 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Sildenafil has low water solubility and oral bioavailability. Dry foam formulations of solid dosage forms of poorly water-soluble drugs may exhibit improved dissolution and bioavailability. We previously developed a sildenafil citrate dry foam tablet formulation and found that it had an improved dissolution profile. In this study, we investigated the pharmacokinetic parameters of sildenafil dry foam tablets in rats after oral administration (at a dose equivalent to 20 mg/kg of sildenafil) and compared them with those of commercial sildenafil tablet and dry powder formulations. LC/MS/MS analysis of plasma sildenafil concentration revealed that the AUCs of sildenafil and N-desmethyl sildenafil in the sildenafil citrate dry foam tablet group were significantly higher (150% and 110%, respectively; P < 0.05) than those in the commercial tablet group and (190% and 120%, respectively; P < 0.05) in the sildenafil citrate powder group. The systemic bioavailability (F value) of sildenafil citrate dry foam tablet was 1.5 and 1.9 times higher than that of commercial sildenafil film-coated tablet and sildenafil powder, respectively. This indicates that the systemic bioavailability of sildenafil was increased when it was prepared as a dry foam tablet formulation.
Subjects:
PUBLIC INTEREST STATEMENT
Sildenafil citrate is a first-generation phosphodiesterase-5 inhibitor. It is used for the treatment of erectile dysfunction in males and pulmonary hypertension. The problem with this molecule is its low oral bioavailability due to poor aqueous solubility. We developed sildenafil citrate as a dry foam tablet to solve this problem. The tablets, formulated using a high content of surfactant, had high porosity causing them to undergo rapid dissolution. We investigated the bioavailability of our novel dry foam tablet formulation in rats and found that the dry foam tablet formulation significantly enhanced oral bioavailability compared to the commercial tablet and powder formulations. This system could also be potentially useful to increase the bioavailability of other poorly-water soluble drugs and increase their oral bioavailability. This article is the final part of our research activities conducted on solid dosage forms of sildenafil citrate.
Competing interests
The authors declare no competing interest.
1. Introduction
Sildenafil or (1-[[3-(6,7-dihydro-1-methyl-7-oxo-3-propyl-1H-pyrazolo[4,3-d] pyrimidin-5-yl)-4-ethoxyphenyl] sulfonyl]-4-methyl-piperazine) is classified as a first-generation phosphodiesterase-5 inhibitor. It is used for the treatment of erectile dysfunction in males and pulmonary hypertension (Chocalingam et al., Citation2005; Ghofrani, Osterloh, & Grimminger, Citation2006). Sildenafil has high membrane permeability but poor aqueous solubility; it is categorized as a class 2 drug according to the biopharmaceutical classification system. Although the conventional sildenafil tablet has been developed as a citrate salt to increase water solubility (4.1 mg/mL), the oral bioavailability is about 40% and provides a late onset of action (Jung et al., Citation2011; Nichols et al., Citation2002; Ramirez et al., Citation2010). Dry foam technology was developed to overcome such problems of poorly water-soluble drugs. This technology aims at enhancing dissolution rate by avoiding agglomeration and floating of the non-wetted particles of the active ingredient (Dischinger, Page, & Kleinebudde, Citation2015; Lenz, Sprunk, Kleinebudde, & Page, Citation2015; Sprunk, Page, & Kleinebudde, Citation2013; Thompson, Weatherley, Pukadyil, & Sheskey, Citation2012). Recently, we reported that the dissolution rate of sildenafil citrate prepared as a dry foam tablet was enhanced compared with that of sildenafil commercial tablets (Sawatdee, Atipairin, SaeYoon, & Srichana, Citation2016; Sawatdee, Atipairin, Sae Yoon, Srichana, & Changsan, Citation2017). Although the oral bioavailability of the drug depends on aqueous solubility and dissolution rate, drug permeability, first-pass metabolism, and susceptibility to efflux mechanisms are also important parameters that can influence oral bioavailability (Khadka et al., Citation2014). Therefore, we investigated the oral bioavailability of the prepared dry foam tablet formulation in rats and compared it with that of the conventional sildenafil citrate tablet and powder formulations.
2. Materials
Sildenafil citrate and a reference standard (potency 99.4%) were obtained from Smilax Laboratories Limited (Andhra Pradesh, India). N-desmethyl sildenafil was obtained from Cayman Chemical (USA). Omeprazole sodium was purchased from Aastrid International Pvt. Ltd. (Mumbai, India). Croscarmellose sodium was obtained as a gift from Maxway Co., Ltd., Thailand. Sodium dodecyl sulfate, potassium dihydrogen orthophosphate, and disodium hydrogen orthophosphate anhydrous were obtained from Ajex Finechem Pty Ltd. (Australia). Magnesium stearate and mannitol were purchased from P.C. Drug Center Co., Ltd. (Bangkok, Thailand). Maltodextrin was a gift from Brentag Ingredients Public Co. Ltd., Thailand. Ammonium acetate (98% purity), methanol, and acetonitrile were obtained from RCI Labscan (Bangkok, Thailand). Formic acid was purchased from Tariko Co., Ltd., Thailand. All solvents used were of HPLC grade. All other chemicals used in this study were of pharmaceutical grade or analytical grade and used as received without further purification. Sildenafil citrate 100 mg film-coated tablets (Viagra®, Pfizer Inc.) were obtained against a prescription from a drugstore in Thailand.
3. Methods
3.1. Preparation of sildenafil dry foam tablets
Dry foam tablets were prepared according to a previously described method (Sawatdee et al., Citation2017). Briefly, sodium dodecyl sulfate was dissolved in purified water using a magnetic stirrer. In this solution, sildenafil citrate was suspended. A 1:1 mixture of maltodextrin and mannitol was then added to the sildenafil citrate suspension using a planetary kneader at a mixing speed of 100 rpm until a homogeneous paste (or slurry) was obtained. The resulting slurry was filled into a stainless steel bottle connected with spray nozzle orifice of 2 mm and then sprayed to produce a foam on a stainless steel plate. The foam was dried in a vacuum oven at 70°C for 12 h to keep the moisture content below 2%. The dried foam product was passed through a sieve (1.4 mm) to produce dried foam granules. Magnesium stearate was passed through a sieve (0.25 mm) before use. Dry foam granules were blended with croscarmellose sodium for 5 min in a plastic bottle, and then sieved magnesium stearate was added and blended for another 3 min in the same bottle. The final granule mixtures were compressed into 600-mg tablets using a single punch tableting machine (Small tablet press machine model SP-KR, Charatchai machinery, Bangkok, Thailand) with a round punch and a die diameter of 12 mm. The list of formulation ingredients is shown in Table .
Table 1. All ingredients of sildenafil dry foam tablet formulation used in this study
3.2. Animals
Male Wistar rats (250–300 g) were purchased from National Laboratory Animal Center, Mahidol University, Thailand. The rats were housed in clean polypropylene or corrugated paper cages at controlled room temperature (25 ± 2°C) and humidity at 50–60% with 12-h light and dark cycle throughout the experiment. All rats had free access to pelleted food and tap water ad libitum before the experiments. The experimental procedures were approved by the Ethical Committee of the Animal, Walailak University, Nakhon Si Thammarat, Thailand (approval no. 003/2558).
3.3. Drug administration and blood sampling
After overnight fasting, the rats were divided into three groups with five rats in each group. Sildenafil citrate dry foam tablet, commercial sildenafil citrate film-coated tablet, or sildenafil citrate powder was orally administered to rats as a single dose (equivalent to 20 mg/kg sildenafil) using a gastric gavage tube (Ahn et al., Citation2011). The tablets or powder were each dispersed in 2 mL of distilled water and mixed homogeneously prior to oral administration. Blood samples (0.5 mL) were collected via the tail vein at 0, 15, 30, 60, 90, 120, 180, and 240 min after the oral administration of sildenafil citrate. The blood samples were immediately transferred to heparinized microcentrifuge tubes and centrifuged at 4000 × g for 20 min at 4°C. The separated plasma samples were transferred to Eppendorf tubes and stored at −70°C until further use.
3.4. Preparation of samples
The test plasma samples (50 μL) were spiked with omeprazole sodium (internal standard; 0.2 μg/200 μL acetonitrile). Each sample was mixed for 10 s by vortexing and centrifugation at 9000 × g for 10 min. The supernatant was transferred to another Eppendorf tube for further analysis.
3.5. Instrumentation and chromatographic conditions
The plasma concentrations of sildenafil and N-desmethyl sildenafil were determined using a liquid chromatography–mass spectrometry–mass spectrometry (LC/MS/MS) method according to previous reports (Alkharfy, Citation2009; Sawatdee, Hiranphan, Laphanayos, & Srichana, Citation2013; Tripathi, Mazumder, & Chandewar, Citation2014). The method was validated for six parameters: specificity, linearity, precision, accuracy, limit of detection, and limit of quantitation. In addition, system suitability was also analyzed. An Agilent 1260 Infinity HPLC system (Agilent, Singapore) was used. The HPLC system consisted of a solvent delivery pump equipped with an in-line degasser and an autosampler. The mobile phase used was 90% acetonitrile plus 10% ammonium acetate (20 mM) containing 0.02% formic acid, which was first filtered and degassed using a 0.22-μm nylon membrane filter before use. The flow rate was 0.2 μL/min (isocratic), and the injection volume was 25 μL. The MS was equipped with an ESI probe and a quadrupole mass analyzer (ABSCIEX API 3200™ LC/MS/MS system, Singapore). The optimized ion spray voltage and temperature were set at 5500 V and 500°C, respectively. The parameters of multiple reaction monitoring were: declustering potential (91 V), entrance potential (6.5 V), collision cell entrance potential (28 V), collision energy potential (41 V), and collision cell exit potential (6V). Nitrogen was used as the nebulizer gas, curtain gas, and collision-activated dissociation gas. Quantification was performed by multiple reaction monitoring of the protonated precursor ion and the related product ion for sildenafil and N-desmethyl sildenafil, using the internal standard method with peak area ratios. Both sildenafil and omeprazole were eluted at room temperature within 1 min. The mass transitions as the [MH]+ molecular ions used for sildenafil, N-desmethyl sildenafil, and internal standard were m/z 475.3 → 100.1, 460.9 → 283.2, and 346.1 → 197.8, respectively, with a dwell time of 150 ms per transition. The control of the LC/MS/MS system and the data acquisition was performed using the Analyst® version 1.6 software (Applied Biosystems). A calibration curve was prepared in the range of 0.5–2000 ng/mL.
3.6. Pharmacokinetics and statistical analysis of data
The plasma concentration versus time curves obtained after each treatment in individual animals were fitted with a software program (WinNonlin, version 5.2, Pharsight Corp, Mountain View, California) and reported as mean ± SD. The following pharmacokinetic parameters for each animal were analyzed via non-compartmental model analysis: maximum concentration (Cmax), time to reach maximum concentration (Tmax), half-life (t1/2), and the area under the concentration–time curve (AUC0–6h). The trapezoidal rule was used to calculate AUC. Systemic bioavailability (F) was calculated by the following equation:
where AUC of the reference is the AUC of sildenafil commercial tablet formulation, and AUC(test formulation) is the AUC of sildenafil citrate dry foam tablet or sildenafil citrate powder.
The pharmacokinetic parameters are reported as mean ± SD. Pharmacokinetic parameters were statistically compared using one-way ANOVA. Mean values were considered significantly different at P < 0.05.
4. Results and discussion
In our previous study, the dissolution of the prepared sildenafil dry foam tablets was two times faster than that of the commercial sildenafil tablet formulation (Sawatdee et al., Citation2017). This may be because the high amount of surfactant used in the formulation might have increased the porosity of the tablet during the manufacturing process and because of the amorphous properties of the sildenafil citrate powders (Sawatdee et al., Citation2017). This prompted us to further probe into the bioavailability of the dry foam tablets in vivo. The specifications and physicochemical properties of the sildenafil citrate dry foam tablet used in this study are shown in Table .
Table 2. Finished product specification and physicochemical properties of sildenafil citrate dry foam tablets
After the oral administration of sildenafil citrate dry foam tablet, sildenafil citrate film-coated tablet (commercial product), and sildenafil citrate powder to rats at a dose of 20 mg/kg sildenafil, the mean plasma concentration–time curves of sildenafil and N-desmethyl sildenafil were plotted as shown in Figure and , respectively. The pharmacokinetic parameters are listed in Table .
Table 3. Comparative mean (± SD) of pharmacokinetic parameters of sildenafil and N-desmethyl sildenafil in rats after oral administration
Figure 1. Mean plasma concentration—time profiles of sildenafil (A) and N-desmethyl sildenafil (B) after oral administration of sildenafil at a dose of 20 mg/kg in dry foam tablet (solid circles, n = 5), commercial film coated tablets (open circles, n = 5) and sildenafil powder (solid triangles, n = 5) in rats. Data are the mean ± standard deviation (SD).
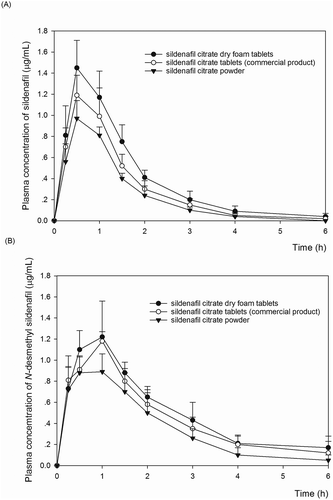
N-desmethyl sildenafil is the major metabolite of sildenafil formed by the N-demethylation of its piperazine (Walker et al., Citation1999). The AUCs of sildenafil and N-desmethyl sildenafil in the sildenafil citrate dry foam tablet group were significantly higher (150% and 110%, respectively; P < 0.05) than those in the commercial tablet group and (190% and 120%, respectively; P < 0.05) in the sildenafil citrate powder group. The systemic bioavailability (F value) of sildenafil citrate dry foam tablet was 1.5 and 1.9 times higher than that of commercial sildenafil film-coated tablet and sildenafil powder, respectively.
The use of water-soluble ingredients or surfactants such as PEG and croscarmellose sodium (super disintegrant) increases the dissolution of sildenafil tablets (Adena et al., Citation2016; Degim, Tugcu-Demiröz, Tamer-Ílbasmi§, & Acartürk, Citation2008). These reports indicate that formulation and dosage form influence the bioavailability of this drug.
Although enhancement in the oral bioavailability of sildenafil was achieved by the dry foam tablet formulation, some pharmacokinetic values differed from those reported previously. The Tmax observed in this study was longer than that reported (Ahn et al., Citation2011; Shin, Bae, & Lee, Citation2006; Tripathi et al., Citation2014). This may be because of the variation in animals used and because the dissolution and absorption profiles were altered as the tablets were pulverized and dispersed in water prior to administration. The Cmax was higher than that reported may cause form the high oral dose administration and variation of animal used (Ahn et al., Citation2011; Tripathi et al., Citation2014; Walker et al., Citation1999). Although the bioavailability was observed to be enhanced by the dry foam tablet formulation in this study, the bioavailability was still lower than that reported for the sublingual formulation in rabbits (Sheu, Hsieh, Chen, Chou, & Ho, Citation2016) and human (Zayed, Kamel, Shukr, & El-Shamy, Citation2012). Because sublingual route is rich supply by blood vessels and avoidance of hepatic first pass metabolism (Khan, Kingsley, & Caroline, Citation2017). In addition, it may cause from the variation of the animal species.
5. Conclusions
The oral bioavailability of sildenafil citrate can be significantly enhanced by formulating it as dry foam tablets. This formulation strategy may also be used to increase the oral bioavailability of other poorly water-soluble drugs.
Author Contributions
Somchai Sawatdee, Apichart Atipairin, Attawadee Sae Yoon, Teerapol Srichana and Tan Suwandecha have contributed to the concept, design of the study, data analysis including interpretation of data. All the authors contributed to prepare the manuscript. Somchai Sawatdee, Apichart Atipairin, Attawadee Sae Yoon, Narumon Changsan, Tan Suwandecha, Wirot Chantorn and Atchara Phoem have contributed to the carrying out of experiments.
Acknowledgements
The authors acknowledge the Drug and Cosmetics Excellence Center, Walailak University, Thailand. The authors would like to thank research assistants of Drug and Cosmetics Excellence Center and staff of Drug Delivery System Excellence Center (DDSEC), Faculty of Pharmaceutical Sciences, Prince of Songkla University, Hat Yai, Songkhla, Thailand, who assisted in the experiments.
Additional information
Funding
Notes on contributors

Somchai Sawatdee
Somchai Sawatdee obtained his Bachelor of Pharmacy degree in 2003 from Prince of Songkla University, Songkhla, Thailand and received his Master of Pharmacy (Pharmaceutical Sciences) degree in 2005 from the same university. He has been the research and development manager in pharmaceutical industry. He established and managed research and development functions to develop pharmaceutical products from its inception through FDA registration till commercialization. He earned his Ph.D. Degree in Pharmaceutical Sciences in 2013 from Prince of Songkla University before moving to Walailak University. Dr. Somchai Sawatdee is an assistant professor at the School of Pharmacy. He has published more than 20 research papers in international journals and has presented his research at international conferences. His multi-disciplinary research interests include novel drug delivery systems (especially pulmonary drug delivery systems), solid dosage forms, and aerosol sciences. Currently, he is the director of Drug and Cosmetics Excellence Center at Walailak University.
References
- Adena, S. K. R., Viswanadh, M. K., Krishna, P. R., & Induri, A. (2016). Formulation and evaluation of sildenafil citrate fast dissolving tablets using crospovidone and croscarmellose sodium superdisintegrants. Journal of Pharmacy Research, 10(7), 484–492.
- Ahn, C. Y., Bae, S. K., Bae, S. H., Kang, H. E., Kim, S. H., Lee, M. G., & Shin, W. G. (2011). Pharmacokinetics of sildenafil and its metabolite, N-desmethylsildenafil, in rats with liver cirrhosis and diabetes mellitus, alone and in combination. Xenobiotica, 41(2), 164–174. doi:10.3109/00498254.2010.532885
- Alkharfy, K. M. (2009). Simple and sensitive LC-ESI-MS method for the quantitation of sildenafil in plasma samples. Journal of Separation Science, 32(22), 3866–3870. doi:10.1002/jssc.200900469
- Chocalingam, A., Gnanavelu, G., Venkatesan, S., Elangovan, S., Jagannathan, V., Subramaniam, T., … Dorairajan, S. (2005). Efficacy and optimal dose of sildenafil in primary pulmonary hypertension. International Journal of Cardiology, 99(1), 91–95. doi:10.1016/j.ijcard.2003.12.023
- Degim, I. T., Tugcu-Demiröz, F., Tamer-Ílbasmi§, S., & Acartürk, F. (2008). Development of controlled release sildenafil formulations for vaginal administration. Drug Delivery, 15(4), 259–265. doi:10.1080/10717540802006781
- Dischinger, A., Page, S., & Kleinebudde, P. (2015). Fast dissolving fillers in dry foam formulation. Powder Technology, 270, 494–501. doi:10.1016/j.powtec.2014.06.036
- Ghofrani, H. A., Osterloh, I. H., & Grimminger, F. (2006). Sildenafil: From angina to erectile dysfunction to pulmonary hypertension and beyond. Nature Review Drug Discovery, 5(8), 689–702. doi:10.1038/nrd2030
- Jung, S., Seo, Y., Kim, G. K., Woo, J. S., Yong, C. S., & Choi, H. (2011). Comparison of solubility and pharmacokinetics of sildenafil salts. Archives Pharmacol Research, 34(3), 451–454. doi:10.1007/s12272-011-0313-y
- Khadka, P., Ro, J., Kim, H., Kim, I., Kim, J., Kim, H., … Lee, J. (2014). Pharmaceutical particle technologies: An approach to improve drug solubility, dissolution and bioavailability. Asian Journal of Pharmaceutical Sciences, 9, 304–316. doi:10.1016/j.ajps.2014.05.005
- Khan, A. B., Kingsley, T., & Caroline, P. (2017). Sublingual tablets and the benefits of the sublingual route of administration. Journal of Pharmaceutical Research, 16(3), 257–267. doi:10.18579/jpcrkc/2017/16/3/118766
- Lenz, E., Sprunk, A., Kleinebudde, P., & Page, S. (2015). Impact of fillers on dissolution kinetic of fenofibrate dry foams. Pharmaceutical Development and Technology, 20(5), 570–578. doi:10.3109/10837450.2014.908301
- Nichols, D.J, Muirhead, G.J, & Harness, J.A. (2002). Pharmacokinetics of sildenafil citrate after single oral doses in healthy male subjects: absolute bioavailability, food effects and dose proportionality. British Journal of Clinical Pharmacology, 53 (Suppl1), 5s-12s. doi: 10.1046/j.0306-5251.2001.00027.x
- Ramirez, E., Laosa, O., Guerra, P., Duque, B., Mosquera, B., Borobia, A. M., … Frias, J. (2010). Acceptability and characteristics of 124 human bioequivalence studies with active substances classified according to the biopharmaceutic classification system. British Journal of Clinical Pharmacology, 70(5), 694–702. doi:10.1111/j.1365-2125.2010.03757.x
- Sawatdee, S., Atipairin, A., Sae Yoon, A., Srichana, T., & Changsan, N. (2017). Enhanced dissolution of sildenafil citrate as dry foam tablets. Pharmaceutical Development and Technology, In press. doi:10.1080/10837450.2017.1281952
- Sawatdee, S., Atipairin, A., SaeYoon, A., & Srichana, T. (2016). Enhanced dissolution of sildenafil dry foam tablet. Asian Journal of Pharmaceutical Sciences, 11, 191–192. doi:10.1016/j.ajps.2015.11.042
- Sawatdee, S., Hiranphan, P., Laphanayos, K., & Srichana, T. (2013). Evaluation of sildenafil pressurized metered dose inhalers as a vasodilator in umbilical blood vessels of chicken egg embryos. European Journal of Pharmaceutics and Biopharmaceutics, 86(1), 90–97. doi:10.1016/j.ejpb.2013.09.001
- Sheu, M., Hsieh, C., Chen, R., Chou, P., & Ho, H. (2016). Rapid-onset sildenafil sublingual drug delivery systems: In vitro evaluation and in vivo pharmacokinetic studies in rabbits. Journal of Pharmaceutical Sciences, 105(9), 2774–2781. doi:10.1016/j.xphs.2016.01.015
- Shin, H. S., Bae, S. K., & Lee, M. G. (2006). Pharmacokinetics of sildenafil after intravenous and oral administration in rats: Hepatic and intestinal first-pass effects. International Journal of Pharmaceutics, 320(1–2), 64–70. doi:10.1016/j.ijpharm.2006.04.005
- Sprunk, A., Page, S., & Kleinebudde, P. (2013). Influence of process parameters and equipment on dry foam formulation properties using indomethacin as model drug. International Journal of Pharmaceutics, 455(1–2), 189–196. doi:10.1016/j.ijpharm.2013.07.039
- Thompson, M. R., Weatherley, S., Pukadyil, R. N., & Sheskey, P. J. (2012). Foam granulation: New developments in pharmaceutical solid oral dosage forms using twin screw extrusion machinery. Drug Development and Industrial Pharmacy, 38(7), 771–784. doi:10.3109/03639045.2011.633265
- Tripathi, A. S., Mazumder, P. M., & Chandewar, A. V. (2014). Changes in the pharmacokinetic of sildenafil citrate in rats with Streptozotocin-induced diabetic nephropathy. Journal of Diabetes and Metabolic Disorders, 13(1), 1–4. doi:10.1186/2251-6581-13-8
- Walker, D. K., Ackland, M. J., James, G. C., Muiehead, G. J., Rance, D. J., Wastall, P., & Wright, P. A. (1999). Pharmacokinetics and metabolism of sildenafil in mouse, rat, rabbit, dog and man. Xenobiotica, 29(3), 297–310. doi:10.1080/004982599238687
- Zayed, R., Kamel, A. O., Shukr, M., & El-Shamy, A. E. (2012). An in vitro and in vivo comparative study of directly compressed solid dispersions and freeze dried sildenafil citrate sublingual tablets for management of pulmonary arterial hypertension. Acta Pharmaceutica, 62(3), 411–432. doi:10.2478/v10007-012-0027-9

