reason for this is currently unknown. Interactions between the immune system and the enteric nervous system have been suggested to be of importance for the pathophysiology of IBD (Gross & Pothoulakis, Citation2007; Villanacci et al., Citation2008) and approximately 1% of the cells lining the intestinal lumen are EECs (Schonhoff, Giel-Moloney, & Leiter, Citation2004). It has previously been shown that serum and faecal CgA levels are increased in IBD patients compared to healthy controls (Sciola et al., Citation2009; Strid et al., Citation2013; Wagner et al., Citation2013; Zissimopoulos et al., Citation2014) and that serum CgA may be used as a marker for disease activity in IBD patients receiving biologic therapy (Zissimopoulos et al., Citation2014). Conflicting data have been reported for CgB and SN where Wagner et al. showed similar levels of faecal CgB and lower levels of SN for IBD patients with active disease as compared to healthy subjects (Wagner et al., Citation2013) while our group recently reported higher levels of faecal CgB, SN and Sg3 for UC patients as compared to healthy subjects (Strid et al., Citation2013). It has also been shown that faecal granin levels increase over time the first year after UC diagnosis and that levels are increased in patients treated with thiopurines (Strid et al., Citation2013). Recent data, using CgA-/- mice with colitis, have shown that CgA regulates classical macrophage activation, chemotaxis and apoptosis (Eissa et al., Citation2018). In addition, in patients with UC, CgA correlated positively with classical macrophage markers and negatively with alternatively activated macrophages (Eissa et al., Citation2018) indicating a role for CgA during early innate immune events and gut tolerance.
Due to the inconclusive data of granins as possible biomarkers of disease activity in patients with UC, we performed a longitudinal study to evaluate the usefulness of faecal SN, Sg3, CgA and CgB in prediction of disease outcome. We hypothesized that high granin expression would be related to a more severe disease profile. Therefore, the aim of this study was to study the dynamics in SN, Sg3, CgA and CgB levels in stool samples from UC patients obtained over a period of up to 18 months and correlate these data with disease history from the time of diagnosis and disease outcome the coming 3 years.
2. Materials and methods
2.1. Study population
This study included a subset of UC patients (n = 40) initially recruited into a 5-ASA intervention study performed on 91 UC patients from August 2009 to December 2012 (Lasson et al., Citation2015). In the initial study, UC patients in remission, with at least one relapse the previous year, were included. All patients were >18 years of age, were on maintenance treatment with oral 5ASA (Asacol, Pentasa or Colazid) and were recruited from five gastroenterology units in Western Sweden. Exclusion criteria were ongoing anti-TNF, corticosteroid or non-steroidal anti-inflammatory drug (NSAID) treatment, pregnancy, prior colon resection or comorbid diseases that could affect compliance of the study protocol. At inclusion, patient demographic data and disease characteristics were collected and disease activity was evaluated according to the Mayo score (Schroeder, Tremaine, & Ilstrup, Citation1987). Remission was defined as a Mayo score of ≤2, with no single variable >1. Patients were asked to send stool samples by regular mail every month for 1.5 years and to contact their outpatient clinic if bowel symptoms occurred, to confirm a possible relapse. Faecal samples were thus collected plus/minus a maximum of 2 weeks from the start of the flare. Stool samples arrived within 24 hours to the clinic and were immediately frozen.
For the present study, patients were included from the original cohort who did or did not present with a clinical relapse during the study period. Selection criteria for patients with a relapse included clinically diagnosed relapse with one to three stool samples before the relapse (1–6 months prior), one stool sample at the relapse and one to three stool samples after the relapse (2–12 months after start of the relapse) and 20 patients met these criteria from the original cohort. After this, the 20 first enrolled patients who did not relapse during the study period, and met the selection criteria, were included. Selection criteria for patients without a relapse were that there should be three stool samples during three consecutive months (n = 10) or four to six stool samples during a period of 12 months. No matching concerning age, sex, disease extent or disease duration was performed. After the last stool sample, disease characteristics (numbers of relapses and changes in treatment) were monitored for an additional 3 years.
2.2. Faecal sample analyses
Stool samples were analysed for calprotectin, SN, Sg3, CgA and CgB. Proteins were extracted using 1:50 volumes (v/w) of faeces and extraction buffer from the Buhlmann Calprotectin ELISA Kit (Buhlmann Laboratories, Shönenbuch, Switzerland). Calprotectin was analysed with the Buhlmann Calprotectin ELISA Kit according to the manufacturer’s instructions. SN and Sg3 were measured with in-house radioimmunoassays (RIA), as previously described (Stridsberg, Eriksson, & Janson, Citation2008). CgA and CgB were measured using commercial RIA (Eurodiagnostica, Malmö, Sweden), according to the manufacturer’s instructions. All standards and samples were analysed in duplicates. For all analyses, the % CV for intra- and inter-assay variability was <7.3%. No reference values for healthy subjects for faecal SN, Sg3, CgA and CgB exist, but values have been reported in other studies (Strid et al., Citation2013; Wagner et al., Citation2013).
2.3. Statistical and data analyses
The Mann-Whitney U test was used to evaluate differences between two groups and Spearman’s rank correlation coefficient was used to test the association between two variables. For testing of differences between related samples, Wilcoxon signed rank test (two groups) and Friedman’s test with Dunn’s multiple comparison test (several groups) were used. The Chi-square test was used for testing of independence between two categorical variables. All statistical analyses were performed using IBM SPSS Statistics 23; p-values <0.05 were considered as statistically significant. Data are shown as median (range). Non-parametric analyses were used since the data were not normally distributed.
Principal component analysis (PCA) and unsupervised hierarchical clustering analysis (HCA) using SN, Sg3, CgA and CgB as X-variables were performed using SIMCA 14.1 (MKS Umetrics, Umeå, Sweden). Granin expression levels used for the PCA were calculated as median of three to seven different stool sample measurements.
Power analysis to estimate size of patient cohorts was not included in the experimental design.
2.4. Ethical considerations
All patients provided written informed consent, according to the Declaration of Helsinki. The study was approved by the Regional Ethical Review Board at the University of Gothenburg.
3. Results
3.1. Faecal granins and patient demographics
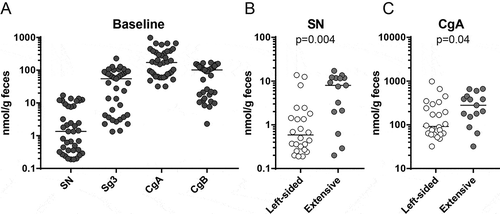
UC patients in remission on a stable dose of 5-ASA who experienced a relapse during the sample collection time of 18 months were included into the study (n = 20). In addition, UC patients in remission on a stable dose of 5-ASA who did not experience a relapse during the sample collection time were included (n = 20). There were no demographic differences between the patient groups (relapse vs. remission) at baseline (Table ). Faecal samples were collected monthly and analysed for calprotectin, SN, Sg3, CgA and CgB. All patients had calprotectin values <250 μg/g faeces at baseline except one patient who presented with a value of 492 μg/g despite having Mayo 0. Calprotectin values for this patient stayed >400 μg/g at remission and increased to >700 μg/g during a flare. Granin values were measured and ranged between 0.1 and 1000 nmol/g faeces with CgA being the most abundant (Figure ). There were no differences for levels of SN, Sg3, CgA or CgB based on sex, smoking or AZA treatment at inclusion and no correlations to age, disease duration, Mayo score, months in remission or dose of 5-ASA at inclusion (data not shown). Levels of Sg3 and CgB were similar in patients with left-sided colitis versus extensive colitis (20.8 nmol/g [1.4–227.9] vs. 63.5 nmol/g [1.3–128.2], p = 0.66 and 47.6 nmol/g [2.3–170.1] vs. 112.6 nmol/g [7.6–164.8], p = 0.09, respectively) while levels of SN and CgA were higher in patients with extensive colitis compared to left-sided colitis (Figure and ).
Table 1. Demographics at baseline for patients with UC who stayed in remission (n = 20) or relapsed (n = 20) during the sample collection time
Figure 1. Faecal granin levels at baseline and relation to disease extent. Faecal samples from UC patients were obtained at baseline and analysed for secretogranins and chromogranins using RIA. (A) Levels of SN, Sg3, CgA and CgB for UC patients in remission (n = 40). (B) Comparison of levels of SN (left) and CgA (right) in UC patients with left-sided colitis (n = 25) and extensive colitis (n = 15). Each symbol represents one individual and horizontal lines indicate median of the group.
3.2. Faecal granin levels over time during remission
Next, we analysed faecal granin levels over time during a short interval (three samples, obtained at baseline and at 2 consecutive months, n = 10) and during a long interval (two samples, obtained at baseline and after 8–10 months, n = 10). During these periods, none of the patients experienced any relapses and 5ASA treatment was kept constant. Faecal levels of SN, Sg3, CgA and CgB persist over time during both short interval (Figure ) and long interval (Figure ). Calprotectin levels were <250 for all samples at all time points (data not shown).
Figure 2. Faecal granin levels stay constant over time during remission. Faecal samples from UC patients were obtained at different time points and analysed for secretogranins and chromogranins using RIA. Levels of SN, Sg3, CgA and CgB for UC patients in remission were compared at three time points during a short interval (A) and at two time points during a long interval (B). Each symbol represents one individual (A: n = 10, B: n = 10).
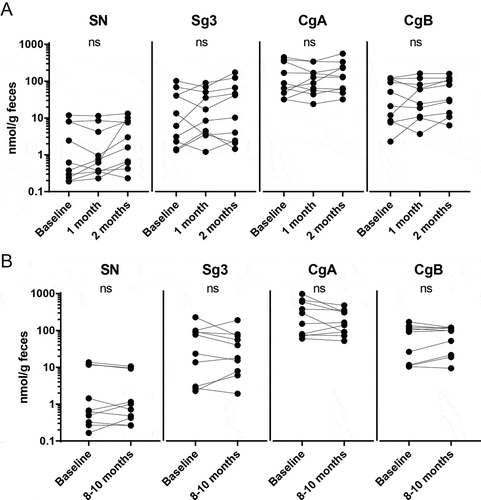
For patients in the long interval group, faecal sampling was also performed between baseline and 8–10 months resulting in a total of four to six samples per patient. There was a fluctuation of granin levels over time, but no statistically confirmed decrease or increase could be detected for any of the granins during the sampling period (Supplementary Figure 1).
3.3. Faecal granin levels over time during inflammation
To evaluate whether faecal granin levels were affected by inflammation or altered 5-ASA treatment, samples were obtained 1–3 months before a clinically defined relapse, at the relapse and 2–12 months after the relapse when symptoms no longer were present (n = 20). At the relapse, appropriate treatment, in accordance to conventional practice (Dignass et al., Citation2012), was prescribed. All patients received high-dose 5ASA treatment (oral n = 8, topical n = 1, both n = 11), nine received corticosteroid treatment (oral n = 3, topical n = 5, both n = 2) and one patient received thiopurines.
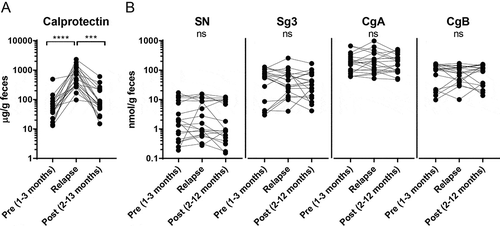
Faecal calprotectin levels were increased at the relapse (Figure ) while no alterations could be detected for any of the granins before, during or after the relapse (Figure ). Granin levels did not show any correlation to calprotectin levels at inflammation (data not shown). Further, no differences in faecal granin levels could be detected after relapse in patients who were (n = 10) or were not (n = 10) taking corticosteroids in addition to 5-ASA during the relapse (data not shown).
Figure 3. Faecal granin levels stay constant over time before, during and after a relapse. Faecal samples from UC patients were obtained at different time points and analysed for calprotectin, secretogranins and chromogranins using ELISA and RIA. Levels of calprotectin (A) and SN, Sg3, CgA and CgB (B) for UC patients 1–3 months before, during and 2–12 months after a relapse. Each symbol represents one individual (n = 20). ****p-value < 0.0001 and ***p-value < 0.001.
Faecal sampling was also performed one to three times before a relapse (1–6 months prior) and one to three times after a relapse (2–12 months after start of the flare), yielding a total of four to seven samples per patient. Again, there was a fluctuation of granin levels over time, but no statistically verified decrease or increase could be detected for any of the granins preceding or succeeding the relapse (Supplementary Figure 2). However, the fluctuation in granin levels in patients experiencing a relapse was greater as compared to patients without a relapse (compare data spread in Supplementary Figures 1 and 2), which may be due to differences in stool consistency at the actual relapse.
3.4. Covariation of granin expression defines three different groups
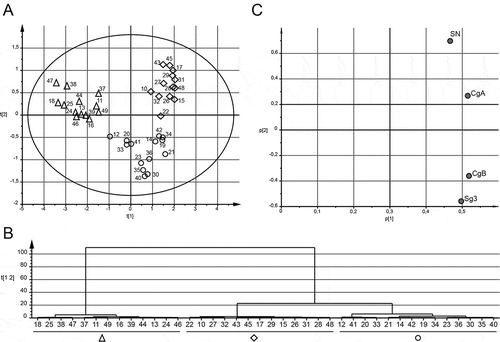
Since the expression pattern of the granins showed uneven distributions with possible subgroups (Figure ), correlation analyses were performed to study co-expression. Data showed that all granins correlated strongly to each other with highest correlation coefficients for SN versus CgA and Sg3 versus CgB (Table ). To further evaluate this covariation, we performed a multivariate analysis using all four granins based on median values from the three to seven samples analysed for each patient. A PCA together with an HCA were performed for unsupervised generation of groups based on multiple parameters, resulting in three distinct groups (Figure and , shapes in A were defined by the result of the HCA in ). As defined by the loading scatter plot (Figure ), the cluster to the left (Figure , triangles, n = 13) had low levels of all granins, the cluster at top right (Figure , diamonds, n = 13) had high levels of SN and CgA and the cluster at low right (Figure , circles, n = 14) had high levels of both Sg3 and CgB. The groups were termed LOW (triangles), SN/CgA (diamonds) and Sg3/CgB (circles). There were no differences in age, sex, disease duration, smoking, months in remission, dose of 5ASA or AZA treatment at inclusion between the three groups (data not shown). However, extensive disease was more common in patients with SN/CgA (10 of 13) as compared to LOW (3 of 13) and Sg3/CgB (2 of 14), p = 0.002. These data together indicate that SN and CgA covariate and are found in a higher level in patients with extensive disease.
Table 2. Correlations between SN, Sg3, CgA and CgB levels in faecal samples obtained at baseline1
Figure 4. UC patients cluster into three different groups dependent on faecal granin levels. Median levels of faecal SN, Sg3, CgA and CgB from three to seven samples were used for principle component analysis (PCA) and hierarchical clustering analysis (HCA). Score scatter plot (A), HCA (B) and loading scatter plot (C) for the PCA are shown. The shapes from the groups defined by the HCA are highlighted in (A). Each symbol in A represents one individual. Patients are numbered 10–49.
3.5. Faecal granin levels and prediction of disease outcome
In order to analyse if different levels of granins could predict disease outcome the coming 3 years, the three groups defined in Figure were utilized. Presence of IBD symptoms, numbers of relapses, need for immunomodulators or biologics or colectomy the coming 3 years were defined for patients being LOW, SN/CgA and Sg3/CgB but no differences could be detected between the groups (Table ). Taken together, this shows that faecal granins cannot be used for prediction of disease outcome.
Table 3. Relation between faecal granin expression and disease outcome
4. Discussion
We have characterized the dynamics of faecal granin levels in patients with UC during a period of up to 18 months, both during remission and during a relapse. Even though large individual differences in faecal granin levels were observed between patients with long-standing UC, faecal granin levels were not related to disease history and could not be used for prediction of disease outcome.
There is a great need for biomarkers that can predict disease outcome in UC, and granins as messenger molecules within the nervous system are plausible markers of disease activity (past, present or future) due to the involvement of the brain-gut axis (Bonaz & Bernstein, Citation2013). The large differences in faecal granin levels between patients, 1 to >2 logs difference between patients with high and low levels, respectively, indicated that faecal granins might be useful as biomarkers. Also, in previous studies, bimodal distributions have been detected for faecal CgA, Sg2 and Sg3 levels for patients with UC (Sciola et al., Citation2009; Strid et al., Citation2013), while this was not apparent for non-inflamed study subjects or patients with irritable bowel syndrome (Ohman, Stridsberg, Isaksson, Jerlstad, & Simrén, Citation2012; Sciola et al., Citation2009).
To begin with, we evaluated if faecal granins were related to patient characteristics or disease parameters in the past or presence. The only link found was that patients with extensive disease had higher faecal levels of SN and CgA as compared to patients with left-sided colitis. A similar trend for serum CgA has been described previously (Sciola et al., Citation2009). Reasons may be that SN and CgA secreting cells in different parts of the intestine are affected or simply that a larger area of the colon is active, resulting in higher faecal concentrations for patients with an extensive disease. However, there is no clear use of faecal granins as markers for disease extent in UC.
Next, we analysed the dynamics of granin expression over time. An increase in faecal granin levels has been identified for newly diagnosed UC patients during the first year of disease (Strid et al., Citation2013) but a further increase during 3-year follow-up could not be shown. Instead, levels were surprisingly stable over time both at remission and during a relapse; however, it is important to note that sample size was quite small which may influence the data. In the present study, a fluctuation in granin levels, sometimes up to one log, was apparent on the individual level. These differences may be due to stool consistency, dietary intake or other causes further discussed below.
In order to evaluate granins as predictors of disease outcome, patients were clustered into three groups dependent on the pattern of granin expression. The reason for this was the apparent covariation of the granins with parameters that varied 1–2 logs in concentration. The PCA showed clear groups among the patients according to faecal granin levels. The HCA clustering of SN with CgA and Sg3 and CgB in the HCA was in agreement with the correlation analyses where these presented with the highest correlation coefficients. However, this pattern was not able to predict the disease course of UC the coming 3 years.
What is then related to differences in granin expression? Multiple factors influence production and release of granins, and elevated serum SN levels have been detected in patients with acute heart failure (Ottesen et al., Citation2015) and elevated serum CgA levels can be detected during various conditions including treatment with proton pump inhibitors (PPIs) (Pregun et al., Citation2011), treatment with serotonin-norepinephrine reuptake inhibitors (Karger et al., Citation2014), renal failure (Hsiao, Mezger, & O’Connor, Citation1990), heart failure (Ceconi et al., Citation2002), hyperthyroidism (Al-Shoumer & Vasanthy, Citation2009) and rheumatoid arthritis (Di Comite et al., Citation2009). Indeed, six patients in the study cohort were prescribed PPIs and had high granin levels (SN/CgA n = 3 and Sg3/CgB n = 3), but whether they were taking the prescribed medicine throughout the study is unknown. However, PPIs are also sold over the counter and since patients were not asked if they were taking PPIs or not, no conclusions can be made. It has been proposed that corticosteroids increase CgA expression by binding to the glucocorticoid response element of the CgA promoter (Rozansky, Wu, Tang, Parmer, & O’Connor, Citation1994), but no link between CgA expression and corticosteroids could be shown. As mentioned previously, serum CgA levels are increased in patients with neuroendocrine tumours and high serum CgA levels seem to be increased in some IBD patients with gastrointestinal carcinoids, but no real conclusions can be made concerning the link between CgA and cancer (Sciola et al., Citation2009).
Limitations of this study include the relatively low number of patients, in particular for the dynamics over time, and that samples were not obtained exactly at the same time intervals for all participants. The study design relied on the willingness of the patients to provide faecal samples every month and this was not fully achieved. Also, enough sample material had to be provided in order to perform the assays. Fortunately, up to seven serial samples could be obtained which gave a possibility to study the dynamics of faecal granins. Longer intervals would have been interesting but was not a part of this study setup. The handling of the samples is also a limitation with up to 24 hour in room temperature before being frozen. However, granins have high stability and loss via degradation should be minor (M. Stridsberg, personal communication). Other limitations are that we do not know when during the day the samples were taken, and intake of a meal could alter granin levels, and that gastritis or infections cannot be ruled out. In addition, watery stools during active disease could influence granin concentrations, but comparison of faecal granin levels in patients with irritable bowel syndrome with either constipation or diarrhoea is comparable (Ohman et al., Citation2012), indicating that watery stools may have minor effects on granin levels. Concerning biologic treatment, only 3 out of 40 patients started anti-TNF treatment the coming 3 years making the relation between biologic therapy and granins uncertain. Likewise, few patients underwent colectomy making it difficult to draw any conclusions. Furthermore, patients on anti-TNF at baseline were excluded from entering the study making a comparison between patients with or without anti-TNF treatment impossible.
In conclusion, we have shown that faecal granin levels are stable over time in patients with UC, irrespective of disease activity, and that faecal granin levels are unrelated to UC disease history or disease outcome the coming 3 years. Since granins are needed for granule biogenesis and secretion of other proteins, such as hormones, growth factors or enzymes, maybe the pattern seen here is linked to other factors, yet to be identified.
Author contribution
MKM, LÖ and AL contributed to the conception and design of the study. MS and SI performed the experiments. AL and HS recruited and enrolled patients in the study. MKM and LÖ contributed to the analysis and interpretation of data. MKM and LÖ wrote the manuscript and AL, MS, SI and HS critically reviewed it and approved the final draft.
Supplemental Material
Download ()Supplementary material
Supplemental material for this article can be accessed here.
Additional information
Funding
Notes on contributors
Maria K. Magnusson
Our translational research group, with clinical and preclinical expertise, has a long-standing interest in the immunopathology of inflammatory bowel diseases, aiming for improved understanding of the natural disease course and mechanisms involved in the fluctuation between active and quiescent disease. Especially, the work of the project group includes the identification of local and systemic biomarkers potentially predicting disease course and therapy response.
References
- Al-Shoumer, K. A., & Vasanthy, B. A. (2009). Serum chromogranin A concentration in hyperthyroidism before and after medical treatment. The Journal of Clinical Endocrinology and Metabolism, 94(7), 2321–2324. doi:10.1210/jc.2008-2231
- Bartolomucci, A., Possenti, R., Mahata, S. K., Fischer-Colbrie, R., Loh, Y. P., & Salton, S. R. J. (2011). The extended granin family: Structure, function, and biomedical implications. Endocrine Reviews, 32(6), 755–797. doi:10.1210/er.2010-0027
- Baudin, E., Bidart, J. M., Bachelot, A., Ducreux, M., Elias, D., Ruffié, P., & Schlumberger, M. (2001). Impact of chromogranin A measurement in the work-up of neuroendocrine tumors. Annals of Oncology, 12(Suppl 2), S79–S82.
- Bonaz, B. L., & Bernstein, C. N. (2013). Brain-gut interactions in inflammatory bowel disease. Gastroenterology, 144(1), 36–49. doi:10.1053/j.gastro.2012.10.003
- Ceconi, C., Ferrari, R., Bachetti, T., Opasich, C., Volterrani, M., Colombo, B., … Corti, A. (2002). Chromogranin A in heart failure; a novel neurohumoral factor and a predictor for mortality. European Heart Journal, 23(12), 967–974. doi:10.1053/euhj.2001.2977
- Di Comite, G., Rossi, C. M., Marinosci, A., Lolmede, K., Baldissera, E., Aiello, P., … Manfredi, A. A. (2009). Circulating chromogranin A reveals extra-articular involvement in patients with rheumatoid arthritis and curbs TNF-alpha-elicited endothelial activation. Journal of Leukocyte Biology, 85(1), 81–87. doi:10.1189/jlb.0608358
- Dignass, A., Lindsay, J. O., Sturm, A., Windsor, A., Colombel, J.-F., Allez, M., … Van Assche, G. (2012). Second European evidence-based consensus on the diagnosis and management of ulcerative colitis part 2: Current management. Journal of Crohn’s & Colitis, 6(10), 991–1030. doi:10.1016/j.crohns.2012.09.002
- Egger, M., Beer, A. G., Theurl, M., Schgoer, W., Hotter, B., Tatarczyk, T., … Kirchmair, R. (2008). Monocyte migration: A novel effect and signaling pathways of catestatin. European Journal of Pharmacology, 598(1–3), 104–111. doi:10.1016/j.ejphar.2008.09.016
- Eissa, N., Hussein, H., Kermarrec, L., Ali, A. Y., Marshall, A., Metz-Boutigue, M.-H., … Ghia, J.-E. (2018). Chromogranin-A regulates macrophage function and the apoptotic pathway in murine DSS colitis. Journal of Molecular Medicine (Berlin, Germany), 96(2), 183–198. doi:10.1007/s00109-017-1613-6
- Gross, K. J., & Pothoulakis, C. (2007). Role of neuropeptides in inflammatory bowel disease. Inflammatory Bowel Diseases, 13(7), 918–932. doi:10.1002/ibd.20129
- Hsiao, R. J., Mezger, M. S., & O’Connor, D. T. (1990). Chromogranin A in uremia: Progressive retention of immunoreactive fragments. Kidney International, 37(3), 955–964.
- Karger, S., Wiesner, T., Kersting, A., Braun, M., Ebert, T., Wurst, U., … Fasshauer, M. (2014). Increased chromogranin a and carcinoid syndrome-like symptoms in a patient treated with duloxetine. Endocrine Practice, 20(11), e215–e218. doi:10.4158/EP14162.CR
- Lasson, A., Öhman, L., Stotzer, P.-O., Isaksson, S., Überbacher, O., Ung, K.-A., & Strid, H. (2015). Pharmacological intervention based on fecal calprotectin levels in patients with ulcerative colitis at high risk of a relapse: A prospective, randomized, controlled study. United European Gastroenterology Journal, 3(1), 72–79. doi:10.1177/2050640614560785
- Ohman, L., Stridsberg, M., Isaksson, S., Jerlstad, P., & Simrén, M. (2012). Altered levels of fecal chromogranins and secretogranins in IBS: Relevance for pathophysiology and symptoms? The American Journal of Gastroenterology, 107(3), 440–447. doi:10.1038/ajg.2011.458
- Ottesen, A. H., Louch, W. E., Carlson, C. R., Landsverk, O. J. B., Kurola, J., Johansen, R. F., … Røsjø, H. (2015). Secretoneurin is a novel prognostic cardiovascular biomarker associated with cardiomyocyte calcium handling. Journal of the American College of Cardiology, 65(4), 339–351. doi:10.1016/j.jacc.2014.10.065
- Pregun, I., Herszényi, L., Juhász, M., Miheller, P., Hritz, I., Patócs, A., … Tulassay, Z. (2011). Effect of proton-pump inhibitor therapy on serum chromogranin a level. Digestion, 84(1), 22–28. doi:10.1159/000321535
- Reinisch, N., Kirchmair, R., Kähler, C. M., Hogue-Angeletti, R., Fischer-Colbrie, R., Winkler, H., & Wiedermann, C. J. (1993). Attraction of human monocytes by the neuropeptide secretoneurin. FEBS Letters, 334(1), 41–44.
- Rossi, R. E., Garcia-Hernandez, J., Meyer, T., Thirlwell, C., Watkins, J., Martin, N. G., … Toumpanakis, C. (2015). Chromogranin A as a predictor of radiological disease progression in neuroendocrine tumours. Annals of Translational Medicine, 3(9), 118.
- Rozansky, D. J., Wu, H., Tang, K., Parmer, R. J., & O’Connor, D. T. (1994). Glucocorticoid activation of chromogranin A gene expression. Identification and characterization of a novel glucocorticoid response element. The Journal of Clinical Investigation, 94(6), 2357–2368. doi:10.1172/JCI117601
- Schonhoff, S. E., Giel-Moloney, M., & Leiter, A. B. (2004). Minireview: Development and differentiation of gut endocrine cells. Endocrinology, 145(6), 2639–2644. doi:10.1210/en.2004-0051
- Schroeder, K. W., Tremaine, W. J., & Ilstrup, D. M. (1987). Coated oral 5-aminosalicylic acid therapy for mildly to moderately active ulcerative colitis. A randomized study. The New England Journal of Medicine, 317(26), 1625–1629. doi:10.1056/NEJM198712243172603
- Sciola, V., Massironi, S., Conte, D., Caprioli, F., Ferrero, S., Ciafardini, C., … Piodi, L. (2009). Plasma chromogranin a in patients with inflammatory bowel disease. Inflammatory Bowel Diseases, 15(6), 867–871. doi:10.1002/ibd.20851
- Shooshtarizadeh, P., Zhang, D., Chich, J.-F., Gasnier, C., Schneider, F., Haïkel, Y., … Metz-Boutigue, M.-H. (2010). The antimicrobial peptides derived from chromogranin/secretogranin family, new actors of innate immunity. Regulatory Peptides, 165(1), 102–110. doi:10.1016/j.regpep.2009.11.014
- Strid, H., Simrén, M., Lasson, A., Isaksson, S., Stridsberg, M., & Öhman, L. (2013). Fecal chromogranins and secretogranins are increased in patients with ulcerative colitis but are not associated with disease activity. Journal of Crohn’s & Colitis, 7(12), e615–e622. doi:10.1016/j.crohns.2013.04.019
- Stridsberg, M., Eriksson, B., & Janson, E. T. (2008). Measurements of secretogranins II, III, V and proconvertases 1/3 and 2 in plasma from patients with neuroendocrine tumours. Regulatory Peptides, 148(1–3), 95–98. doi:10.1016/j.regpep.2008.03.007
- Villanacci, V., Bassotti, G., Nascimbeni, R., Antonelli, E., Cadei, M., Fisogni, S., … Geboes, K. (2008). Enteric nervous system abnormalities in inflammatory bowel diseases. Neurogastroenterology and Motility, 20(9), 1009–1016. doi:10.1111/j.1365-2982.2008.01146.x
- Wagner, M., Stridsberg, M., Peterson, C. G., Sangfelt, P., Lampinen, M., & Carlson, M. (2013). Increased fecal levels of chromogranin A, chromogranin B, and secretoneurin in collagenous colitis. Inflammation, 36(4), 855–861. doi:10.1007/s10753-013-9612-4
- Welin, S., Stridsberg, M., Cunningham, J., Granberg, D., Skogseid, B., Oberg, K., … Janson, E. T. (2009). Elevated plasma chromogranin A is the first indication of recurrence in radically operated midgut carcinoid tumors. Neuroendocrinology, 89(3), 302–307. doi:10.1159/000179900
- Zissimopoulos, A., Vradelis, S., Konialis, M., Chadolias, D., Bampali, A., Constantinidis, T., … Kouklakis, G. (2014). Chromogranin A as a biomarker of disease activity and biologic therapy in inflammatory bowel disease: A prospective observational study. Scandinavian Journal of Gastroenterology, 49(8), 942–949. doi:10.3109/00365521.2014.920910
