Abstract
Objective. In 1998, Felitti and colleagues published the first study of the Adverse Childhood Experiences-Questionnaire (ACE-Q), a 10-item scale used to correlate childhood maltreatment and adverse rearing contexts with adult health outcomes. This paper qualitatively reviews nearly two decades of research utilizing the ACE-Q, highlighting its contribution to our understanding of the causal roots of common, interlinked comorbidities of the brain and body.
Methods. An OVID/PubMed search was conducted for English language articles published before 2016, containing the phrase “Adverse Childhood Experiences” in which the ACE-Q was utilized. Source review included a manual search of bibliographies, resulting in 134 articles, including 44 based on the original ACE-Q study population.
Results. ACE-Q research has demonstrated that exposures to adverse childhood experiences converge dose-dependently to potently increase the risk for a wide array of causally interlinked mental illnesses, addictions, and multi-organ medical diseases. The intergenerational transmission of this disease burden via disrupted parenting and insecure rearing contexts is apparent throughout this literature. However, the ACE-Q does not tease out genetic or fetal drug exposure components of this transmission.
Conclusions. Adverse childhood experiences and rearing may generate a public health burden that could rival or exceed all other root causes. Translating this information to health-care reform will require strengthening brain-behavioral health as core public and preventative health-care missions. Greater integration of mental health and addiction services for parents should be accompanied by more research into brain mechanisms impacted by different forms and interactions between adverse childhood experiences.
PUBLIC INTEREST STATEMENT
Twenty years of research using the Adverse Childhood Experiences (ACE) Questionnaire reveals that a short list of traumatic childhood experiences, neglect, and stressful rearing contexts associates with an incredibly wide array of serious adult-age diseases. Mental illnesses, addictions, and multi-organ diseases of the body occur in comorbid patterns in a dose-dependent way with greater convergence of multiple types of ACEs. The intergenerational transmission from parents with high ACE-scores to children who are not well attached, protected and nurtured—and grow up more sick and less able to parent—is evident in this literature. This evidence indicates the need for more investigations on how ACEs generate mental illness and addictions as modifiable biological processes just as important as psychiatric genetics. Additionally, this literature highlights the imperative for greater integration of mental health and addiction services especially for parents with young children, as core, rather than marginal public and preventative health missions.
Disclosures
None of the authors have financial or commercial interests that pertain to the work of authorship, content, or conclusions of this manuscript. Dr. Zarse is an addiction psychiatrist at Community Health Network, Indianapolis; Drs. Neff and Yoder are child psychiatrists employed by Indiana University School of Medicine (IUSM); Dr. Hulvershorn is a combined child/addiction psychiatrist employed by IUSM; Dr. J Chambers, is a general adult psychiatrist employed by IUSM; and Dr. RA Chambers an addiction psychiatrist employed by IUSM who has received independent reimbursement for consultations to the U.S. DEA, and has consulting contracts with Enfoglobe and Indigobio, (clinical data analytic software companies).
1. Introduction
Child abuse, neglect and unusually stressful or traumatic rearing conditions are common pediatric health problems with a majority of Americans having experienced at least one of these kinds of adverse childhood experiences (ACE) (CDC, Citation2010; Flaherty et al., Citation2009). While it has long been known that behavioral problems in children can result from maltreatment and/or deprivation (Rutter et al., Citation1999), our understanding of these experiences as key determinants of adult health has been a fairly recent topic of neurobiological, clinical, and epidemiological investigation (Anda et al., Citation2006; Chambers, Citation2017; Flaherty et al., Citation2006).
Since the mid 1900s, animal and human studies have provided increasing evidence that experiencing especially high or sustained levels of psychosocial stress, primary attachment deprivation and/or maltreatment in childhood severely impacts adult behavior (Bowlby, Citation1984; Citation2017; Bruskas & Tessin, Citation2013; Harlow, Citation1962; Sheridan & McLaughlin, Citation2014; Sullivan, Citation2017). Neuroscience has identified many brain systems impacted by adverse experiences, including those involved in stress responsivity and social interaction, suggesting that these experiences are developmentally neurotoxic, producing long-lasting changes in brain architecture and function, contributing to adult psychiatric syndromes (CitationBenedetti et al., Citation2012, Citation2014; Heim, Shugart, Craighead, & Nemeroff, Citation2010; Teicher, Anderson, & Polcari, Citation2012). When occurring at high levels, and in the absence of resilience factors, early adverse experiences can damage later parental attachment, nurturing, and protective behaviors, producing transgenerational cycles of trauma-spectrum neuropsychiatric illness (Chung et al., Citation2009; Logan-Greene, Green, Nurius, & Longhi, Citation2014; Stamoulis, Vanderwert, Zeanah, Fox, & Nelson, Citation2017). Thus, children growing up in families with high ACE exposure levels can expect to have much higher rates of psychiatric (Gould et al., Citation1994; Van Niel, Pachter, Wade, Felitti, & Stein, Citation2014), addictive (Anda, Citation1999; Cavanaugh, Petras, & Martins, Citation2015; McCauley et al., Citation1997) and medical diseases (Felitti, Citation1991, Citation1993; Springs & Friedrich, Citation1992) that impair parental skills needed for protecting offspring from these same illness-risks.
The Adverse Childhood Experience Questionnaire (ACE-Q) is a brief rating scale designed and first published by Felitti et al., that has provided substantial epidemiological evidence concerning the link between adverse childhood experiences and adult mental and physical illnesses (Felitti et al., Citation1998). This paper reviews the literature published prior to 2016 showing how the ACE-Q has helped characterize exposure to ACEs as a core public health challenge of remarkable scope in terms of the diversity and severity of adult brain and body diseases that ACEs link with. A search of OVID/PubMed English language articles containing the phrase “Adverse Childhood Experiences” in which the ACE-Q was utilized for data collection or analysis was conducted, followed by a manual search of bibliographies. This resulted in 134 articles of which 44 were based on the original population of the first ACE-Q study (Felitti et al., Citation1998) and 90 on other populations. We overview the breadth of mental health, substance disorders, and medical conditions that high ACE-Q scores are linked with, while highlighting the diverse populations and more specific scientific applications the ACE-Q instrument has been implemented for. We conclude with a discussion of the overall contributions, strengths, and weaknesses of the ACE-Q, and how the ACE-Q literature may be integrated with, and interpreted in light of the emerging neuroscience characterizing causal neurodevelopmental interlinkages between ACEs, adult age mental illness, addictions and ultimately, a wide scope of multi-organ diseases. Finally, we consider how this literature can inform future directions and applications needed for advancing behavioral healthcare and research as a core component of public health.
1.1. Origins and attributes of the ACE-Q
Physicians at a large American Health Care consortium, Kaiser Permanente, developed the ACE-Q from several previously researched assessment tools. The 10-item questionnaire checks for the subject’s recall of pre-age 19 exposure to psychological, physical, and sexual abuse as well as household dysfunction including domestic violence, substance use, and incarceration (Figure ). In 1996, surveys assessing current medical symptoms and past adverse experience were sent to 13,494 Kaiser patients resulting in a 70.5% response sample with a mean age of 56.1 years that was 79.4% white and 43% college educated. It was from this sample that the seminal ACE-Q study was published (Felitti et al., Citation1998) showing that increased ACE scores corresponded to greater degrees of adult illness burden. Notably, this predictive relationship was found based on the increased number of different types of adverse experiences a person was exposed to, and not by the severity of any one kind of adverse childhood event (which the ACE-Q is not designed to quantify). This is a defining aspect of the ACE-Q, and also its major strength and core weakness: it is a relatively rapid tool for quantifying overall degree of convergence of different ACEs and contexts onto one individual, but it does not measure the degree, duration, severity, timing, or quality of each of these ACE components for the individual.
Figure 1. Adverse Childhood Experience (ACE) Questionnaire (Felitti et al., Citation1998).

Prior to the Felitti 1998 study, different forms of child maltreatment were mainly studied independently with little attention to their co-occurrence or the impact of other household factors on outcomes (Dong et al., Citation2004). But since then, several studies have examined the interrelatedness of various forms of abuse (Edwards, Holden, Felitti, & Anda, Citation2003; Flaherty et al., Citation2009; Thompson et al., Citation2014). A study of 8,629 survey respondents showed that more than 81% who reported experiencing one type of maltreatment also reported at least one other type of adverse experience (Dong et al., Citation2004). The increased risk of experiencing at least one other event has been found looking specifically at childhood sexual trauma (Dong, Anda, Dube, Giles, & Felitti, Citation2003; Easton, Citation2012) and witnessing domestic violence (Dube, Anda, Felitti, Edwards, & Williamson, Citation2002). This clustering of different types of abuse, neglect, or adverse contexts highlights the importance of considering the effects of multiple adverse childhood experiences in research and practice (Anda, Citation1999; Dube, Felitti, Dong, Giles, & Anda, Citation2003; Van Niel et al., Citation2014).
There are several other notable self-report abuse screening questionnaires (e.g. the Child Abuse and Trauma Scale, the parent to child Conflict Tactics Scale, the Childhood Trauma Questionnaire, Traumatic Experience Checklist, and the Childhood Experiences Scale (Bernstein, Ahluvalia, Pogge, & Handelsman, Citation1997; Nijenhuis, Van der Hart, & Kruger, Citation2002; Sanders & Becker-Lausen, Citation1995; Straus, Citation1998; Zanarini, Citation2000)). However, the ACE-Q has been uniquely productive in revealing a strong “dose-response” relationship where greater convergence of different forms of ACEs lead to increasingly poor adult health spanning many major behavioral and medical diseases (Felitti et al., Citation1998; Hughes et al., Citation2017).
Since its introduction in 1998, the ACE-Q has been used in research to assess a diverse set of subpopulations throughout the United States, Europe, and Asia (Matsuura, Hashimoto, & Toichi, Citation2009; Ramiro, Madrid, & Brown, Citation2010). Validation studies suggest that ACE-Q scoring remains strong as a predictive measure despite potential distortion due to historical self-reporting-memory artifacts (Dube, Williamson, Thompson, Felitti, & Anda, Citation2004; Hardt, Vellaisamy, & Schoon, Citation2010). Within a population sample, the scale can characterize different overall scores or rates of different adverse experiences in different demographic subgroups. For instance, the ACE-Q has shown higher rates of adverse early experiences in gay, lesbian, and bisexual vs. heterosexual populations (Andersen & Blosnich, Citation2013). Women also report higher numbers of overall adverse childhood experiences on the ACE-Q, including the specific category of sexual abuse, although several studies have shown that the two genders suffer similar rates of exposure to physical abuse (R.F. Anda, Citation1999; Dube et al., Citation2001, Citation2002, Citation2005, Citation2003, Citation2006; Edwards, Anda, Gu, Dube, & Felitti, Citation2007).
Utilization of the ACE-Q as a tool for intervention-outcomes research has also been an application of growing interest, e.g. for measuring how social work interventions may ameliorate the long-term consequences of adverse childhood experiences (Larkin, Felitti, & Anda, Citation2014). Other studies have examined how the sensitivity and/or specificity of the ACE-Q to particular long-term health outcomes might be augmented with additional queries, e.g., that examine peer rejection or victimization, community violence exposure, school performance, and socioeconomic status (Finkelhor, Shattuck, Turner, & Hamby, Citation2013). The public health relevance and versatility of research applications of the ACE-Q, are reflected by the adaptation of ACE questions into the CDC’s behavioral risk factor surveillance system, the largest ongoing health survey in the world, and its use by many state health departments in the U.S. (CDC, Citation2010; Ye & Reyes-Salvail, Citation2014).
1.2. The ACE-Q as a core risk measure of premature illness and death
The following sections will outline the literature demonstrating the relationships between adverse childhood experiences measured by the ACE-Q, and long-term adult health outcomes spanning 1) mental health; 2) substance use disorders; and 3) general medical/somatic conditions. It is important to keep in mind that this evidence indicates that the convergence of several different categories of adverse experiences most strongly conveys adult illness risk, while at the same time, studies spanning different populations and nationalities have consistently shown that the majorities of all adults (52%–75%) score one or higher on the ACE-Q (Anda, Citation1999; CDC, Citation2010; Dube et al., Citation2002, Citation2003, Citation2003, Citation2006; Edwards et al., Citation2007; Ford et al., Citation2011; Ramiro et al., Citation2010; Rothman, Bernstein, & Strunin, Citation2010). It is a 5%–10% minority of the general population who carry an ACE-Q score of 4 plus, where the general long-term health consequences become most pronounced (Felitti et al., Citation1998; Hughes et al., Citation2017).
2. The ACE-Q and mental health
2.1. General mental health indicators
A graded dose–response association has been found between ACE-Q score and relationship problems, emotional distress, worker performance, financial problems, current family problems, high stress, and inability to control anger (Anda et al., Citation2004; Hillis et al., Citation2004; Nurius, Logan-Greene, & Green, Citation2012; Ramiro et al., Citation2010). High ACE scores also predict risk for homelessness which is especially prevalent in individuals with comorbid substance use disorders and mental illness (Montgomery, Cutuli, Evans-Chase, Treglia, & Culhane, Citation2013; Patterson, Moniruzzaman, & Somers, Citation2014; Wu, Schairer, Dellor, & Grella, Citation2010). Findings also suggest that people cannot merely “age out” of the mental health effects of ACEs; adults over the age of 65 with higher ACEs have increased odds of mood and personality disorders (Raposo, Mackenzie, Henriksen, & Afifi, Citation2014). However, the effects of different ACEs may be modifiable by interventions that can support better health behaviors, and increased quality of life measures (Whitaker et al., Citation2014).
Positive relationships between ACE-Q scores and risk of psychiatric illness have also been identified in terms of being prescribed a diversity of psychiatric medications. A study of 15,000 adults prospectively examined over 7 years, showed that prescription rates for psychotropic medications increased yearly and in a graded fashion as the ACE score increased (Anda et al., Citation2007). A score above 5 was associated with an increased likelihood of being prescribed antidepressant, anxiolytic, antipsychotic, or mood-stabilizing medications. The strength of this relationship was not substantially changed even when the history of mental illness in the home was excluded from the ACE score. A separate study showed that in females with obsessive-compulsive disorder, being on psychotropic medications was associated with higher ACE scores compared to un-medicated patients (Benedetti et al., Citation2014).
2.2. Depression
The risk for depression increases in a dose–response fashion with ACE-Q scores, and history of childhood exposure to emotional abuse may especially accentuate this effect (Anda et al., Citation2002; Chapman et al., Citation2004; Edwards et al., Citation2003; Lu, Mueser, Rosenberg, & Jankowski, Citation2008; Ramiro et al., Citation2010). While some studies have not identified a statistically significant dose–response relationship, a categorical link between depression or components of depression (e.g. hopelessness or suicidal ideation) and elevated ACE-Q scores has been shown in women (Corcoran, Gallagher, Keeley, Arensman, & Perry, Citation2006; Haatainen et al., Citation2003; Honkalampi et al., Citation2005) and other special populations including impoverished women seeking prenatal care (Chung, Mathew, Elo, Coyne, & Culhane, Citation2008), Native Americans over 50 years old (Roh et al., Citation2015), Native Americans with family histories of forced relocations (Bombay, Matheson, & Anisman, Citation2011), active duty soldiers (Gahm, Lucenko, Retzlaff, & Fukuda, Citation2007), and female Japanese prisoners (Matsuura et al., Citation2009).
The association between ACEs and depression is detectable across adult ages including elderly samples (Ege, Messias, Thapa, & Krain, Citation2015) allowing cohort studies to examine the potential influence of growing up in specific social, historical, or cultural eras on the predictive link between ACE-Q and depression. A study of four birth cohorts: 1900–31, 1932–46, 1947–61, and 1962–78, found a similar positive correlation between ACE-Q score and depression risk in all four cohorts (Dube et al., Citation2003). The intergenerational effects of ACEs to increase depression risk has been shown in a sample of 143 Native Americans, in which 67 of subjects had parents that were forced to attend Indian Residential Schools, where many individuals experienced neglect, abuse, and the trauma of separation from families and culture (Bombay et al., Citation2011). In this study, ACE-Q scores were higher in the offspring of those who attended the Schools, and were correlated with more depressive symptoms.
2.3. Post-traumatic stress disorder (PTSD)
Exposure to higher levels of ACEs predisposes to onset and greater severities of PTSD (Swopes, Simonet, Jaffe, Tett, & Davis, Citation2013; Yehuda, Citation2001), and witnessing interpersonal violence (IPV) may especially generate this risk (Lamers-Winkelman, Willemen, & Visser, Citation2012). In a sample of adult victims of IPV, in which 75% met criteria for PTSD, the median ACE-Q score was 3.5, with a third having a score of 5 or more, and 18% reporting a score of 7 (Corbin et al., Citation2013). In a general population study in which 18% of the sample reported having child autobiographical memory disturbance (CAMD), where they are unable to recount large portions of their childhood presumably because of traumatic experiences, there was a strong graded correlation between ACE-Q score and risk of CAMD that was consistent across four birth cohorts (Brown et al., Citation2007).
In active duty soldiers, both ACE-Q score and history of being deployed to a combat zone correlated with increased risk for positive PTSD screening (Gahm et al., Citation2007). ACEs also increase the likelihood of suffering with any mental illness and homelessness in military veterans (Montgomery et al., Citation2013). Interestingly, volunteers for military experience (as compared to conscripts) appear to have higher overall ACE-Q scores, suggesting that voluntary enlistment could represent an escape from a dysfunctional home life (Blosnich, Dichter, Cerulli, Batten, & Bossarte, Citation2014), which could set up for greater biological risk for acquiring PTSD.
Surprisingly, and reflecting a major gap in the ACE-Q literature, research on the association between ACE-Q scores, and risk of trauma-spectrum personality disorders (e.g. borderline personality) is lacking although childhood sexual, emotional, and physical abuse are well-known risk factors for developing borderline psychopathology (Zanarini, Citation2000).
2.4. Psychosis
Although studies have suggested a causal link between experiencing childhood sexual abuse and developing adult psychosis (e.g. (Bebbington et al., Citation2011)) little research has been done using the ACE-Q in patients with psychotic spectrum disorders. However, in the initial ACE cohort, 2% of the sample reported ever having a hallucination, and for each ACE-Q point the risk of ever hallucinating increased by 1.2–2.5 fold in a dose–response manner; those with 7 or more ACEs had a 5-fold increase in risk of reporting hallucinations (Whitfield, Dube, Felitti, & Anda, Citation2005). This relationship was found independent of a history of substance abuse.
3. The ACE-Q and substance use disorders
3.1. Nicotine
Multiple studies have shown a relationship between ACE score and smoking (Anda, Citation1999; Bellis, Lowey, Leckenby, Hughes, & Harrison, Citation2014; Dube et al., Citation2003; Edwards et al., Citation2007; Ford et al., Citation2011; Fuller-Thomson, Filippelli, & Lue-Crisostomo, Citation2013; Ramiro et al., Citation2010; Sacco et al., Citation2007; Vander Weg, Citation2011; Walsh & Cawthon, Citation2014; Wu et al., Citation2010; Yeoman, Safranek, Buss, Cadwell, & Mannino, Citation2013). Exposure to any category of ACE shows a graded relationship with ever smoking, heavy smoking, and early smoking initiation (Anda, Citation1999; Ramiro et al., Citation2010). These associations appeared to strengthen in samples who grew up after the surgeon general’s warning on smoking, suggesting that ACEs confer vulnerability to nicotine addiction that is relatively impervious to anti-smoking social pressures and educational initiatives (Anda, Citation1999).
Exposure to each category of ACE significantly increases the risk of lifetime and current cigarette use, such that likelihood of smoking increases by 20–30% for each increase in ACE-Q score (Dube et al., Citation2003). A different population showed similar findings, with higher ACE-Q scores in current vs. former smokers suggesting that ACEs confer increased difficulty in smoking cessation (Vander Weg, Citation2011). This relationship holds true even when controlling for socioeconomic status (Ford et al., Citation2011). Increasing ACE-Q score is also associated not only with lower cessation rates in people with serious health problems due to smoking, but also increased the risk of having more smoking-induced medical diseases (Edwards et al., Citation2007).
When examining smoking rates in patients with mental illness, several ACE categories, including histories of verbal, physical, and sexual abuse all increase the likelihood of smoking (Sacco et al., Citation2007). Increasing ACE-Q scores were also ordered according to degree of dual diagnosis comorbidity: Mentally ill smokers demonstrated the highest mean ACE-Q scores, followed by mentally ill non-smokers, well smokers, and well, non-smokers, suggesting that ACEs produce independent and compounding effects on risk of both mental illness and addiction (Sacco et al., Citation2007). As with mental illness, female gender may also modulate the degree to which ACEs associate with smoking (Strine et al., Citation2012).
3.2. Alcohol
Based on the original ACE-Q data set, each ACE type, except physical neglect, was associated with a 1.6- to 2.4-fold increased likelihood of drinking alcohol (Dube et al., Citation2006). This finding was consistent across several birth cohorts (Dube et al., Citation2003) and is replicated by later studies (Ramiro et al., Citation2010; Strine et al., Citation2012; Young, Hansen, Gibson, & Ryan, Citation2006). Bellis et al. found that those with ACE scores greater than four were 3.72 times as likely to be heavy drinkers (Bellis et al., Citation2014).
As with smoking, ACE-Q score varies inversely with age of drinking initiation, and multiple ACE categories are associated with an increased likelihood of starting drinking during early adolescence rather than adulthood (Dube et al., Citation2006; Rothman et al., Citation2010; Rothman, Edwards, Heeren, & Hingson, Citation2008).
Several studies have investigated how parental alcohol use affected an individual’s use of alcohol and the connection between parental alcohol use and ACE score, suggesting that alcohol use disorders in parents facilitate the transgenerational flow of elevated ACE-Q across multiple categories (Anda et al., Citation2002; Clarke-Walper, Riviere, & Wilk, Citation2014; Dube et al., Citation2002; Xiao, Dong, Yao, Li, & Ye, Citation2008). A combination of elevated ACE scores ≥4 and a history of parental alcohol misuse produces the highest risk of heavy and problem drinking (Dube et al., Citation2002). Similarly, in a veteran sample, having an alcoholic in the household and experiencing sexual abuse specifically appears to compound the risk of alcohol misuse (Clarke-Walper et al., Citation2014).
3.3. Illicit drug use
As with nicotine and alcohol, the original Kaiser sample (Dube et al., Citation2003) and Philippine data (Ramiro et al., Citation2010) indicate that the odds of illicit drug use and addiction increases with the number of positive ACE categories. Moreover, higher ACE-Q predicts earlier initiation of illicit drug use (Dube et al., Citation2003). Higher ACE scores also appear to elevate the risk not only of drug addiction, but the adverse psychiatric consequences associated with drug use: Among methamphetamine users, those with three or more ACEs compared to those with none were 4.5 times more likely to have a history of psychosis (Ding, Lin, Zhou, Yan, & He, Citation2014). In female prisoners, the increased ACE score was associated with increased levels of drug use and suicidal ideation (Friestad, Ase-Bente, & Kjelsberg, Citation2014).
4. The ACE-Q and non-specific neuropsychiatric symptoms and behaviors
4.1. Suicide
General and specific population studies have shown that ACE score correlates with both suicidal ideation and attempts (Corcoran et al., Citation2006; De Ravello, Abeita, & Brown, Citation2008; Friestad et al., Citation2014). In the initial ACE study cohort, while 3.8% reported ever having attempted suicide, the risk of attempt during childhood or adolescence increased in a dose–response manner from OR 1.4 for ACE score of 1 to OR 50.7 for ACE score of 7 or more, with the risk increasing by about 60% for each increased ACE score (Dube et al., Citation2001). A similar association was found when the sample was analyzed for the risk of suicide attempt during adulthood, demonstrating the persistent effects of ACEs through adulthood. Adjustment for illicit drug use, alcoholism, and depressed affect reduced the strength of the relationship, but still found a significant correlation (Dube et al., Citation2001).
4.2. Aggression, victimhood, and perpetration
A study assessing just the areas of physical abuse, sexual abuse, and having a battered mother showed that the odds of being a female victim of intimate partner violence (IPV), or being a male perpetrator of IPV, increased in a graded fashion based on the ACE-Q scores 1, 2, or 3 for the above items (Whitfield, Anda, Dube, & Felitti, Citation2003). In female Japanese juvenile detainees, a higher ACE score correlated with higher levels of measured aggression (Matsuura et al., Citation2009). Dutch youth who witnessed IPV against their mothers had a higher risk of other ACE categories, with 20% of this population having ACE scores ≥7 (Lamers-Winkelman et al., Citation2012). In an incarcerated American Indian/Alaskan Native sample, ACE exposure increased the risk of being involved in IPV in adulthood and committing violent offenses (De Ravello et al., Citation2008). A more general sample of offenders, including nonsexual child abusers, sexual offenders, and domestic violence perpetrators, had four times as many ACEs as controls (Reavis, Looman, Franco, & Rojas, Citation2013). Notably, aggression is also linked to other mental health problems; among IPV aggressors, higher ACEs, PTSD, and aggression are all interrelated (Swopes et al., Citation2013).
4.3. Pain
Pain has a prominent relationship with mood and other medical conditions. In a group of 416 psychiatric inpatients, of which 31% reported substantial pain, the subjective report of pain intensity was significantly related to higher ACE scores among both women and men (Greggersen et al., Citation2010). In women, a diagnosis of PTSD further contributed to more reporting of substantial pain (Greggersen et al., Citation2010). Back pain and chronic pain in general was also associated with having higher ACE scores (McCall-Hosenfeld, Winter, Heeren, & Liebschutz, Citation2014; Ramiro et al., Citation2010) as are headaches (R. Anda, Tietjen, Schulman, Felitti, & Croft, Citation2010; Ramiro et al., Citation2010). Within the headache spectrum, migraines as opposed to tension headaches are more tightly linked to elevated ACEs (Tietjen et al., Citation2015), also in association with elevations of general serum inflammatory biomarkers such as C-reactive protein (Tietjen, Khubchandani, Herial, & Shah, Citation2012).
4.4. Insomnia
Sleep disturbances are associated with a wide range of medical and psychiatric diseases and poor health outcomes. In a 2011 survey, in which 33% of adults self-reported poor sleep, 8 ACE categories were associated with sleep disturbances, and the odds of having sleep disturbances increased in a dose–response manner with higher ACE scores (Chapman et al., Citation2011; Ramiro et al., Citation2010). A replication of this finding also suggests linkages between smoking, frequent mental distress and sleep disturbances in adults with high ACE scores (Chapman et al., Citation2013). In a sample of patients diagnosed with primary insomnia monitored for a week with nocturnal wrist actigraphs, higher ACE scores was a predictor of problems with sleep onset latency, sleep efficiency, and increased nocturnal body movements (Bader et al., Citation2007).
4.5. Sexually transmitted diseases (STDs), sexual risk behaviors & unintended pregnancy
A study of 9,328 survey respondents found a strong, graded relationship between ACE scores and self-reported history of STDs (Hillis, Anda, Felitti, Nordenberg, & Marchbanks, Citation2000). This association was replicated across four birth cohorts (spanning 1900 to 1978), where the odds of having a STD history increased at least 1.5 times with each unit increase in ACE score (Dube et al., Citation2003).
In terms of high risk sexual behavior more generally, a study of 5,060 female survey respondents found the relative risk of having intercourse before age 15, perceiving oneself as being at risk for AIDS, and reporting 30 or more lifetime sexual partners was significantly increased by several ACE categories and total ACE score (Hillis, Anda, Felitti, & Marchbanks, Citation2001). Again, the four birth cohort study by Dube et al., found that elevated ACEs produced increased likelihoods of having >30 lifetime sexual partners (Dube et al., Citation2003).
As a reflection of early sexual activity and unprotected sex, higher ACE-Q scores are also associated with greater risk of adolescent pregnancy, or becoming a biological father in adolescence (Bellis et al., Citation2014; Hillis et al., Citation2004; Zapata et al., Citation2011). These associations are detectable in European and U.S. samples with elevations in ACE ≥1 and as a graded increase in pregnancy risk with greater ACE scores (Hillis et al., Citation2004; Zapata et al., Citation2011). In young men, higher ACE scores are also correlated in a graded relationship with the risk of impregnating an adolescent girl so that ACE-Q ≥5 more than doubles this risk (Anda et al., Citation2002). In an earlier study, the risk that a boy would impregnate an adolescent girl was strongly linked to the ACE-Q components of the boy having witnessing maternal battery and/or experiencing physical and sexual abuse; this led to the recommendation that ACE-Qs should be used with men in primary care to better target patients who should be counseled on contraception and sexual risk behavior (Anda et al., Citation2001).
Adverse childhood experiences have also been shown to affect risk of unintended pregnancies (Dietz et al., Citation1999), premature delivery (Christiaens, Hegadoren, & Olson, Citation2015; Leeners, Rath, Block, Gorres, & Tschudin, Citation2014), high-risk behaviors during pregnancy (Chung et al., Citation2010), and risk of fetal demise (Hillis et al., Citation2004). A survey of 1,476 women in a federally qualified health center encountered at a prenatal visit, and at 3 and 11 months postpartum, found that exposure to several ACE-Q categories and scores ≥2 were associated with higher rates of smoking, alcohol, and illicit drug use during pregnancy (Chung et al., Citation2010). Exposure to childhood sexual abuse carried the highest rates of risky behavior during pregnancy. Another study of 1,193 adult females with ACE-Q scores ≥4 were 1.5 times more likely to experience an unintended pregnancy, which is linked with maternal complications and poorer infant outcomes (Dietz et al., Citation1999). A sample of 9,159 women indicated that the risk of fetal demise in adolescent pregnancy is more closely to elevated ACE-Q scores in that age group, than from the biophysical risks associated purely with childbirth at this age (Hillis et al., Citation2004).
Recent Investigations have begun to characterize some of the complex neuropsychiatric and biomarker correlates of ACE exposures in expecting parents. Pregnant women with histories of childhood sexual abuse (detected by ACE-Q) and poorer perceived family functioning, show increased salivary cortisol-awakening levels (Bublitz, Parade, & Stroud, Citation2014). Pregnant women with ACE scores ≥1 also experience higher pain intensities and larger pain distributions (across body regions) in late pregnancy, which are premorbid risk factors for postpartum chronic pain syndromes (Drevin et al., Citation2015). In expecting fathers, greater ACE scores are associated with increased depression and anxiety during multiple time points of the expecting mother’s pregnancy (Skjothaug, Smith, Wentzel-Larsen, & Moe, Citation2015).
5. The ACE-Q and general health/somatic diseases
5.1. General health indicators
Higher ACE scores correlate with several poor general health-status indicators in children (Burke, Hellman, Scott, Weems, & Carrion, Citation2011; Wing, Gjelsvik, Nocera, & McQuaid, Citation2015) and adults, including increased use of prescription medications (Anda, Brown, Felitti, Dube, & Giles, Citation2008), lower quality of life measures (Corso, Edwards, Fang, & Mercy, Citation2008), lower self-appraisal of general health (Mostoufi et al., Citation2013), physical disability (Schussler-Fiorenza Rose, Xie, & Stineman, Citation2014) and increased risk of premature death (Brown et al., Citation2009). Higher ACE scores are also associated with less specific indicators of poor health including measures of mobility, homelessness, and unemployment (Dong et al., Citation2005; Dube, Cook, & Edwards, Citation2010; Liu et al., Citation2013; Patterson et al., Citation2014). Frequently moving to new homes and neighborhoods (e.g. moving more than 8 times prior to the age of 18), are associated with higher ACE scores and a range of health risk behaviors (Dong et al., Citation2005).
Poor diet, low exercise, and various indicators of allostatic load (exposure to experiences that produce physiological wear-and-tear) are also associated with elevated ACE scores (Barboza Solis et al., Citation2015; Bellis, Hughes, Leckenby, Perkins, & Lowey, Citation2014). Using a modified version of the ACE-Q international questionnaire (ACE-IQ) in a Saudi Arabian study, ACE scores ≥4 were associated with 2 to 11 fold increased the risk for chronic medical diseases and poor health behaviors (Almuneef, Qayad, Aleissa, & Albuhairan, Citation2014). Unsurprisingly, these data go along with increased ACEs predicting earlier mortality. In a sample of 17,337 adults followed prospectively for a decade (1997–2006), respondents with ACE scores ≥6 were 2.4 times more likely to die when aged ≤65 and 1.7 times more likely to die when aged ≤75 (Brown et al., Citation2009). This effect also seems to run in families, as respondents with an ACE score ≥4 were 1.8 times more likely to report a family member who died before age 65 (Anda et al., Citation2009).
5.2. Obesity
A study of 13,177 respondents showed that multiple ACE categories are associated with increased weight during adulthood even after adjustment for demographic covariates, smoking, physical activity, and alcohol consumption (Williamson, Thompson, Anda, Dietz, & Felitti, Citation2002). This study showed that an ACE-Q >4 produces relative risks of 1.22 of having a BMI ≥30 (obesity) and 1.8 of having a BMI ≥40 (severe obesity). A replication showed that ACE scores ≥4 increases the risk of morbid obesity by 3-fold (Bellis et al., Citation2014).
5.3. Autoimmune and gastro-intestinal diseases
In the original ACE cohort, there was an increased chance of being hospitalized for any of 21 autoimmune diseases as assessed via 7-year follow-up of women with ACE scores ≥3 (Dube et al., Citation2009). In a Filipino sample, an ACE score ≥4 was associated with an odds ratio of 1.5 for self-reported diabetes (Ramiro et al., Citation2010).
In a sample of 17,337 respondents in which 6.8% reported having a history of liver disease, an ACE score ≥6 was associated with an elevated risk of engaging in multiple behaviors that are major risk factors for liver disease (Dong, Dube, Felitti, Giles, & Anda, Citation2003). After controlling for those behaviors, elevated ACE scores still produced an odds ratio of 1.8 for having liver disease. A Filipino study showed similar findings (Ramiro et al., Citation2010).
5.4. Pulmonary disease
In a sample of 15,472 from the original ACE study, ACE-Q ≥5 produced an odds ratio of 2.1 for self-reported chronic obstructive pulmonary disease (COPD), even after adjusting for diabetes, obesity, smoking, and demographics; this risk increased in a dose–response manner with increasing ACE-Q score (Anda et al., Citation2008). Suggesting a gender bias in this risk, an ACE-Q ≥6 in women (but not men; N > 19,000 in both groups) that involved childhood exposure to verbal abuse, sexual abuse, living with a substance using household member, witnessing domestic violence, and parental separation/divorce, produced an increased likelihood for COPD (Cunningham et al., Citation2014).
5.5. Cardiovascular disease
Higher ACE-Q scores are correlated with cardiovascular disease risk generally, even as early as young adulthood with respect to hypertension (Su et al., Citation2014, Citation2015). In a study of 17,337 patients in which 10.6% self-reported ischemic heart disease (IHD), there was a dose-dependent relationship between IHD risk and ACE scores and significant predictive relationship between most of the ACE items and IHD risk (Dong et al., Citation2004). The adjusted odds of having IHD for subjects with ACE-Q ≥7 was 3.6. Interestingly, this study also suggested that psychiatric risk factors for IHD (e.g. depression, anger problems) were stronger mediators of the predictive relationship between ACE score and IHD compared to the more traditional risk factors (smoking, obesity, physical inactivity, diabetes, hypertension) (Dong et al., Citation2004). In replication of these findings, a Filipino survey showed the ORs of having self-reported IHD, hypertension, and stroke for respondents with ACE score ≥4 were 3.5, 1.6, and 1.2, respectively (Ramiro et al., Citation2010). Suggesting a connection between cardiovascular health, stress response physiology, and the pathogenesis of trauma-spectrum mental disorders, a study of young healthy adult women, showed that elevated ACE scores are associated with blunted corticosteroid and heart rate reactivity to psychological stress (Voellmin et al., Citation2015).
5.6. Cancer
As would be expected from associations between elevated ACE scores and health behaviors that contribute to higher risk of cancer (e.g. nicotine addiction), elevated ACE scores are associated with increased risk of cancer and worse outcomes including earlier death due to lung cancer (Brown et al., Citation2010). The risk of cancer due to ACEs does generalize to cancers beyond those involving lung tissue (Brown, Thacker, & Cohen, Citation2013; Felitti et al., Citation1998; Fuller-Thomson & Brennenstuhl, Citation2009; Holman et al., Citation2016) and are likely mediated in part (e.g. as with smoking) through high risk psychiatric and/or addiction-related behaviors. However, cancer risk in those with higher ACE scores may not be completely mediated by high-risk behaviors, e.g., childhood physical abuse is associated with significantly increased lung cancer risk, even when adjusted for smoking (Fuller-Thomson & Brennenstuhl, Citation2009). Furthermore, the adverse experience itself can be a risk factor for cancer, as is the case in women with higher ACE scores involving childhood sexual abuse who have increased the risk of developing invasive cervical cancer (Coker, Hopenhayn, DeSimone, Bush, & Crofford, Citation2009).
6. Discussion
Over two decades since the development and first use of the ACE-Q in a large population study, research using the ACE-Q and its variants has repeatedly demonstrated a linkage between ACEs and increased risk of acquiring a wide range of psychiatric disorders, addictions, and medical (multi-organ) illnesses in adulthood that ultimately involve high-cost medical care and premature death (Brown et al., Citation2009; Mokdad, Marks, Stroup, & Gerberding, Citation2004; Wilkes, Guyn, Li, Lu, & Cawthorpe, Citation2012). This literature supports the original assertion by Felitti that “adverse childhood experiences have a profound effect half a century later…transmutated from psychosocial experience into organic disease” (Felitti, Citation2002). The impact of early adverse experiences on damaging the expression of healthy parenting behavior and capacity for supporting secure rearing environments—which perpetuates the inter-generational transmission of multi-illness comorbidities associated with adverse childhood experiences—is also consistently evident in this literature (Holman et al., Citation2016; Hughes et al., Citation2017; Skjothaug et al., Citation2015).
The astounding scope of brain and body diseases that associates with high ACE-Q scores begs two key questions that relate to the instrument’s strength and weakness as a measure of a confluence of multiple types of ACEs onto an individual. First, to the extent that a confluence of ACEs is a root cause of such an incredible scope of secondary diseases, by what shared pathological mechanisms and/or developmental pathways could this occur? Second, and on the other hand, could such a diversity of secondary brain and body disease outcomes, merely reflect the non-specificity of the ACE-Q in capturing so many (and an increasing number) of genetic and environmental risk factors as ACE-Q scores increase? Next, we consider both of these as potentially concurrently true perspectives that are each important to understanding how this literature should be interpreted, applied and built upon.
Broad Implications and Theory Building: Extrapolating from Associations to Causality as Informed by Developmental Neuroscience of Trauma, Mental Illness, and Addiction
The causal connections between various forms of childhood trauma and deprivation and a wide spectrum of adult diseases are undoubtedly complex and likely involve multiple pathways. However, a wealth of animal modeling and human data suggests that the neurotoxic effects of neglect, abuse and attachment failure during childhood and adolescence—on neuroplasticity, neurogenesis, neurohormonal and neuroimmunological regulation of brain circuits that regulate emotion and motivation—biologically underpins both mental illness and addiction vulnerability (Chambers, Citation2017; Chambers, Citation2013b;Chambers, Taylor, & Potenza, Citation2003; Korosi et al., Citation2012; Oomen et al., Citation2010; Rothman et al., Citation2010; Teicher, Samson, Anderson, & Ohashi, Citation2016). At the heart of this evidence is a rapidly growing body of research revealing that early abuse and neglect negatively impacts the long-term function and development of both the 1) immune system, as it operates in both the brain and the body (Danese & Lewis, Citation2017) and the 2) neurohormonal system, inclusive of both stress and social-affiliative response systems (Heim et al., Citation2009; Pervanidou et al., Citation2007). Such an impact on just these 2 systems provides broad explanatory power in understanding how ACEs lead to such a wide array of adult co-morbidities: The immune and neurohormonal systems (inclusive of the Hypothalamic-Pitutary-Adrenal Axis) not only have key roles within both brain and multiple body organs, but they each determine how brain and body systems interact with each other, and in response to a wide array of external (environmental/biological) threats. Thus, adaptation to adverse experiences in childhood can be viewed as a multi-organ insult that can exact a price on brain and body health that becomes more broadly clinically evident with age. In terms of brain function, this cost may be reflected as impaired capacities for making healthy motivational and social decisions in the face of significant psychosocial stressors and change (Chambers, Citation2013b; Chambers & Wallingford, Citation2017). Thus, many genes and cellular systems that mediate both immune function and stress responses are also expressed in the brain as mediators of neuronal function and plasticity, especially in cognitive and motivational circuits the are disordered in mental illness and addiction (Blank & Prinz, Citation2013; Chambers et al., Citation2013). Similarly, throughout body organs, the cost of early ACEs may be exacted not only as a reflection of how brain and behavioral problems pose significant secondary risks to the health of the body (e.g. via injuries), but in terms of ACE-induced impairments in fighting off infections and cancer, and increased vulnerability to autoimmune diseases (Miller, Chen, & Parker, Citation2011; O’Mahony et al., Citation2009).
Together with the ACEs literature reviewed here, this broad-based neuroscientific evidence suggests the existence of a general causal chain and unfolding comorbidity pathway that courses up through the lifespan in four stages from (1) the social-emotional experiences of the child, to (2) the impact of these experiences on the biology and development of the brain up through adolescence, to (3) the emergence of psychiatric and substance use disorders and impulsive behaviors of young adulthood, culminating in (4) an increasingly wide range and worsening severity of toxicological damage and injuries to the brain and body organs that accumulate secondary to the underlying mental illness and addictive disorders. As suggested in Figure , which summarizes this brain-body pathogenesis in a visual-conceptual format, the ACEs-induced pathway of complex comorbidities can be conceptualized as “bugle shaped”. That is, the brain-body pathogenesis not only broadens in terms of the individual acquiring an increasingly complex array of illness comorbidities with age, but it is trans-generationally renewed and reinforced downward to the offspring. So, due to mental illnesses and addictions that resulted in part from adverse rearing conditions experienced in Stage 1, reproductively active adults in Stages 2 and 3 show deficits in parenting, protection, and attachment formation with respect to their own children.
Figure 2. Bugle-shaped ACES comorbidity pathway. Diagrammatic conceptualization of a neuroscience-informed causal pathway through developmental stages that explains how and why the ACE-Q literature broadly associates adverse childhood experiences with a wide range of adult mental illnesses, addictions, and medical comorbidities. In (1) childhood and (2) adolescence, adverse rearing environments, impaired parental behavior, and attachment failures are biologically neurotoxic to the developing brain, resulting in preclinical or emerging signs of mental illness (a arrow). In turn, mental illness-induced neurobiological vulnerability to drug addiction, leads to the onset of one or more addictions in adolescence and/or (3) young adulthood (b arrows), which further exacerbates the neurobiological and clinical dimensions of the underlying mental illness (c arrow). The mental illness/addiction comorbidity experienced during young adulthood results in chaotic reproduction and propagates down to the offspring, a new cycle of exposure to adverse rearing environments and parenting impairments. The later causal dynamic (handle of the Bugle shaped horn) represents both a transgenerational and trans-environmental-neurobiological cycle: the brain illness of the parent generates an adverse environment for the child; the adverse environment for the child causes brain illness that later manifests as adult mental illness, addiction, and impairments in parenting capabilities. With increasing age into older adulthood, the scope and severity of addictions and mental illness comorbidities worsen (the girth of the bugle enlarges) so that a larger scope and greater severity of multi-organ toxicities and injuries (i.e. chronic medical diseases) accumulate as consequences of addictions (d arrows), mental illness-induced behaviors (e arrows) and related neuroimmunological insults from early trauma.
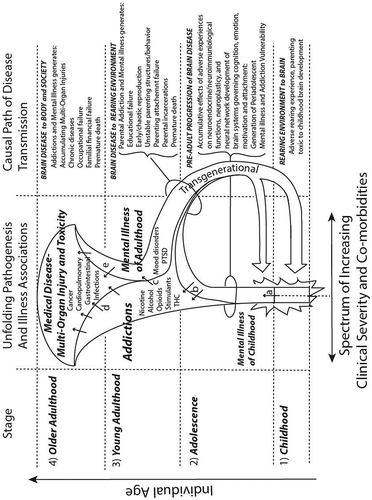
6.1. Limitations and shortfalls of the ACE-Q
A key limitation of both the ACE-Q instrument (Figure ) and the bugle-shaped comorbidity pathway hypothesis drawn from the ACEs literature (Figure ) is that they do not separate out, or comparatively quantify the different contributions of genetic, individual traumatic experiences (e.g. child sexual abuse vs. physical abuse), or general environmental circumstances (e.g. poverty, premature death of parental figures) that contribute to the overall causal pathway (Anda et al., Citation2009; Lipina & Posner, Citation2012). Indeed, an examination of individual questions in the ACE-Q (see Figure ) quickly reveals that many of the items are confounded by, or bundled together with multiple forms of quite different kinds and sources of adverse childhood exposures. For example, item 5 actually has two questions, either of which could be true to earn a single point, but with the first part talking about recalling lack of enough food and shelter, and the second part, asking about the recall of parents being negligent due to intoxication. Thus, while the ACE-Q scale is sensitive to recall of neglect broadly speaking, it sacrifices an ability to specify the form of neglect, its root causes (e.g. whether it arises from simple poverty or from the parents having genetic loading for addictions or either or both) or the overall developmental timing, and duration of these stressors. Moreover, a significant component of early developmental neuropsychiatric injury may result from exposure of fetal brains to nicotine, alcohol, opioids and many other addictive drugs, and/or psychiatric treatment medications (Behnke, Smith, Committee on Substance, Committee on, & Newborn, Citation2013; Pearlstein, Citation2013), which are all more likely to be prevalent in pre-partum women with high ACE scores, and which also can also raise the risk of peri-partum complications that impact newborn brain health.
More research using alternative or more advanced adverse experience rating instruments (e.g. as informed by the ACE-Q) and concurrent genetic testing approaches in populations assayed with the ACE-Q, are needed to separate out more fine-structural contributions of various genotypes and ecophenotypic pathways (Teicher & Samson, Citation2013) on overall adult behavioral phenotypes. This area of research holds potential for revealing a number of complex relationships between genetics and early life experiences that determine resilience, including the possibility that some genes may actually promote better brain function conditional on the individual having had adverse child experiences (Chung et al., Citation2008; Douglas et al., Citation2011; Hillis et al., Citation2010; Southwick & Charney, Citation2012). Further studies using more precise instruments than what the ACE-Q screens for, are also needed to understand how the developmental timing of adverse contexts or events, and the dose or duration of more specific ACE categories may interact with other ACEs in contributing to the bugle-shaped comorbidities pathway (Figure ). This work, which should span both animal modeling and human observational studies should also examine how neurodevelopmentally impactful events that are not directly captured on the ACE-Q scale (e.g. traumatic brain injury; in utero exposure to psychoactive substances) pathologically synergize with ACE-Q items. Finally, although the ACE-Q has been applied to studying (and has been found to show strong correlations) with a wide array of psychiatric and medical conditions, its application to understanding the more narrow set of mental health conditions that are otherwise well known to be related to traumatic experiences and problems with attachment (e.g. borderline personality) are surprisingly lacking. Future studies should do more to examine ACE-Q data in light of other attachments experiences, biomarkers, and key clinical features of the major personality disorders.
6.2. Moving beyond the ACE-Q
Regardless of these more detailed scientific questions pertaining to the nature vs. nurture debate, which remains unresolved by the ACE-Q literature, the centrality of psychiatric health to overall public health is clearly reflected in the ACEs literature. The strong dependency of adult psychiatric health and parenting capacity on freedom from exposure to high levels of adverse childhood experiences is pronounced. Yet, the U.S. health care and insurance coverage systems remain disproportionately oriented to delivering high-cost procedure and specialty care to address diseases and injuries caused by untreated mental illnesses and addictions, while at the same time the population has relatively limited access to quality services and expertise for addiction and mental health services (Chambers, Citation2013a). The public health effects of this lack of parity between medical-somatic vs. brain-behavioral health services (Wen, Cummings, Hockenberry, Gaydos, & Druss, Citation2013), may be compounded by a number of problems intrinsic to contemporary behavioral health care and research. These include the widespread segregation of mental health from addiction services and research, child from adult psychiatric services and research, and the predominant focus of modern research on discovering and addressing the genetic determinants of psychiatric disease, to the exclusion of investigations on the neurobiological impacts of adverse psychosocial experiences.
Enacting a clinical translation of conclusions we can draw from the ACEs literature, may require a significant rethinking and repositioning of behavioral health care within the core of public health and primary preventative medicine. Achieving greater integration of mental health and addiction services, and parity of these services with the rest of medical and surgical care, may be critical not only for the prevention of neuropsychiatric disorders, but an even wider range of chronic multi-organ diseases of the body, traditionally (but incorrectly) thought of as unrelated to psychiatric diseases (R. Andrew Chambers, Citation2018; Felitti, Citation2009; Hughes et al., Citation2017; Lesesne & Kennedy, Citation2005; Whitfield, Citation1998). In this rebuilding, greater integration of trauma-related mental health and addiction services and expertise, psychotherapies and medication treatments (R. A. Chambers & Wallingford, Citation2017; Sanford, Donahue, & Cosden, Citation2014) will be important. More clinical and research resources should also be directed to the care of reproductively active young adults with young children (i.e. for Stage 3 patients in Figure ), that aim to enhance both resilience and parenting skills (Larkin, Beckos, & Shields, Citation2012; Larkin & MacFarland, Citation2012; Marvin, Cooper, Hoffman, & Powell, Citation2002; Southwick & Charney, Citation2012; Ungar, Citation2013; Whitaker et al., Citation2014).
In conclusion, this review of two decades of ACE-Q research, suggests the pervasiveness and centrality of detrimental childhood experiences, and their secondary effects on brain development and parenting behavior, as key and perhaps unparallelled root causes of overall disease burden and mortality in the general population (CDC, Citation2010; Foege, Citation1998).
Additional information
Funding
Notes on contributors
R. Andrew Chambers
R. Andrew Chambers, MD (senior/corresponding author) is Associate Professor of Psychiatry, and Director of Addiction Psychiatry Training at the Indiana University (IU) School of Medicine in Indianapolis. Andy’s research on the developmental neuroscience of addiction vulnerability in mental illness seeks to combine expertise from the fields of Addiction Psychiatry (Drs. Zarse, Hulvershorn), Child Psychiatry (Drs. Neff, Yoder, Hulvershorn) and Perinatal/Attachment Psychiatry (Dr. J. Chambers). Andy’s research has recently been translated into a clinical guide: “The 2 × 4 Model: a Neuroscience-based Blueprint for the Modern Integrated Addiction and Mental Health Treatment System” Routledge, New York, 2018. The ACE-Q literature suggesting the deep and broad impact of childhood abuse and neglect on generating adult mental illness, addiction and medical diseases in large population samples, reflects routine observations in individual patients suffering with comorbid addictions and mental illnesses encountered in the IU-affiliated 2 × 4 Model Clinic in Indianapolis.
References
- Almuneef, M., Qayad, M., Aleissa, M., & Albuhairan, F. (2014). Adverse childhood experiences, chronic diseases, and risky health behaviors in Saudi Arabian adults: A pilot study. Child Abuse & Neglect, 38(11), 1787–24. doi:10.1016/j.chiabu.2014.06.003
- Anda, R., Tietjen, G., Schulman, E., Felitti, V., & Croft, J. (2010). Adverse childhood experiences and frequent headaches in adults. Headache, 50(9), 1473–1481. doi:10.1111/j.1526-4610.2010.01756.x
- Anda, R. F. (1999). Adverse childhood experiences and smoking during adolesence and adulthood. JAMA. doi:10.1001/jama.282.17.1652
- Anda, R. F., Brown, D. W., Dube, S. R., Bremner, J. D., Felitti, V. J., & Giles, W. H. (2008). Adverse childhood experiences and chronic obstructive pulmonary disease in adults. American Journal of Preventive Medicine, 34(5), 396–403. doi:10.1016/j.amepre.2008.02.002
- Anda, R. F., Brown, D. W., Felitti, V. J., Bremner, J. D., Dube, S. R., & Giles, W. H. (2007). Adverse childhood experiences and prescribed psychotropic medications in adults. American Journal of Preventive Medicine, 32(5), 389–394. doi:10.1016/j.amepre.2007.01.005
- Anda, R. F., Brown, D. W., Felitti, V. J., Dube, S. R., & Giles, W. H. (2008). Adverse childhood experiences and prescription drug use in a cohort study of adult HMO patients. BMC Public Health, 8, 198. doi:10.1186/1471-2458-8-198
- Anda, R. F., Chapman, D. P., Felitti, V. J., Edwards, V., Williamson, D. F., Croft, J. B., & Giles, W. H. (2002). Adverse childhood experiences and risk of paternity in teen pregnancy. Obstetrics and Gynecology, 100(1), 37–45.
- Anda, R. F., Dong, M., Brown, D. W., Felitti, V. J., Giles, W. H., Perry, G. S., … Dube, S. R. (2009). The relationship of adverse childhood experiences to a history of premature death of family members. BMC Public Health, 9, 106. doi:10.1186/1471-2458-9-106
- Anda, R. F., Felitti, V. J., Bremner, J. D., Walker, J. D., Whitfield, C., Perry, B. D., … Giles, W. H. (2006). The enduring effects of abuse and related adverse experiences in childhood. A convergence of evidence from neurobiology and epidemiology. European Archives of Psychiatry and Clinical Neuroscience, 256(3), 174–186. doi:10.1007/s00406-005-0624-4
- Anda, R. F., Felitti, V. J., Chapman, D. P., Croft, J. B., Williamson, D. F., Santelli, J., … Marks, J. S. (2001). Abused boys, battered mothers, and male involvement in teen pregnancy. Pediatrics, 107(2), E19.
- Anda, R. F., Fleisher, V. I., Felitti, V. J., Edwards, V. J., Whitfield, C. L., Dube, S. R., & Williamson, D. F. (2004). Childhood abuse, household dysfunction, and indicators of impaired adult worker performance. The Permanente Journal, 8(1), 30–38.
- Anda, R. F., Whitfield, C. L., Felitti, V. J., Chapman, D., Edwards, V. J., Dube, S. R., & Williamson, D. F. (2002). Adverse childhood experiences, alcoholic parents, and later risk of alcoholism and depression. Psychiatric Services (Washington, D.C.), 53(8), 1001–1009. doi:10.1176/appi.ps.53.8.1001
- Andersen, J. P., & Blosnich, J. (2013). Disparities in adverse childhood experiences among sexual minority and heterosexual adults: Results from a multi-state probability-based sample. PLoS One, 8(1), e54691. doi:10.1371/journal.pone.0054691
- Bader, K., Schafer, V., Schenkel, M., Nissen, L., Kuhl, H. C., & Schwander, J. (2007). Increased nocturnal activity associated with adverse childhood experiences in patients with primary insomnia. The Journal of Nervous and Mental Disease, 195(7), 588–595. doi:10.1097/NMD.0b013e318093ed00
- Barboza Solis, C., Kelly-Irving, M., Fantin, R., Darnaudery, M., Torrisani, J., Lang, T., & Delpierre, C. (2015). Adverse childhood experiences and physiological wear-and-tear in midlife: Findings from the 1958 British birth cohort. Proceedings of the National Academy of Sciences of the United States of America, 112(7), E738–746. doi:10.1073/pnas.1417325112
- Bebbington, P., Jonas, S., Kuipers, E., King, M., Cooper, C., Brugha, T., … Jenkins, R. (2011). Childhood sexual abuse and psychosis: Data from a cross-sectional national psychiatric survey in England. British Journal of Psychiatry, 199(1), 29–37. doi:10.1192/bjp.bp.110.083642
- Committee on Substance, A., Committee on, F., Behnke, M., & Smith, V. C., Newborn. (2013). Prenatal substance abuse: Short- and long-term effects on the exposed fetus. Pediatrics, 131(3), e1009–1024. doi:10.1542/peds.2012-3931.
- Bellis, M. A., Hughes, K., Leckenby, N., Perkins, C., & Lowey, H. (2014). National household survey of adverse childhood experiences and their relationship with resilience to health-harming behaviors in England. BMC Medicine, 12, 72. doi:10.1186/1741-7015-12-72
- Bellis, M. A., Lowey, H., Leckenby, N., Hughes, K., & Harrison, D. (2014). Adverse childhood experiences: Retrospective study to determine their impact on adult health behaviours and health outcomes in a UK population. Journal of Public Health (Oxford, England), 36(1), 81–91. doi:10.1093/pubmed/fdt038
- Benedetti, F., Bollettini, I., Radaelli, D., Poletti, S., Locatelli, C., Falini, A., … Colombo, C. (2014). Adverse childhood experiences influence white matter microstructure in patients with bipolar disorder. Psychological Medicine, 44(14), 3069–3082. doi:10.1017/S0033291714000506
- Benedetti, F., Poletti, S., Radaelli, D., Pozzi, E., Giacosa, C., Ruffini, C., … Smeraldi, E. (2012). Caudate gray matter volume in obsessive-compulsive disorder is influenced by adverse childhood experiences and ongoing drug treatment. Journal of Clinical Psychopharmacology, 32(4), 544–547. doi:10.1097/JCP.0b013e31825cce05
- Benedetti, F., Poletti, S., Radaelli, D., Pozzi, E., Giacosa, C., & Smeraldi, E. (2014). Adverse childhood experiences and gender influence treatment seeking behaviors in obsessive-compulsive disorder. Comprehensive Psychiatry, 55(2), 298–301. doi:10.1016/j.comppsych.2013.08.028
- Bernstein, D. P., Ahluvalia, T., Pogge, D., & Handelsman, L. (1997). Validity of the childhood trauma questionnaire in an adolescent psychiatric population. Journal of the American Academy of Child and Adolescent Psychiatry, 36(3), 340–348. doi:10.1097/00004583-199703000-00012
- Blank, T., & Prinz, M. (2013). Microglia as modulators of cognition and neuropsychiatric disorders. Glia, 61(1), 62–70. doi:10.1002/glia.22372
- Blosnich, J. R., Dichter, M. E., Cerulli, C., Batten, S. V., & Bossarte, R. M. (2014). Disparities in adverse childhood experiences among individuals with a history of military service. JAMA Psychiatry (Chicago, Ill.), 71(9), 1041–1048. doi:10.1001/jamapsychiatry.2014.724
- Bombay, A., Matheson, K., & Anisman, H. (2011). The impact of stressors on second generation Indian Residential School survivors. Transcultural Psychiatry, 48(4), 367–391. doi:10.1177/1363461511410240
- Bowlby, J. (1984). Violence in the family as a disorder of the attachment and caregiving systems. American Journal of Psychoanalysis, 44(1), 9–27, 29–31.
- Bowlby, R. (2017). Growing up with attachment theory-a personal view. Psychodynamic Psychiatry, 45(4), 431–439. doi:10.1521/pdps.2017.45.4.431
- Brown, D. W., Anda, R. F., Edwards, V. J., Felitti, V. J., Dube, S. R., & Giles, W. H. (2007). Adverse childhood experiences and childhood autobiographical memory disturbance. Child Abuse & Neglect, 31(9), 961–969. doi:10.1016/j.chiabu.2007.02.011
- Brown, D. W., Anda, R. F., Felitti, V. J., Edwards, V. J., Malarcher, A. M., Croft, J. B., & Giles, W. H. (2010). Adverse childhood experiences are associated with the risk of lung cancer: A prospective cohort study. BMC Public Health, 10, 20. doi:10.1186/1471-2458-10-20
- Brown, D. W., Anda, R. F., Tiemeier, H., Felitti, V. J., Edwards, V. J., Croft, J. B., & Giles, W. H. (2009). Adverse childhood experiences and the risk of premature mortality. American Journal of Preventive Medicine, 37(5), 389–396. doi:10.1016/j.amepre.2009.06.021
- Brown, M. J., Thacker, L. R., & Cohen, S. A. (2013). Association between adverse childhood experiences and diagnosis of cancer. PLoS One, 8(6), e65524. doi:10.1371/journal.pone.0065524
- Bruskas, D., & Tessin, D. H. (2013). Adverse childhood experiences and psychosocial well-being of women who were in foster care as children. The Permanente Journal, 17(3), e131–141. doi:10.7812/TPP/12-121
- Bublitz, M. H., Parade, S., & Stroud, L. R. (2014). The effects of childhood sexual abuse on cortisol trajectories in pregnancy are moderated by current family functioning. Biological Psychology, 103, 152–157. doi:10.1016/j.biopsycho.2014.08.014
- Burke, N. J., Hellman, J. L., Scott, B. G., Weems, C. F., & Carrion, V. G. (2011). The impact of adverse childhood experiences on an urban pediatric population. Child Abuse & Neglect, 35(6), 408–413. doi:10.1016/j.chiabu.2011.02.006
- Cavanaugh, C. E., Petras, H., & Martins, S. S. (2015). Gender-specific profiles of adverse childhood experiences, past year mental and substance use disorders, and their associations among a national sample of adults in the United States. Social Psychiatry and Psychiatric Epidemiology, 50(8), 1257–1266. doi:10.1007/s00127-015-1024-3
- CDC. (2010). Adverse childhood experiences reported by adults — Five states, 2009. MMWR. Morbidity and Mortality Weekly Report, 59(49), 1609–1613.
- Chambers, J. (2017). The neurobiology of attachment: From infancy to clinical outcomes. Psychodynamic Psychiatry, 45(4), 542–563. doi:10.1521/pdps.2017.45.4.542
- Chambers, R. A. (2013a). The addiction psychiatrist as dual diagonsis physician: A profession in great need and greatly needed. Journal of Dual Diagnosis, 9(3), 260–266. doi:10.1080/15504263.2013.807072
- Chambers, R. A. (2013b). Adult hippocampal neurogenesis in the pathogenesis of addiction and dual diagnosis disorders. Drug and Alcohol Dependence, 130(1–3), 1–12. doi:10.1016/j.drugalcdep.2012.12.005
- Chambers, R. A. (2018). The 2 × 4 model: A neuroscience-based blueprint for the modern integrated addiction and mental health treatment system (First ed.). New York, NY: Routledge.
- Chambers, R. A., McClintick, J. N., Sentir, A. M., Berg, S. A., Runyan, M., Choi, K. H., & Edenberg, H. J. (2013). Cortical-striatal gene expression in neonatal hippocampal lesion (NVHL)-amplified cocaine sensitization. Genes, Brain, & Behavior, 12(5), 564–575. doi:10.1111/gbb.12051
- Chambers, R. A., Taylor, J. R., & Potenza, M. N. (2003). Developmental neurocircuitry of motivation in adolescence: A critical period of addiction vulnerability. The American Journal of Psychiatry, 160, 1041–1052. doi:10.1176/appi.ajp.160.6.1041
- Chambers, R. A., & Wallingford, S. C. (2017). On mourning and recovery: Integrating stages of grief and change toward a neuroscience-based model of attachment adaptation in addiction treatment. Psychodynamic Psychiatry, 45(4), 451–473. doi:10.1521/pdps.2017.45.4.451
- Chapman, D. P., Liu, Y., Presley-Cantrell, L. R., Edwards, V. J., Wheaton, A. G., Perry, G. S., & Croft, J. B. (2013). Adverse childhood experiences and frequent insufficient sleep in 5 U.S. States, 2009: A retrospective cohort study. BMC Public Health, 13, 3. doi:10.1186/1471-2458-13-3
- Chapman, D. P., Wheaton, A. G., Anda, R. F., Croft, J. B., Edwards, V. J., Liu, Y., … Perry, G. S. (2011). Adverse childhood experiences and sleep disturbances in adults. Sleep Medicine, 12(8), 773–779. doi:10.1016/j.sleep.2011.03.013
- Chapman, D. P., Whitfield, C. L., Felitti, V. J., Dube, S. R., Edwards, V. J., & Anda, R. F. (2004). Adverse childhood experiences and the risk of depressive disorders in adulthood. Journal of Affective Disorders, 82(2), 217–225. doi:10.1016/j.jad.2003.12.013
- Christiaens, I., Hegadoren, K., & Olson, D. M. (2015). Adverse childhood experiences are associated with spontaneous preterm birth: A case-control study. BMC Medicine, 13, 124. doi:10.1186/s12916-015-0353-0
- Chung, E. K., Mathew, L., Elo, I. T., Coyne, J. C., & Culhane, J. F. (2008). Depressive symptoms in disadvantaged women receiving prenatal care: The influence of adverse and positive childhood experiences. Ambulatory Pediatrics: The Official Journal of the Ambulatory Pediatric Association, 8(2), 109–116. doi:10.1016/j.ambp.2007.12.003
- Chung, E. K., Mathew, L., Rothkopf, A. C., Elo, I. T., Coyne, J. C., & Culhane, J. F. (2009). Parenting attitudes and infant spanking: The influence of childhood experiences. Pediatrics, 124(2), e278–286. doi:10.1542/peds.2008-3247
- Chung, E. K., Nurmohamed, L., Mathew, L., Elo, I. T., Coyne, J. C., & Culhane, J. F. (2010). Risky health behaviors among mothers-to-be: The impact of adverse childhood experiences. Academic Pediatrics, 10(4), 245–251. doi:10.1016/j.acap.2010.04.003
- Clarke-Walper, K., Riviere, L. A., & Wilk, J. E. (2014). Alcohol misuse, alcohol-related risky behaviors, and childhood adversity among soldiers who returned from Iraq or Afghanistan. Addictive Behaviors, 39(2), 414–419. doi:10.1016/j.addbeh.2013.05.001
- Coker, A. L., Hopenhayn, C., DeSimone, C. P., Bush, H. M., & Crofford, L. (2009). Violence against women raises risk of cervical cancer. Journal of Women’s Health (2002), 18(8), 1179–1185. doi:10.1089/jwh.2008.1048
- Corbin, T. J., Purtle, J., Rich, L. J., Rich, J. A., Adams, E. J., Yee, G., & Bloom, S. L. (2013). The prevalence of trauma and childhood adversity in an urban, hospital-based violence intervention program. Journal of Health Care for the Poor and Underserved, 24(3), 1021–1030. doi:10.1353/hpu.2013.0120
- Corcoran, P., Gallagher, J., Keeley, H. S., Arensman, E., & Perry, I. J. (2006). Adverse childhood experiences and lifetime suicide ideation: A cross-sectional study in a non-psychiatric hospital setting. Irish Medical Journal, 99(2), 42–45.
- Corso, P. S., Edwards, V. J., Fang, X., & Mercy, J. A. (2008). Health-related quality of life among adults who experienced maltreatment during childhood. American Journal of Public Health, 98(6), 1094–1100. doi:10.2105/AJPH.2007.119826
- Cunningham, T. J., Ford, E. S., Croft, J. B., Merrick, M. T., Rolle, I. V., & Giles, W. H. (2014). Sex-specific relationships between adverse childhood experiences and chronic obstructive pulmonary disease in five states. International Journal of Chronic Obstructive Pulmonary Disease, 9, 1033–1042. doi:10.2147/COPD.S68226
- Danese, A., & Lewis, S. J. (2017). Psychoneuroimmunology of early-life stress: The hidden wounds of childhood trauma? Neuropsychopharmacology, 42(1), 99–114. doi:10.1038/npp.2016.198
- De Ravello, L., Abeita, J., & Brown, P. (2008). Breaking the cycle/mending the hoop: Adverse childhood experiences among incarcerated American Indian/Alaska native women in New Mexico. Health Care for Women International, 29(3), 300–315. doi:10.1080/07399330701738366
- Dietz, P. M., Spitz, A. M., Anda, R. F., Williamson, D. F., McMahon, P. M., Santelli, J. S., … Kendrick, J. S. (1999). Unintended pregnancy among adult women exposed to abuse or household dysfunction during their childhood. JAMA, 282(14), 1359–1364.
- Ding, Y., Lin, H., Zhou, L., Yan, H., & He, N. (2014). Adverse childhood experiences and interaction with methamphetamine use frequency in the risk of methamphetamine-associated psychosis. Drug and Alcohol Dependence, 142, 295–300. doi:10.1016/j.drugalcdep.2014.06.042
- Dong, M., Anda, R. F., Dube, S. R., Giles, W. H., & Felitti, V. J. (2003). The relationship of exposure to childhood sexual abuse to other forms of abuse, neglect, and household dysfunction during childhood. Child Abuse & Neglect, 27(6), 625–639.
- Dong, M., Anda, R. F., Felitti, V. J., Dube, S. R., Williamson, D. F., Thompson, T. J., … Giles, W. H. (2004). The interrelatedness of multiple forms of childhood abuse, neglect, and household dysfunction. Child Abuse & Neglect, 28(7), 771–784. doi:10.1016/j.chiabu.2004.01.008
- Dong, M., Anda, R. F., Felitti, V. J., Williamson, D. F., Dube, S. R., Brown, D. W., & Giles, W. H. (2005). Childhood residential mobility and multiple health risks during adolescence and adulthood: The hidden role of adverse childhood experiences. Archives of Pediatrics & Adolescent Medicine, 159(12), 1104–1110. doi:10.1001/archpedi.159.12.1104
- Dong, M., Dube, S. R., Felitti, V. J., Giles, W. H., & Anda, R. F. (2003). Adverse childhood experiences and self-reported liver disease: New insights into the causal pathway. Archives of Internal Medicine, 163(16), 1949–1956. doi:10.1001/archinte.163.16.1949
- Dong, M., Giles, W. H., Felitti, V. J., Dube, S. R., Williams, J. E., Chapman, D. P., & Anda, R. F. (2004). Insights into causal pathways for ischemic heart disease: Adverse childhood experiences study. Circulation, 110(13), 1761–1766. doi:10.1161/01.CIR.0000143074.54995.7F
- Douglas, K., Chan, G., Gelernter, J., Arias, A. J., Anton, R. F., Poling, J., … Kranzler, H. R. (2011). 5-HTTLPR as a potential moderator of the effects of adverse childhood experiences on risk of antisocial personality disorder. Psychiatric Genetics, 21(5), 240–248. doi:10.1097/YPG.0b013e3283457c15
- Drevin, J., Stern, J., Annerback, E. M., Peterson, M., Butler, S., Tyden, T., … Kristiansson, P. (2015). Adverse childhood experiences influence development of pain during pregnancy. Acta Obstetricia Et Gynecologica Scandinavica, 94(8), 840–846. doi:10.1111/aogs.12674
- Dube, S. R., Anda, R. F., Felitti, V. J., Chapman, D. P., Williamson, D. F., & Giles, W. H. (2001). Childhood abuse, household dysfunction, and the risk of attempted suicide throughout the life span: Findings from the Adverse Childhood Experiences Study. JAMA, 286(24), 3089–3096.
- Dube, S. R., Anda, R. F., Felitti, V. J., Edwards, V. J., & Williamson, D. F. (2002). Exposure to abuse, neglect, and household dysfunction among adults who witnessed intimate partner violence as children: Implications for health and social services. Violence and Victims, 17(1), 3–17.
- Dube, S. R., Anda, R. F., Whitfield, C. L., Brown, D. W., Felitti, V. J., Dong, M., & Giles, W. H. (2005). Long-term consequences of childhood sexual abuse by gender of victim. American Journal of Preventive Medicine, 28(5), 430–438. doi:10.1016/j.amepre.2005.01.015
- Dube, S. R., Cook, M. L., & Edwards, V. J. (2010). Health-related outcomes of adverse childhood experiences in Texas, 2002. Preventing Chronic Disease, 7(3), A52.
- Dube, S. R., Fairweather, D., Pearson, W. S., Felitti, V. J., Anda, R. F., & Croft, J. B. (2009). Cumulative childhood stress and autoimmune diseases in adults. Psychosomatic Medicine, 71(2), 243–250. doi:10.1097/PSY.0b013e3181907888
- Dube, S. R., Felitti, V. J., Dong, M., Chapman, D. P., Giles, W. H., & Anda, R. F. (2003). Childhood abuse, neglect, and household dysfunction and the risk of illicit drug use: The adverse childhood experiences study. Pediatrics, 111(3), 564–572.
- Dube, S. R., Felitti, V. J., Dong, M., Giles, W. H., & Anda, R. F. (2003). The impact of adverse childhood experiences on health problems: Evidence from four birth cohorts dating back to 1900. Preventive Medicine, 37(3), 268–277.
- Dube, S. R., Miller, J. W., Brown, D. W., Giles, W. H., Felitti, V. J., Dong, M., & Anda, R. F. (2006). Adverse childhood experiences and the association with ever using alcohol and initiating alcohol use during adolescence. Journal of Adolescent Health, 38(4), 444 e441–410. doi:10.1016/j.jadohealth.2005.06.006
- Dube, S. R., Williamson, D. F., Thompson, T., Felitti, V. J., & Anda, R. F. (2004). Assessing the reliability of retrospective reports of adverse childhood experiences among adult HMO members attending a primary care clinic. Child Abuse & Neglect, 28(7), 729–737. doi:10.1016/j.chiabu.2003.08.009
- Easton, S. D. (2012). Understanding adverse childhood experiences (ACE) and their relationship to adult stress among male survivors of childhood sexual abuse. Journal of Prevention & Intervention in the Community, 40(4), 291–303. doi:10.1080/10852352.2012.707446
- Edwards, V. J., Anda, R. F., Gu, D., Dube, S. R., & Felitti, V. J. (2007). Adverse childhood experiences and smoking persistence in adults with smoking-related symptoms and illness. The Permanente Journal, 11(2), 5–13.
- Edwards, V. J., Holden, G. W., Felitti, V. J., & Anda, R. F. (2003). Relationship between multiple forms of childhood maltreatment and adult mental health in community respondents: Results from the adverse childhood experiences study. The American Journal of Psychiatry, 160(8), 1453–1460. doi:10.1176/appi.ajp.160.8.1453
- Ege, M. A., Messias, E., Thapa, P. B., & Krain, L. P. (2015). Adverse childhood experiences and geriatric depression: Results from the 2010 BRFSS. The American Journal of Geriatric Psychiatry: Official Journal of the American Association for Geriatric Psychiatry, 23(1), 110–114. doi:10.1016/j.jagp.2014.08.014
- Felitti, V. J. (1991). Long-term medical consequences of incest, rape, and molestation. Southern Medical Journal, 84(3), 328–331.
- Felitti, V. J. (1993). Childhood sexual abuse, depression, and family dysfunction in adult obese patients: A case control study. Southern Medical Journal, 86(7), 732–736.
- Felitti, V. J. (2002). [The relationship of adverse childhood experiences to adult health: Turning gold into lead]. Zeitschrift fur Psychosomatische Medizin und Psychotherapie, 48(4), 359–369.
- Felitti, V. J. (2009). Adverse childhood experiences and adult health. Academic Pediatrics, 9(3), 131–132. doi:10.1016/j.acap.2009.03.001
- Felitti, V. J., Anda, R. F., Nordenberg, D., Williamson, D. F., Spitz, A. M., Edwards, V., … Marks, J. S. (1998). Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults. The Adverse Childhood Experiences (ACE) Study. American Journal of Preventive Medicine, 14(4), 245–258.
- Finkelhor, D., Shattuck, A., Turner, H., & Hamby, S. (2013). Improving the adverse childhood experiences study scale. JAMA Pediatrics, 167(1), 70–75. doi:10.1001/jamapediatrics.2013.420
- Flaherty, E. G., Thompson, R., Litrownik, A. J., Theodore, A., English, D. J., Black, M. M., … Dubowitz, H. (2006). Effect of early childhood adversity on child health. Archives of Pediatrics & Adolescent Medicine, 160(12), 1232–1238. doi:10.1001/archpedi.160.12.1232
- Flaherty, E. G., Thompson, R., Litrownik, A. J., Zolotor, A. J., Dubowitz, H., Runyan, D. K., … Everson, M. D. (2009). Adverse childhood exposures and reported child health at age 12. Academic Pediatrics, 9(3), 150–156. doi:10.1016/j.acap.2008.11.003
- Foege, W. H. (1998). Adverse childhood experiences. A public health perspective. American Journal of Preventive Medicine, 14(4), 354–355.
- Ford, E. S., Anda, R. F., Edwards, V. J., Perry, G. S., Zhao, G., Li, C., & Croft, J. B. (2011). Adverse childhood experiences and smoking status in five states. Preventive Medicine, 53(3), 188–193. doi:10.1016/j.ypmed.2011.06.015
- Friestad, C., Ase-Bente, R., & Kjelsberg, E. (2014). Adverse childhood experiences among women prisoners: Relationships to suicide attempts and drug abuse. The International Journal of Social Psychiatry, 60(1), 40–46. doi:10.1177/0020764012461235
- Fuller-Thomson, E., & Brennenstuhl, S. (2009). Making a link between childhood physical abuse and cancer: Results from a regional representative survey. Cancer, 115(14), 3341–3350. doi:10.1002/cncr.24372
- Fuller-Thomson, E., Filippelli, J., & Lue-Crisostomo, C. A. (2013). Gender-specific association between childhood adversities and smoking in adulthood: Findings from a population-based study. Public Health, 127(5), 449–460. doi:10.1016/j.puhe.2013.01.006
- Gahm, G. A., Lucenko, B. A., Retzlaff, P., & Fukuda, S. (2007). Relative impact of adverse events and screened symptoms of posttraumatic stress disorder and depression among active duty soldiers seeking mental health care. Journal of Clinical Psychology, 63(3), 199–211. doi:10.1002/jclp.20330
- Gould, D. A., Stevens, N. G., Ward, N. G., Carlin, A. S., Sowell, H. E., & Gustafson, B. (1994). Self-reported childhood abuse in an adult population in a primary care setting. Prevalence, correlates, and associated suicide attempts. Archives of Family Medicine, 3(3), 252–256.
- Greggersen, W., Rudolf, S., Findel, C., Burow, J., Stoll, A., Ristow, J., … Schweiger, U. (2010). Pain complaints in a sample of psychiatric inpatients. General Hospital Psychiatry, 32(5), 509–513. doi:10.1016/j.genhosppsych.2010.07.003
- Haatainen, K. M., Tanskanen, A., Kylma, J., Honkalampi, K., Koivumaa-Honkanen, H., Hintikka, J., … Viinamaki, H. (2003). Gender differences in the association of adult hopelessness with adverse childhood experiences. Social Psychiatry and Psychiatric Epidemiology, 38(1), 12–17. doi:10.1007/s00127-003-0598-3
- Hardt, J., Vellaisamy, P., & Schoon, I. (2010). Sequelae of prospective versus retrospective reports of adverse childhood experiences. Psychological Reports, 107(2), 425–440. doi:10.2466/02.04.09.10.16.21.PR0.107.5.425-440
- Harlow, H. F. (1962). The effects of early experiences upon the personal-social, heterosexual, and maternal behavior of rhesus monkeys. Transactions of the American Neurological Association, 87, 9–10.
- Heim, C., Shugart, M., Craighead, W. E., & Nemeroff, C. B. (2010). Neurobiological and psychiatric consequences of child abuse and neglect. Developmental Psychobiology, 52(7), 671–690. doi:10.1002/dev.20494
- Heim, C., Young, L. J., Newport, D. J., Mletzko, T., Miller, A. H., & Nemeroff, C. B. (2009). Lower CSF oxytocin concentrations in women with a history of childhood abuse. Molecular Psychiatry, 14(10), 954–958. doi:10.1038/mp.2008.112
- Hillis, S. D., Anda, R. F., Dube, S. R., Felitti, V. J., Marchbanks, P. A., Macaluso, M., & Marks, J. S. (2010). the protective effect of family strengths in childhood against adolescent pregnancy and its long-term psychosocial consequences. The Permanente Journal, 14(3), 18–27.
- Hillis, S. D., Anda, R. F., Dube, S. R., Felitti, V. J., Marchbanks, P. A., & Marks, J. S. (2004). The association between adverse childhood experiences and adolescent pregnancy, long-term psychosocial consequences, and fetal death. Pediatrics, 113(2), 320–327.
- Hillis, S. D., Anda, R. F., Felitti, V. J., & Marchbanks, P. A. (2001). Adverse childhood experiences and sexual risk behaviors in women: A retrospective cohort study. Family Planning Perspectives, 33(5), 206–211.
- Hillis, S. D., Anda, R. F., Felitti, V. J., Nordenberg, D., & Marchbanks, P. A. (2000). Adverse childhood experiences and sexually transmitted diseases in men and women: A retrospective study. Pediatrics, 106(1), E11. doi:10.1542/peds.106.1.e11
- Holman, D. M., Ports, K. A., Buchanan, N. D., Hawkins, N. A., Merrick, M. T., Metzler, M., & Trivers, K. F. (2016). The association between adverse childhood experiences and risk of cancer in adulthood: A systematic review of the literature. Pediatrics, 138(Suppl 1), S81–S91. doi:10.1542/peds.2015-4268L
- Honkalampi, K., Hintikka, J., Haatainen, K., Koivumaa-Honkanen, H., Tanskanen, A., & Viinamaki, H. (2005). Adverse childhood experiences, stressful life events or demographic factors: Which are important in women’s depression? A 2-year follow-up population study. The Australian and New Zealand Journal of Psychiatry, 39(7), 627–632. doi:10.1111/j.1440-1614.2005.01636.x
- Hughes, K., Bellis, M. A., Hardcastle, K. A., Sethi, D., Butchart, A., Mikton, C., … Dunne, M. P. (2017). The effect of multiple adverse childhood experiences on health: A systematic review and meta-analysis. Lancet Public Health, 2(8), e356–e366. doi:10.1016/S2468-2667(17)30118-4
- Korosi, A., Naninck, E. F., Oomen, C. A., Schouten, M., Krugers, H., Fitzsimons, C., & Lucassen, P. J. (2012). Early-life stress mediated modulation of adult neurogenesis and behavior. Behavioural Brain Research, 227(2), 400–409. doi:10.1016/j.bbr.2011.07.037
- Lamers-Winkelman, F., Willemen, A. M., & Visser, M. (2012). Adverse childhood experiences of referred children exposed to intimate partner violence: Consequences for their wellbeing. Child Abuse & Neglect, 36(2), 166–179. doi:10.1016/j.chiabu.2011.07.006
- Larkin, H., Beckos, B. A., & Shields, J. J. (2012). Mobilizing resilience and recovery in response to adverse childhood experiences (ACE): A Restorative Integral Support (RIS) case study. Journal of Prevention & Intervention in the Community, 40(4), 335–346. doi:10.1080/10852352.2012.707466
- Larkin, H., Felitti, V. J., & Anda, R. F. (2014). Social work and adverse childhood experiences research: Implications for practice and health policy. Social Work in Public Health, 29(1), 1–16. doi:10.1080/19371918.2011.619433
- Larkin, H., & MacFarland, N. S. (2012). Restorative integral support (RIS) for older adults experiencing co-occurring disorders. International Journal of Aging & Human Development, 74(3), 231–241. doi:10.2190/AG.74.3.d
- Leeners, B., Rath, W., Block, E., Gorres, G., & Tschudin, S. (2014). Risk factors for unfavorable pregnancy outcome in women with adverse childhood experiences. Journal of Perinatal Medicine, 42(2), 171–178. doi:10.1515/jpm-2013-0003
- Lesesne, C. A., & Kennedy, C. (2005). Starting early: Promoting the mental health of women and girls throughout the life span. Journal of Women’s Health (2002), 14(9), 754–763. doi:10.1089/jwh.2005.14.754
- Lipina, S. J., & Posner, M. I. (2012). The impact of poverty on the development of brain networks. Frontiers in Human Neuroscience, 6, 238. doi:10.3389/fnhum.2012.00238
- Liu, Y., Croft, J. B., Chapman, D. P., Perry, G. S., Greenlund, K. J., Zhao, G., & Edwards, V. J. (2013). Relationship between adverse childhood experiences and unemployment among adults from five U.S. states. Social Psychiatry and Psychiatric Epidemiology, 48(3), 357–369. doi:10.1007/s00127-012-0554-1
- Logan-Greene, P., Green, S., Nurius, P. S., & Longhi, D. (2014). Distinct contributions of adverse childhood experiences and resilience resources: A cohort analysis of adult physical and mental health. Social Work in Health Care, 53(8), 776–797. doi:10.1080/00981389.2014.944251
- Lu, W., Mueser, K. T., Rosenberg, S. D., & Jankowski, M. K. (2008). Correlates of adverse childhood experiences among adults with severe mood disorders. Psychiatric Services (Washington, D.C.), 59(9), 1018–1026. doi:10.1176/appi.ps.59.9.1018
- Marvin, R., Cooper, G., Hoffman, K., & Powell, B. (2002). The circle of security project: Attachment-based intervention with caregiver-pre-school child dyads. Attachment & Human Development, 4(1), 107–124. doi:10.1080/14616730252982491
- Matsuura, N., Hashimoto, T., & Toichi, M. (2009). Correlations among self-esteem, aggression, adverse childhood experiences and depression in inmates of a female juvenile correctional facility in Japan. Psychiatry and Clinical Neurosciences, 63(4), 478–485. doi:10.1111/j.1440-1819.2009.01996.x
- McCall-Hosenfeld, J. S., Winter, M., Heeren, T., & Liebschutz, J. M. (2014). The association of interpersonal trauma with somatic symptom severity in a primary care population with chronic pain: Exploring the role of gender and the mental health sequelae of trauma. Journal of Psychosomatic Research, 77(3), 196–204. doi:10.1016/j.jpsychores.2014.07.011
- McCauley, J., Kern, D. E., Kolodner, K., Dill, L., Schroeder, A. F., DeChant, H. K., … Bass, E. B. (1997). Clinical characteristics of women with a history of childhood abuse: Unhealed wounds. JAMA, 277(17), 1362–1368.
- Miller, G. E., Chen, E., & Parker, K. J. (2011). Psychological stress in childhood and susceptibility to the chronic diseases of aging: Moving toward a model of behavioral and biological mechanisms. Psychological Bulletin, 137(6), 959–997. doi:10.1037/a0024768
- Mokdad, A. H., Marks, J. S., Stroup, J. S., & Gerberding, J. L. (2004). Actual causes of death in the United States, 2000. Journal of the American Medical Association, 291, 1238–1245. doi:10.1001/jama.291.10.1238
- Montgomery, A. E., Cutuli, J. J., Evans-Chase, M., Treglia, D., & Culhane, D. P. (2013). Relationship among adverse childhood experiences, history of active military service, and adult outcomes: Homelessness, mental health, and physical health. American Journal of Public Health, 103(Suppl 2), S262–268. doi:10.2105/AJPH.2013.301474
- Mostoufi, S. M., Strachan, E., Chopko, L., Succop, A., Martinez, B., Ahumada, S. M., & Afari, N. (2013). Adverse childhood experiences, health perception, and the role of shared familial factors in adult twins. Child Abuse & Neglect, 37(11), 910–916. doi:10.1016/j.chiabu.2013.06.005
- Nijenhuis, E. R. S., Van der Hart, O., & Kruger, K. (2002). The psychometric characteristics of the Traumatic Experiences Questionnaire (TEC): First findings among psychiatric outpatients. Clinical Psychology and Psychotherapy, 9(3), 200–210. doi:10.1002/cpp.332
- Nurius, P. S., Logan-Greene, P., & Green, S. (2012). Adverse childhood experiences (ACE) within a social disadvantage framework: Distinguishing unique, cumulative, and moderated contributions to adult mental health. Journal of Prevention & Intervention in the Community, 40(4), 278–290. doi:10.1080/10852352.2012.707443
- O’Mahony, S. M., Marchesi, J. R., Scully, P., Codling, C., Ceolho, A. M., Quigley, E. M., … Dinan, T. G. (2009). Early life stress alters behavior, immunity, and microbiota in rats: Implications for irritable bowel syndrome and psychiatric illnesses. Biological Psychiatry, 65(3), 263–267. doi:10.1016/j.biopsych.2008.06.026
- Oomen, C. A., Soeters, H., Audureau, N., Vermunt, L., van Hasselt, F. N., Manders, E. M., … Krugers, H. (2010). Severe early life stress hampers spatial learning and neurogenesis, but improves hippocampal synaptic plasticity and emotional learning under high-stress conditions in adulthood. The Journal of Neuroscience: the Official Journal of the Society for Neuroscience, 30(19), 6635–6645. doi:10.1523/JNEUROSCI.0247-10.2010
- Patterson, M. L., Moniruzzaman, A., & Somers, J. M. (2014). Setting the stage for chronic health problems: Cumulative childhood adversity among homeless adults with mental illness in Vancouver, British Columbia. BMC Public Health, 14, 350. doi:10.1186/1471-2458-14-350
- Pearlstein, T. (2013). Use of psychotropic medication during pregnancy and the postpartum period. Womens Health (London), 9(6), 605–615. doi:10.2217/whe.13.54
- Pervanidou, P., Kolaitis, G., Charitaki, S., Lazaropoulou, C., Papassotiriou, I., Hindmarsh, P., … Chrousos, G. P. (2007). The natural history of neuroendocrine changes in pediatric posttraumatic stress disorder (PTSD) after motor vehicle accidents: Progressive divergence of noradrenaline and cortisol concentrations over time. Biological Psychiatry, 62(10), 1095–1102. doi:10.1016/j.biopsych.2007.02.008
- Ramiro, L. S., Madrid, B. J., & Brown, D. W. (2010). Adverse childhood experiences (ACE) and health-risk behaviors among adults in a developing country setting. Child Abuse & Neglect, 34(11), 842–855. doi:10.1016/j.chiabu.2010.02.012
- Raposo, S. M., Mackenzie, C. S., Henriksen, C. A., & Afifi, T. O. (2014). Time does not heal all wounds: Older adults who experienced childhood adversities have higher odds of mood, anxiety, and personality disorders. The American Journal of Geriatric Psychiatry: Official Journal of the American Association for Geriatric Psychiatry, 22(11), 1241–1250. doi:10.1016/j.jagp.2013.04.009
- Reavis, J. A., Looman, J., Franco, K. A., & Rojas, B. (2013). Adverse childhood experiences and adult criminality: How long must we live before we possess our own lives? The Permanente Journal, 17(2), 44–48. doi:10.7812/TPP/12-072
- Roh, S., Burnette, C. E., Lee, K. H., Lee, Y. S., Easton, S. D., & Lawler, M. J. (2015). Risk and protective factors for depressive symptoms among American Indian older adults: Adverse childhood experiences and social support. Aging & mental health, 19(4), 371–380. doi:10.1080/13607863.2014.938603
- Rothman, E. F., Bernstein, J., & Strunin, L. (2010). Why might adverse childhood experiences lead to underage drinking among US youth? Findings from an emergency department-based qualitative pilot study. Substance Use & Misuse, 45(13), 2281–2290. doi:10.3109/10826084.2010.482369
- Rothman, E. F., Edwards, E. M., Heeren, T., & Hingson, R. W. (2008). Adverse childhood experiences predict earlier age of drinking onset: Results from a representative US sample of current or former drinkers. Pediatrics, 122(2), e298–304. doi:10.1542/peds.2007-3412
- Rutter, M., Andersen-Wood, L., Beckett, C., Bredenkamp, D., Castle, J., Groothues, C., … O’Connor, T. G. (1999). Quasi-autistic patterns following severe early global privation. English and Romanian Adoptees (ERA) Study Team. Journal of Child Psychology and Psychiatry, and Allied Disciplines, 40(4), 537–549.
- Sacco, K. A., Head, C. A., Vessicchio, J. C., Easton, C. J., Prigerson, H. G., & George, T. P. (2007). Adverse childhood experiences, smoking and mental illness in adulthood: A preliminary study. Annals of Clinical Psychiatry: Official Journal of the American Academy of Clinical Psychiatrists, 19(2), 89–97. doi:10.1080/10401230701334762
- Sanders, B., & Becker-Lausen, E. (1995). The measurement of psychological maltreatment: Early data on the child abuse and trauma scale. Child Abuse & Neglect, 19(3), 315–323.
- Sanford, A., Donahue, M., & Cosden, M. (2014). Consumer perceptions of trauma assessment and intervention in substance abuse treatment. Journal of Substance Abuse Treatment, 47(3), 233–238. doi:10.1016/j.jsat.2014.05.011
- Schussler-Fiorenza Rose, S. M., Xie, D., & Stineman, M. (2014). Adverse childhood experiences and disability in U.S. adults. PM & R: the Journal of Injury, Function, and Rehabilitation, 6(8), 670–680. doi:10.1016/j.pmrj.2014.01.013
- Sheridan, M. A., & McLaughlin, K. A. (2014). Dimensions of early experience and neural development: Deprivation and threat. Trends in Cognitive Sciences, 18(11), 580–585. doi:10.1016/j.tics.2014.09.001
- Skjothaug, T., Smith, L., Wentzel-Larsen, T., & Moe, V. (2015). Prospective fathers’ adverse childhood experiences, pregnancy-related anxiety, and depression during pregnancy. Infant Mental Health Journal, 36(1), 104–113. doi:10.1002/imhj.21485
- Southwick, S. M., & Charney, D. S. (2012). The science of resilience: Implications for the prevention and treatment of depression. Science, 338(6103), 79–82. doi:10.1126/science.1222942
- Springs, F. E., & Friedrich, W. N. (1992). Health risk behaviors and medical sequelae of childhood sexual abuse. Mayo Clinic Proceedings. Mayo Clinic, 67(6), 527–532.
- Stamoulis, C., Vanderwert, R. E., Zeanah, C. H., Fox, N. A., & Nelson, C. A. (2017). Neuronal networks in the developing brain are adversely modulated by early psychosocial neglect. Journal of Neurophysiology, 118(4), 2275–2288. doi:10.1152/jn.00014.2017
- Straus, M. A. (1998). Identification of child maltreatment with the parent-child conflict tactics scales. Child Abuse & Neglect, 22(4), 249–270.
- Strine, T. W., Dube, S. R., Edwards, V. J., Prehn, A. W., Rasmussen, S., Wagenfeld, M., … Croft, J. B. (2012). Associations between adverse childhood experiences, psychological distress, and adult alcohol problems. American Journal of Health Behavior, 36(3), 408–423. doi:10.5993/AJHB.36.3.11
- Strine, T. W., Edwards, V. J., Dube, S. R., Wagenfeld, M., Dhingra, S., Prehn, A. W., … Croft, J. B. (2012). The mediating sex-specific effect of psychological distress on the relationship between adverse childhood experiences and current smoking among adults. Substance Abuse Treatment, Prevention, and Policy, 7, 30. doi:10.1186/1747-597X-7-30
- Su, S., Wang, X., Kapuku, G. K., Treiber, F. A., Pollock, D. M., Harshfield, G. A., … Pollock, J. S. (2014). Adverse childhood experiences are associated with detrimental hemodynamics and elevated circulating endothelin-1 in adolescents and young adults. Hypertension, 64(1), 201–207. doi:10.1161/HYPERTENSIONAHA.113.02755
- Su, S., Wang, X., Pollock, J. S., Treiber, F. A., Xu, X., Snieder, H., … Harshfield, G. A. (2015). Adverse childhood experiences and blood pressure trajectories from childhood to young adulthood: The Georgia stress and Heart study. Circulation, 131(19), 1674–1681. doi:10.1161/CIRCULATIONAHA.114.013104
- Sullivan, R. M. (2017). Attachment figure’s regulation of infant brain and behavior. Psychodynamic Psychiatry, 45(4), 475–498. doi:10.1521/pdps.2017.45.4.475
- Swopes, R. M., Simonet, D. V., Jaffe, A. E., Tett, R. P., & Davis, J. L. (2013). Adverse childhood experiences, posttraumatic stress disorder symptoms, and emotional intelligence in partner aggression. Violence and Victims, 28(3), 513–530.
- Teicher, M. H., Anderson, C. M., & Polcari, A. (2012). Childhood maltreatment is associated with reduced volume in the hippocampal subfields CA3, dentate gyrus, and subiculum. Proceedings of the National Academy of Sciences of the United States of America, 109(9), E563–572. doi:10.1073/pnas.1115396109
- Teicher, M. H., & Samson, J. A. (2013). Childhood maltreatment and psychopathology: A case for ecophenotypic variants as clinically and neurobiologically distinct subtypes. American Journal of Psychiatry, 170(10), 1114–1133. doi:10.1176/appi.ajp.2013.12070957
- Teicher, M. H., Samson, J. A., Anderson, C. M., & Ohashi, K. (2016). The effects of childhood maltreatment on brain structure, function and connectivity. Nature Reviews. Neuroscience, 17(10), 652–666. doi:10.1038/nrn.2016.111. [pii].
- Thompson, R., Flaherty, E. G., English, D. J., Litrownik, A. J., Dubowitz, H., Kotch, J. B., & Runyan, D. K. (2014). Trajectories of adverse childhood experiences and self-reported health at age 18. Academic Pediatrics. doi:10.1016/j.acap.2014.09.010
- Tietjen, G. E., Buse, D. C., Fanning, K. M., Serrano, D., Reed, M. L., & Lipton, R. B. (2015). Recalled maltreatment, migraine, and tension-type headache: Results of the AMPP study. Neurology, 84(2), 132–140. doi:10.1212/WNL.0000000000001120
- Tietjen, G. E., Khubchandani, J., Herial, N. A., & Shah, K. (2012). Adverse childhood experiences are associated with migraine and vascular biomarkers. Headache, 52(6), 920–929. doi:10.1111/j.1526-4610.2012.02165.x
- Ungar, M. (2013). Resilience after maltreatment: The importance of social services as facilitators of positive adaptation. Child Abuse & Neglect, 37(2–3), 110–115. doi:10.1016/j.chiabu.2012.08.004
- Van Niel, C., Pachter, L. M., Wade, R., Jr., Felitti, V. J., & Stein, M. T. (2014). Adverse events in children: Predictors of adult physical and mental conditions. Journal of Developmental and Behavioral Pediatrics: JDBP, 35(8), 549–551. doi:10.1097/DBP.0000000000000102
- Vander Weg, M. W. (2011). Adverse childhood experiences and cigarette smoking: The 2009 Arkansas and Louisiana behavioral risk factor surveillance systems. Nicotine & Tobacco Research, 13(7), 616–622. doi:10.1093/ntr/ntr023
- Voellmin, A., Winzeler, K., Hug, E., Wilhelm, F. H., Schaefer, V., Gaab, J., … Bader, K. (2015). Blunted endocrine and cardiovascular reactivity in young healthy women reporting a history of childhood adversity. Psychoneuroendocrinology, 51, 58–67. doi:10.1016/j.psyneuen.2014.09.008
- Walsh, E. G., & Cawthon, S. W. (2014). The mediating role of depressive symptoms in the relationship between adverse childhood experiences and smoking. Addictive Behaviors, 39(10), 1471–1476. doi:10.1016/j.addbeh.2014.05.020
- Wen, H., Cummings, J. R., Hockenberry, J. M., Gaydos, L. M., & Druss, B. G. (2013). State parity laws and access to treatment for substance use disorder in the United States: Implications for federal parity legislation. JAMA Psychiatry (Chicago, Ill.), 70(12), 1355–1362. doi:10.1001/jamapsychiatry.2013.2169
- Whitaker, R. C., Dearth-Wesley, T., Gooze, R. A., Becker, B. D., Gallagher, K. C., & McEwen, B. S. (2014). Adverse childhood experiences, dispositional mindfulness, and adult health. Preventive Medicine, 67, 147–153. doi:10.1016/j.ypmed.2014.07.029
- Whitfield, C. L. (1998). Adverse childhood experiences and trauma. American Journal of Preventive Medicine, 14(4), 361–364.
- Whitfield, C. L., Dube, S. R., Felitti, V. J., & Anda, R. F. (2005). Adverse childhood experiences and hallucinations. Child Abuse & Neglect, 29(7), 797–810. doi:10.1016/j.chiabu.2005.01.004
- Whitfield, C. L., Anda, RF, Dube, SR, & Felitti, VJ (2003). Violent childhood experiences and the risk of intimate partner violence in adults: Assessment in a large health maintenance organization. Journal of Interpersonal Violence, 18(2), 166–185. doi:10.1177/0886260502238733
- Wilkes, T. C., Guyn, L., Li, B., Lu, M., & Cawthorpe, D. (2012). Association of child and adolescent psychiatric disorders with somatic or biomedical diagnoses: Do population-based utilization study results support the adverse childhood experiences study? The Permanente Journal, 16(2), 23–26.
- Williamson, D. F., Thompson, T. J., Anda, R. F., Dietz, W. H., & Felitti, V. (2002). Body weight and obesity in adults and self-reported abuse in childhood. International Journal of Obesity and Related Metabolic Disorders: Journal of the International Association for the Study of Obesity, 26(8), 1075–1082. doi:10.1038/sj.ijo.0802038
- Wing, R., Gjelsvik, A., Nocera, M., & McQuaid, E. L. (2015). Association between adverse childhood experiences in the home and pediatric asthma. Annals of Allergy, Asthma & Immunology: Official Publication of the American College of Allergy, Asthma, & Immunology, 114(5), 379–384. doi:10.1016/j.anai.2015.02.019
- Wu, N. S., Schairer, L. C., Dellor, E., & Grella, C. (2010). Childhood trauma and health outcomes in adults with comorbid substance abuse and mental health disorders. Addictive Behaviors, 35(1), 68–71. doi:10.1016/j.addbeh.2009.09.003
- Xiao, Q., Dong, M. X., Yao, J., Li, W. X., & Ye, D. Q. (2008). Parental alcoholism, adverse childhood experiences, and later risk of personal alcohol abuse among Chinese medical students. Biomedical and Environmental Sciences: BES, 21(5), 411–419. doi:10.1016/S0895-3988(08)60062-8
- Ye, D., & Reyes-Salvail, F. (2014). Adverse childhood experiences among Hawai’i adults: Findings from the 2010 behavioral risk factor survey. Hawai’i Journal of Medicine & Public Health: a Journal of Asia Pacific Medicine & Public Health, 73(6), 181–190.
- Yehuda, R. (2001). Childhood trauma and risk for PTSD: Relationship to intergenerational effects of trauma, parental PTSD, and cortisol excretion. Development and Psychopathology, 13, 733–753.
- Yeoman, K., Safranek, T., Buss, B., Cadwell, B. L., & Mannino, D. (2013). Adverse childhood experiences and adult smoking, Nebraska, 2011. Preventing Chronic Disease, 10, E159. doi:10.5888/pcd10.130009
- Young, S. Y., Hansen, C. J., Gibson, R. L., & Ryan, M. A. (2006). Risky alcohol use, age at onset of drinking, and adverse childhood experiences in young men entering the US Marine Corps. Archives of Pediatrics & Adolescent Medicine, 160(12), 1207–1214. doi:10.1001/archpedi.160.12.1207
- Zanarini, M. C. (2000). Childhood experiences associated with the development of borderline personality disorder. The Psychiatric Clinics of North America, 23(1), 89–101.
- Zapata, L. B., Kissin, D. M., Robbins, C. L., Finnerty, E., Skipalska, H., Yorick, R. V., … Hillis, S. D. (2011). Multi-city assessment of lifetime pregnancy involvement among street youth, Ukraine. Journal of Urban Health: Bulletin of the New York Academy of Medicine, 88(4), 779–792. doi:10.1007/s11524-011-9596-z

