Abstract
Objective: The Japanese drug use system allowed the “once-daily use” of inhaled corticosteroid (ICS) fluticasone furoate (FF) combined with a long acting beta-2 agonist (LABA) vilanterol (VI) against asthma for the first time in 2013. Until then, patients with asthma had to use ICS at least twice-daily. We investigated the real-world efficacy and problems of this drug (FF/VI).
Methods: This was an open-label, uncontrolled, within-group time-series (before-after) design. Prior treatments of asthma (twice-daily use of ICS with or without LABA) were switched to once-daily use of FF/VI (200 μg/25 μg). Subjects were evaluated by lung function tests prior to, and 2–3 months after, the initiation of FF/VI. Questions on the asthma control test (ACT) and preference of drugs were asked to patients.
Results: One hundred and twenty-eight Japanese asthma outpatients were enrolled from 2014–2018 and 107 subjects completed the study. Peak flow, instantaneous flow at 75% of the forced vital capacity (V75), V50, maximum mid-expiratory flow rate, forced expiratory volume in 1 s, and ACT score in FF/VI-using subjects were significantly increased (all p < 0.01). The percent predicted vital capacity and the inspiratory reserve volume were also increased significantly (all 0.01 < p < 0.05). Ninety-three percent of subjects declared they wanted to continue FF/VI in the future. Adverse effects including hoarseness and/or uncomfortable sensations in the throat were increased (16%).
Conclusions: Once-daily use of FF/VI is a potent and effective treatment. Its effect was marked on larger airways and yielded a greater satisfaction in patients despite a higher incidence of local steroid effects.
PUBLIC INTEREST STATEMENT
Currently, the most important drug to control asthma is inhaled corticosteroids combined with a long acting beta-2 agonist (ICS/LABA). In December 2013, The Japanese drug use system approved the “once-daily use” of ICS/LABA consisting of fluticasone furoate/vilanterol (FF/VI) for the first time against asthma. Until then, patients with asthma had to use ICS/LABA twice-daily. “This is a great change,” we thought and started this study. We have investigated the real-world efficacy and problems of FF/VI since 2014. Prior treatments for asthma were switched to the once-daily use of FF/VI. Various airflow data were improved and the FF/VI target sites of bronchodilation were thought to be the larger airways. This once-daily FF/VI is a convenient, potent and long-acting drug. Many patients will be satisfied with this drug, but the strong effects related to the steroid component may increase local side effects such as hoarseness and/or uncomfortable sensation in the throat. Patients with asthma should try FF/VI at least once.
1. Introduction
Asthma is defined by a history of respiratory symptoms including wheezing, shortness of breath, chest tightness, and cough that vary over time and in intensity, together with variable expiratory airflow limitation (Global Initiative for Asthma (GINA), Citation2018; CitationWorld Health Organization). This condition is due to inflammation of the air passages in the lungs (CitationWorld Health Organization). According to World Health Organization estimates, 235 million people suffer from asthma (CitationWorld Health Organization). Complications of asthma include rhinosinusitis, gastro-esophageal reflux disease, and asthma-chronic obstructive pulmonary disease (COPD) overlap, which was recently termed ACO (Global Initiative for Asthma (GINA), Citation2018). The daily administration of inhaled corticosteroids (ICS) is recommended for the treatment of patients with persistent asthma (Global Initiative for Asthma (GINA), Citation2018; Ernst et al., Citation1992). There is an increased risk of life-threatening attacks of asthma (exacerbations) if not treated appropriately with ICS (Ernst et al., Citation1992). The Japanese drug use system approved the use of beclomethasone dipropionate, an ICS, for the first time in 1978. This first-generation ICS needed to be used four-times daily. The Japanese drug use system then approved a second ICS, fluticasone propionate (FP), in 1998 and a third ICS, budesonide, in 2002, which needed to be used twice-daily.
Maintenance treatment using a combination of ICS with long-acting β2-agonists (LABA) is currently recommended for patients at high risk of asthma exacerbations (Global Initiative for Asthma (GINA), Citation2018). In 2007, the Japanese drug use system approved the first combination of an ICS and LABA (ICS/LABA), fluticasone propionate/salmeterol (FP/S). The use of combined ICS/LABA allowed many patients with asthma to control their symptoms, but these patients had to use ICS/LABA twice-daily. In December 2013, the Japanese drug use system allowed the “once-daily use” of ICS/LABA against asthma for the first time. A newly introduced ICS/LABA consists of a combination of fluticasone furoate (FF) and vilanterol (VI). Previous reports indicate that FF is clearly different from previous ICS including fluticasone propionate, budesonide, and beclomethasone dipropionate (Allen, Bareille, & Rousell, Citation2013; Daley-Yates, Citation2015; Rossios et al., Citation2011; Salter et al., Citation2007). FF was reported to show higher glucocorticoid receptor binding affinity, and to have a stronger and longer action than previous ICS.
The combined use of FF plus VI (FF/VI) has been used against asthma worldwide (Bernstein et al., Citation2015; Devillier et al., Citation2018; Kerwin et al., Citation2018; Lin et al., Citation2015; O’Byrne et al., Citation2014; Woodcock et al., Citation2017). A randomized trial by the Salford Lung Study on asthma (n = 4725) showed that FF/VI was superior to usual care (optimized by the patient’s general practitioner) in controlling asthma as assessed by the asthma control test (ACT), without serious adverse events (Woodcock et al., Citation2017).
The aim of this study was to evaluate the real-world efficacy and problems of FF/VI for Japanese patients with asthma. A reduction in the frequency of taking medication has been demonstrated to help adherence (Coleman et al., Citation2012; Price et al., Citation2010). Better adherence would improve the condition of patients with asthma and decrease the risk of exacerbations (Global Initiative for Asthma (GINA), Citation2018; Ernst et al., Citation1992). Therefore, the once-daily use of ICS/LABA would be a good option for asthma patients if it has sufficient efficacy. Because we wanted all the patients with asthma to experience FF/VI, we performed an uncontrolled “real-world” study. After the use of FF/VI, subjects were asked which drug was better. Our hypothesis was that FF is more potent than all previous ICS, but that it might cause new problems when switching from a previous ICS. This is an observational study under the control of the Japanese drug use system since 2014.
2. Methods
2.1. Enrollment
We recruited asthma outpatients at the IUHW Shioya Hospital (in Yaita-City, Tochigi Prefecture, Japan) from September 2014 to October 2018 (IUHW ethics committee approval number according to the Declaration of Helsinki: 13-B-66). Recruitment was conducted during the outpatient service by physicians of this hospital. All subjects provided written consent before participating in this study. The registration site and number: UMIN000034466
Inclusion criteria included: age ≥ 20 years; stable asthma with the use of ICS alone or ICS/LABA. Patients had to be diagnosed with asthma by pulmonary physicians. Patients had to be already satisfied with their current treatment with regular maintenance inhalation therapy with ICS alone or ICS/LABA.
Exclusion criteria were pregnancy, unstable asthma that needed an increase in systemic steroids, a recent history of life-threatening asthma, and/or concomitant life-threatening disease.
2.2. Study procedures
This study’s design was an open-label, uncontrolled, within-group time-series (before-after). Previous treatments against asthma (twice daily use of ICS or ICS/LABA) were switched to the once-daily use of FF/VI (200 μg/25 μg). Subjects were evaluated by lung function tests prior to, and 2–3 months after the initiation of FF/VI. CHESTAC-8900 (CHEST M.I., INC., Tokyo, Japan) was used for the lung function tests. At the same time, ACT scores were recorded (Global Initiative for Asthma (GINA), Citation2018; Woodcock et al., Citation2017; Nathan et al., Citation2004). ACT is a commonly used questionnaire for patients with asthma. A higher ACT score indicates better control of asthma. At 2–3 months after the initiation of FF/VI, subjects were asked which treatment was better. The response to the question on the patient’s decision making was determined by providing qualitative responses during a conversation with the physician. The response to the questions of ACT was determined by selecting responses in a questionnaire. All adverse events and respiratory symptoms were recorded.
2.3. Data analysis
Data are shown as the mean ± standard deviation (SD). A Student’s paired t-test was used for comparisons between baseline and after FF/VI use (two-tailed). Statistical significance was set at p < 0.05. For the statistical analyses, Ekuseru-Toukei 2010 (Social Survey Research Information Co., Ltd., Tokyo, Japan) was used. The sample size was estimated according to our pilot study using data from the initial thirty subjects. The primary endpoint was peak flow data by spirometry. The expected effect size was 0.34 L/s with an SD of 0.78 L/s; therefore, the standardized effect size was 0.44 (Hulley, Cummings, Browner, Grady, & Newman, Citation2013). To have 90% power to detect significance at the 5% level (two-sided) for a one-sample t-test, approximately 57 samples was calculated to be necessary. To evaluate many other parameters, we increased the sample size to 107.
3. Results
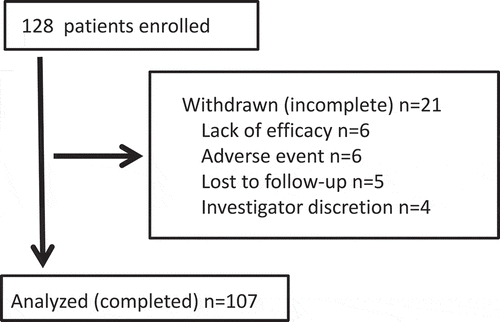
A total of 128 Japanese adult patients with asthma (63 males, 65 females; mean age: 65.6 ± 17.6 years) were enrolled from September 2014 to October 2018. Patient characteristics (male/female) at baseline included: age 65.7 ± 16.8/65.4 ± 18.6 years, height 164 ± 9/153 ± 9 cm, weight 63.6 ± 11.2/64.1 ± 55.8 kg, body mass index 23.6 ± 4.7/27.2 ± 21.1 kg/m2; frequency of concurrent smoking 5%/2%, duration of asthma ≥5years 75%/59%, number of exacerbations for 12 months before enrollment 0.3 ± 0.6/0.3 ± 0.5; comorbidity of emphysema 43%/9%, heart disease 14%/22%, cerebrovascular disease 11%/8%, hypertension 56%/51%, hyperlipidemia 30%/35%, and diabetes mellitus 24%/12%. The progress of this study is shown in Figure . One hundred and seven subjects completed the study. Among these, 93% of subjects declared that they wanted to continue FF/VI in the future. Exacerbation with the intravenous steroid treatment was observed in 2 completed subjects. An increase in the use of rescues by short acting beta-2 agonists was seen in 4 subjects, but 2 wanted to continue FF/VI. Compliance with the prescribed drug was good, but the contribution of pharmacists to this was more influential (e.g., explanations to aged patients). Among 21 incomplete subjects, 10 subjects clearly preferred previous drugs to FF/VI. Five subjects discontinued their visits; therefore, their decisions, use of rescue by short acting beta-2 agonists, and occurrence of exacerbations were unknown.
Figure 1. Progress of this study.
Among 21 incomplete subjects, 6 subjects withdrew consent because of lack of efficiency and returned to their previous drugs. Six subjects discontinued the study because of the prominent adverse effects and 5 subjects discontinued their visits.
3.1. Previous drugs
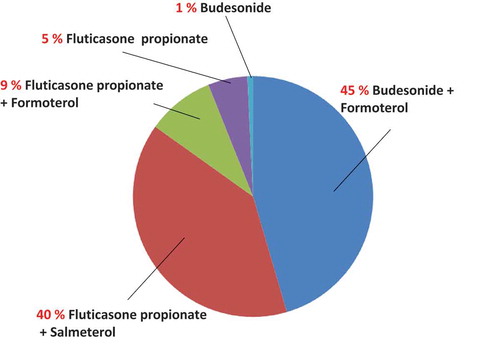
Previous ICS or ICS/LABA drugs used before the use of FF/VI are shown in Figure . Budesonide 160 μg with formoterol fumarate dihydrate 4.5 μg, 1–4 blisters, twice-daily was the most frequently used drug (45%), followed by FP 250–500 μg with salmeterol xinafoate 50 μg, 1 blister, twice-daily (40%), FP 125 μg with formoterol fumarate dehydrate 5 μg, 2–4 blisters, twice-daily (9%), and FP 200μg, twice-daily (5%).
Figure 2. Previous drugs before the use of fluticasone furoate with vilanterol.
Previous inhaled corticosteroids with or without long-acting beta-2 agonists before the use of fluticasone furoate with vilanterol are shown. Budesonide 160 μg with formoterol fumarate dihydrate 4.5 μg, 1–4 blisters, twice-daily was the most frequent drug (45%), followed by fluticasone propionate 250–500 μg with salmeterol xinafoate 50 μg, 1 blister, twice-daily (40%), fluticasone propionate 125 μg with formoterol fumarate dehydrate 5 μg, 2–4 blisters, twice-daily (9%), fluticasone propionate 200 μg, twice-daily (5%), and budesonide 200 μg, 1 blister, twice-daily (1%).
3.2. Change of various airflow data
Changes in various airflow data are shown in Table . Peak flow in FF/VI-using subjects was significantly increased (p = 0.0005). Instantaneous flow at 75% of the forced vital capacity (V75) and instantaneous flow at 50% of the forced vital capacity (V50) were also significantly increased (p = 0.0097 and p = 0.0007, respectively). The maximum mid-expiratory flow rate (MMF) was also significantly increased (p = 0.0046) after the use of FF/VI. Instantaneous flow at 25% of the forced vital capacity (V25) was increased slightly, but the difference was insignificant.
Table 1. Changes in various air flow data
3.3. Other associated parameter changes
Changes in other lung function data and associated parameters are shown in Table . ACT scores were significantly increased (asthma symptoms were better controlled, p = 0.002). The forced expired volume in the first second (FEV1) was increased significantly (p = 0.006). The inspiratory reserve volume (IRV) was also increased significantly (p = 0.011). The percent predicted vital capacity (%VC) was also increased significantly (p = 0.046). Although statistically insignificant, the vital capacity (VC), forced vital capacity (FVC), the percent predicted FVC (%FVC), the FEV1 to FVC (FEV1/FVC), and the inspiratory capacity (IC) had a tendency to be increased.
Table 2. Changes in other parameters
3.4. Newly seen adverse effects
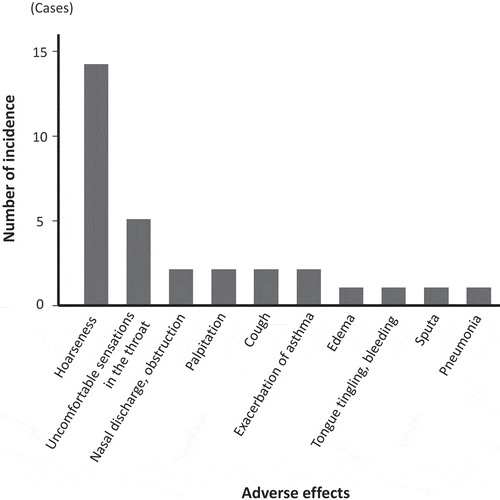
Newly seen adverse effects are shown in Figure . The most frequent adverse effect was hoarseness. Uncomfortable sensations in the throat, nasal discharge and/or obstruction, palpitation, cough, and exacerbation of asthma were also noted. The incidence of newly seen hoarseness and/or uncomfortable sensations in the throat was 16%.
Figure 3. Newly seen adverse effects after the initiation of fluticasone furoate with vilanterol.
The most frequent newly seen adverse effect was hoarseness (14 cases), followed by uncomfortable sensations in the throat (5 cases), nasal discharge and/or obstruction (2 cases), palpitation (2 cases), cough (2 cases), and exacerbation of asthma (2 cases).
3.5. Reasons for decision making
The main reason why all subjects preferred FF/VI was because it required less effort (a smaller number of necessary inhalations) (Table ). Thirty-eight patients answered that FF/VI itself was a more powerful drug than previous ICS or ICS/LABA they had taken.
Table 3. Reasons for decision making (continue fluticasone furoate with vilanterol or return to previous drugs)
4. Discussion
The most striking finding in this study is the prominent increase in airflow data of peak flow, V75, V50 and MMF, by the treatment switch from previous drugs to FF/VI. In addition, there were also significant increases in FEV1, %VC, IRV, and ACT score although the increase in V25 or FEV1/FVC was statistically insignificant. Peak flow data has been reported to reflect larger airway (central or upper airway) patency compared with FEV1 or lower flow indexes (Empey, Citation1972; Hayes & Kraman, Citation2009; Mink, Greville, Gomez, & Eng, Citation1988). Here, the p-value for peak flow was smaller than the p-value for FEV1, V75, V50, and MMF. In selective alveolobronchography for patients with asthma, it was reported that a narrowing of central airways was more severe than in peripheral airways (Nomura et al., Citation1978). Our hypothesis is that FF/VI itself is a potent long-acting drug and sufficiently dilates the central airways. The improving effects of FF 200 μg once-daily on FEV1 and peak flow in asthma patients was reported to be more than for FP 100 or 250 μg twice-daily, although this was statistically insignificant (Bateman et al., Citation2012; Bleecker et al., Citation2012). Studies comparing the effects of FF/VI (100 μg/25 μg) once-daily vs FP/S (250 μg/50 μg) twice-daily demonstrated that these drugs had a similar effect on the FEV1 improvement in asthma patients (Bernstein, Andersen, Forth, Jacques, & Yates, Citation2018; Woodcock et al., Citation2013). To the best of our knowledge, this is the first report to show the effectiveness of FF/VI on various airflow data of asthma patients including V75, V50, V25, and MMF.
Significant increases in %VC and IRV, and an insignificant increase in FEV1/FVC were unexpected for the treatment of patients with obstructive lung diseases. IC and VC, which are often used as index of restrictive impairment of the lung, were reported to increase by bronchodilators in asthmatic patients with severe airflow obstruction (Azevedo et al., Citation2012; Des Jardins & Burton, Citation1995; Szidon & Fishman, Citation1988). Although insignificant, FVC was also increased by a switch from previous drugs to FF/VI. This may explain why the increase in FEV1/FVC was statistically insignificant. Our hypothesis is that FF/VI increases the air volume in the central airways, which causes a significant increase in the %VC and IRV. One patient commented “Thanks to FF/VI, I feel that I can inhale more air than before.” This patient’s IRV had prominently increased from 1.10 L to 1.55 L and her VC also increased from 2.53 L to 2.72 L (%VC increased from 92.3% to 99.3%). Here, the increase in IRV was significant, but the increase in IC was statistically insignificant. Thus, IRV might be a more sensitive and useful additional parameter to evaluate the effect of ICS/LABA against asthma compared with IC. To the best of our knowledge, this is the first report that found FF/VI significantly increased the IRV and %VC in asthma patients.
ACT scores were also significantly increased, compatible with the findings by the Salford Lung Study (Woodcock et al., Citation2017). Among 107 subjects who completed the study, 93% of patients wanted to continue FF/VI. If all 21 incomplete subjects were counted as subjects who wanted to return to previous drugs, this indicates that 78% of all subjects are likely to continue FF/VI in the future. This difference (93% vs 78%) may be a limitation of this before-after study. The before-after lung function data were available only from completed subjects. Another limitation is the weakness of the “real-word” design. A randomized controlled trial is usually more powerful than a “real-word” uncontrolled trial (Hulley et al., Citation2013). However, the strength of this study is that all the subjects experienced FF/VI. It was evident that the majority of subjects preferred FF/VI to previous drugs.
The most common newly seen adverse effect was hoarseness, followed by uncomfortable sensations in the throat. A potent local effect of FF seemed to increase the ratio of these adverse effects. This phenomenon was thought to be compatible with the hypothesis that FF is more potent than previous ICS drugs. According to previous reports using FF/VI against asthma with a dose of 200 μg/25 μg or 100 μg/25 μg, no increased treatment-related adverse effects by FF/VI were shown (Bernstein et al., Citation2015; Devillier et al., Citation2018; Kerwin et al., Citation2018; Lin et al., Citation2015; O’Byrne et al., Citation2014; Woodcock et al., Citation2017). An explanation for this discrepancy may be partly because the drug doses were different between the studies. Alternatively, the differences in body size and/or sensitivity among ethnic backgrounds might have affected the results. Lower doses of FF/VI (consistently 100 μg/25 μg) have been used against COPD globally, and no reported increase in the rate of adverse events was noted (Martinez et al., Citation2017; Siler, Nagai, Scott-Wilson, Midwinter, & Crim, Citation2017; Vestbo et al., Citation2016, Citation2016). As of September 2018, the Japanese drug use system has not allowed the use of FF/VI against COPD without asthma. Early step down of FF/VI from 200 μg/25 μg to 100 μg/25 μg or FF 100 μg alone may be a good option for Japanese patients with asthma (Adachi, Goldfrad, Jacques, & Nishimura, Citation2016).
In conclusion, the once-daily use of FF/VI is a potent and effective treatment. Its effect was greater on larger airways and yielded greater satisfaction in patients despite a higher incidence of local steroid effects as compared to their prior treatments.
Abbreviations
| ACT | = | asthma control test |
| COPD | = | chronic obstructive pulmonary disease |
| FEV1 | = | forced expiratory volume in 1 s |
| FEV1/FVC | = | forced expiratory volume in 1 s to forced vital capacity |
| FF | = | fluticasone furoate |
| FF/VI | = | combination drug of fluticasone furoate plus vilanterol |
| FP | = | fluticasone propionate |
| FP/S | = | combination drug of fluticasone propionate plus salmeterol |
| FVC | = | forced vital capacity |
| IC | = | inspiratory capacity |
| ICS | = | inhaled corticosteroid |
| ICS/LABA | = | combination drug of inhaled corticosteroid plus long acting beata-2 agonist |
| IRV | = | inspiratory reserve volume |
| LABA | = | long acting beata-2 agonist |
| VC | = | vital capacity |
| VI | = | vilanterol |
| V25 | = | instantaneous flow at 25% of the forced vital capacity |
| V50 | = | instantaneous flow at 50% of the forced vital capacity |
| V75 | = | instantaneous flow at 75% of the forced vital capacity |
| %FVC | = | percent predicted forced vital capacity |
| %VC | = | percent predicted vital capacity. |
Declaration of Interests
None of the authors have potential conflicts to declare.
Author contribution
All authors contributed toward data analysis, drafting, and revising the paper and agree to be accountable for all aspects of the work.
Ethics approval
The institutions’ ethics committee approved the access to patient records and confirmed that patient confidentiality was maintained (IUHW ethics committee approval number: 13-B-66).
Acknowledgements
We thank all the participants of this study. We especially thank Ms. Rena Ishizaki for assisting with the data reduction work.
Additional information
Funding
Notes on contributors
Akira Umeda
Akira Umeda, M.D., Ph.D. graduated from the School of Medicine, Keio University, Tokyo, Japan in 1988, and his first clinical training was at the Lung and Heart Division, Department of Medicine, School of Medicine, Keio University. His Ph.D. was related to immunoelectron microscopy. Currently he is a Professor at the Department of General Medicine, School of Medicine, International University of Health and Welfare (IUHW), Chiba Prefecture, Japan as well as the Director of the Department of Respiratory Medicine, IUHW Shioya Hospital, Tochigi Prefecture, Japan. His main interests are respiratory physiology, pathology, and pharmacological approaches to respiratory diseases. His research team includes pharmacologists. The mission of the team is to find the best drug to treat patients with various disease types.
References
- Adachi, M., Goldfrad, C., Jacques, L., & Nishimura, Y. (2016, Nov). Efficacy and safety comparison: Fluticasone furoate and fluticasone propionate, after step down from fluticasone furoate/vilanterol in Japanese patients with well-controlled asthma, a randomized trial. Respiratory Medicine, 120, 78–11. Epub 2016 Sep 30. doi:10.1016/j.rmed.2016.09.018.
- Allen, A., Bareille, P. J., & Rousell, V. M. (2013). Fluticasone furoate, a novel inhaled corticosteroid, demonstrates prolonged lung absorption kinetics in man compared with inhaled fluticasone propionate. Clinical Pharmacokinetics, 52, 37–42.
- Azevedo, K. S., Luiz, R. R., Rocco, P. R., & Conde, M. B. (2012). Vital capacity and inspiratory capacity as additional parameters to evaluate bronchodilator response in asthmatic patients: A cross sectional study. BMC Pulmonary Medicine, 12, 49.
- Bateman, E. D., Bleecker, E. R., Lötvall, J., Woodcock, A., Forth, R., Medley, H., … Busse, W. W. (2012, May). Dose effect of once-daily fluticasone furoate in persistent asthma: A randomized trial. Respiratory Medicine, 106(5), 642–650. Epub 2012 Feb 18. doi: 10.1016/j.rmed.2012.01.004
- Bernstein, D., Andersen, L., Forth, R., Jacques, L., & Yates, L. (2018, Sep). Once-daily fluticasone furoate/vilanterol versus twice-daily fluticasone propionate/salmeterol in patients with asthma well controlled on ICS/LABA. The Journal of Asthma, 55(9), 984–993. Epub 2018 Apr 13. doi: 10.1080/02770903.2017.1386214
- Bernstein, D. I., Bateman, E. D., Woodcock, A., Toler, W. T., Forth, R., Jacques, L., … O’Byrne, P. M. (2015). Fluticasone furoate (FF)/vilanterol (100/25 mcg or 200/25 mcg) or FF (100 mcg) in persistent asthma. The Journal of Asthma, 52, 1073–1083.
- Bleecker, E. R., Bateman, E. D., Busse, W. W., Woodcock, A., Frith, L., House, K. W., … Lötvall, J. (2012, Nov). Once-daily fluticasone furoate is efficacious in patients with symptomatic asthma on low-dose inhaled corticosteroids. Annals of Allergy, Asthma & Immunology, 109(5), 353–358.e4. doi:10.1016/j.anai.2012.08.017
- Coleman, C. I., Limone, B., Sobieraj, D. M., Lee, S., Roberts, M. S., Kaur, R., & Alam, T. (2012, Sep). Dosing frequency and medication adherence in chronic disease. Journal of Managed Care Pharmacy : JMCP, 18(7), 527–539.
- Daley-Yates, P. T. (2015). Inhaled corticosteroids: Potency, dose equivalence and therapeutic index. British Journal of Clinical Pharmacology, 80, 372–380.
- Des Jardins, T., & Burton, G. G. (1995). Patient Assessment. In Clinical manifestations & assessment of respiratory disease (3rd ed., pp. 3–118). St. Louis: Mosby-Year Book, Inc.
- Devillier, P., Humbert, M., Boye, A., Zachgo, W., Jacques, L., Nunn, C., … Grouin, J. M. (2018). Efficacy and safety of once-daily fluticasone furoate/vilanterol (FF/VI) versus twice-daily inhaled corticosteroids/long-acting β2-agonists (ICS/LABA) in patients with uncontrolled asthma: An open-label, randomized, controlled trial. Respiratory Medicine, 141, 111–120.
- Empey, D. W. (1972, Aug 26). Assessment of upper airways obstruction. British Medical Journal, 3(5825), 503–505.
- Ernst, P., Spitzer, W. O., Suissa, S., Cockcroft, D., Habbick, B., Horwitz, R. I., … Buist, A. S. (1992, Dec 23–30). Risk of fatal and near-fatal asthma in relation to inhaled corticosteroid use. JAMA, 268(24), 3462–3464.
- Global Initiative for Asthma (GINA). (2018). Global strategy for asthma management and prevention. Retrieved from https://ginasthma.org/wp-content/uploads/2018/04/wms-GINA-2018-report-V1.3-002.pdf
- Hayes, D., Jr, & Kraman, S. S. (2009). The physiologic basis of spirometry. Respiratory Care, 54, 1717–1726.
- Hulley, S. B., Cummings, S. R., Browner, W. S., Grady, D. G., & Newman, T. B. (2013). Designing clinical research (4th ed.). Philadelphia, USA: Lippincott Williams & Wilkins, a Wolters Kluwer business.
- Kerwin, E., Barnes, N., Gibbs, M., Leather, D., Forth, R., Jacques, L., & Yates, L. J. (2018). Fluticasone furoate/vilanterol once daily improves night-time awakenings in asthma patients with night symptoms: Post hoc analyses of three randomized controlled trials. The Journal of Asthma, 55, 890–897.
- Lin, J., Kang, J., Lee, S. H., Wang, C., Zhou, X., Crawford, J., … Stone, S. (2015). Fluticasone furoate/vilanterol 200/25 mcg in Asian asthma patients: A randomized trial. Respiratory Medicine, 109, 44–53.
- Martinez, F. J., Vestbo, J., Anderson, J. A., Brook, R. D., Celli, B. R., Cowans, N. J., … Calverley, P. M. (2017). Effect of fluticasone furoate and vilanterol on exacerbations of chronic obstructive pulmonary disease in patients with moderate airflow obstruction. American Journal of Respiratory and Critical Care Medicine, 195, 881–888.
- Mink, S. N., Greville, H., Gomez, A., & Eng, J. (1988, Jan). Expiratory flow limitation in dogs with regional changes in lung mechanical properties. Journal of Applied Physiology (1985), 64(1), 162–173.
- Nathan, R. A., Sorkness, C. A., Kosinski, M., Schatz, M., Li, J. T., Marcus, P., … Pendergraft, T. B. (2004, Jan). Development of the asthma control test: A survey for assessing asthma control. The Journal of Allergy and Clinical Immunology, 113(1), 59–65.
- Nomura, K., Asai, S., Muraoka, F., Kinjo, Y., Kadota, T., Oye, T., … Yamasaki, T. (1978). The relationship between airway lesions and pulmonary function in bronchial asthma. Nihon Kyobu Shikkan Gakkai Zasshi, 16, 173–179.
- O’Byrne, P. M., Bleecker, E. R., Bateman, E. D., Busse, W. W., Woodcock, A., Forth, R., … Lötvall, J. (2014). Once-daily fluticasone furoate alone or combined with vilanterol in persistent asthma. The European Respiratory Journal, 43, 773–782. doi:10.1183/09031936.00064513
- Price, D., Robertson, A., Bullen, K., Rand, C., Horne, R., & Staudinger, H. (2010, Jan 5). Improved adherence with once-daily versus twice-daily dosing of mometasone furoate administered via a dry powder inhaler: A randomized open-label study. BMC Pulmonary Medicine, 10, 1. doi:10.1186/1471-2466-10-1
- Rossios, C., To, Y., To, M., Ito, M., Barnes, P. J., Adcock, I. M., … Ito, K. (2011). Long-acting fluticasone furoate has a superior pharmacological profile to fluticasone propionate in human respiratory cells. European Journal of Pharmacology, 670, 244–251.
- Salter, M., Biggadike, K., Matthews, J. L., West, M. R., Haase, M. V., Farrow, S. N., … Gray, D. W. (2007). Pharmacological properties of the enhanced-affinity glucocorticoid fluticasone furoate in vitro and in an in vivo model of respiratory inflammatory disease. American Journal of Physiology. Lung Cellular and Molecular Physiology, 293, L660–L667. doi:10.1152/ajplung.00108.2007
- Siler, T. M., Nagai, A., Scott-Wilson, C. A., Midwinter, D. A., & Crim, C. (2017). A randomised, phase III trial of once-daily fluticasone furoate/vilanterol 100/25 μg versus once-daily vilanterol 25 μg to evaluate the contribution on lung function of fluticasone furoate in the combination in patients with COPD. Respiratory Medicine, 123, 8–17.
- Szidon, J. P., & Fishman, A. P. (1988). Approach to the pulmonary patient with respiratory signs and symptoms. In A. P. Fishman (Ed.), Pulmonary diseases and disorders (2nd ed., pp. 313–366). New York: McGraw-Hill Book Company.
- Vestbo, J., Anderson, J. A., Brook, R. D., Calverley, P. M., Celli, B. R., Crim, C., … Newby, D. E. (2016). Fluticasone furoate and vilanterol and survival in chronic obstructive pulmonary disease with heightened cardiovascular risk (SUMMIT): A double-blind randomised controlled trial. Lancet, 387(10030), 1817–1826.
- Vestbo, J., Leather, D., Diar Bakerly, N., New, J., Gibson, J. M., McCorkindale, S., … Woodcock, A. (2016). Effectiveness of fluticasone furoate-vilanterol for COPD in clinical practice. The New England Journal of Medicine, 375, 1253–1260.
- Woodcock, A., Bleecker, E. R., Lötvall, J., O’Byrne, P. M., Bateman, E. D., Medley, H., … Busse, W. W. (2013, Oct). Efficacy and safety of fluticasone furoate/vilanterol compared with fluticasone propionate/salmeterol combination in adult and adolescent patients with persistent asthma: A randomized trial. Chest, 144(4), 1222–1229. doi:10.1378/chest.13-0178
- Woodcock, A., Vestbo, J., Bakerly, N. D., New, J., Gibson, J. M., McCorkindale, S., … Leather, D. (2017). Salford lung study investigators. Effectiveness of fluticasone furoate plus vilanterol on asthma control in clinical practice: An open-label, parallel group, randomised controlled trial. Lancet, 390(10109), 2247–2255.
- World Health Organization. (2019). Asthma: Definition. Retrieved from https://www.who.int/respiratory/asthma/definition/en/
- World Health Organization. (2019). Asthma. Retrieved from https://www.who.int/respiratory/asthma/en/
