Abstract
Background: Human bone marrow has shown promise as a minimally invasive approach in treating a variety of musculoskeletal conditions due to the presence of stem cells, platelets, and growth factors in solution. This study examines the clinical effect of whole bone marrow (WBM) and bone marrow concentrate (BMC) injections in patients who were diagnosed with rotator cuff tears or shoulder osteoarthritis. Methods: Forty-seven patients and fifty shoulders with rotator cuff tears or shoulder osteoarthritis underwent one or two BMC or WBM treatments. These patients were grouped based on number of treatments and pathology for analysis. The patients who were injected twice received them approximately 22.33 days apart. Outcomes of resting pain, active pain, upper extremity functionality scale and overall improvement percentage were compared to baseline and between groups. Results: Patients who received either one and two treatments reported significant improvements in resting pain, active pain, and functionality score when compared to baseline. These groups also experienced a 42.25% and 50.17% overall improvement respectively. The group that received two treatments experienced statistically significant improvements in active pain when compared to the group that received one injection. There were no significant outcome differences between RCT and OA patients. Conclusions: Our study demonstrated that patients diagnosed with shoulder osteoarthritis or rotator cuff tears experienced symptomatic improvements in pain and functionality when injected with BMC or WBM. Further randomized control studies are needed to validate these findings.
PUBLIC INTEREST STATEMENT
Chronic shoulder pain is the third most common cause of musculoskeletal pain affecting an approximate 4.5 million patients and costing in excess of $7 billion per year in the United States.
Furthermore, the prevalence of shoulder pain caused by rotator cuff tears or osteoarthritis dramatically increases with age. Human bone marrow presents a promising therapy in treating musculoskeletal shoulder pain due to the presence of stem cells, platelets, and growth factors in solution. The purpose of our study was to report the clinical outcomes of patients diagnosed with shoulder osteoarthritis or rotator cuff tears when injected with bone marrow derived therapies. Our hope is by providing continual research about the efficacy of regenerative medicine it can reach and educate more people.
1. Introduction
Chronic shoulder pain is the third most common cause of musculoskeletal pain affecting approximately 4.5 million patients and costing in excess of $7 billion per year in the United States (Mather et al., Citation2013; Oh, Wolf, Hall, Levy, & Marx, Citation2007; Pope, Croft, Pritchard, & Silman, Citation1997). Common shoulder pathologies include anterior shoulder instability, biceps tendinitis, lesions to the acromioclavicular joint, and proximal humeral fractures; however, the most common pathologies resulting in chronic shoulder pain are rotator cuff tears (RCT) and glenohumeral osteoarthritis (OA) (Herin, Vézina, Thaon, Soulat, & Paris, Citation2012). The prevalence of partial or total rotator cuff tears dramatically increases with age with a prevalence of 4% under the age of 40 to 54% over the age of 60 (Chillemi & Franceschini, Citation2013). OA of the shoulder affects up to 32.4% of individuals over the age of sixty (Kerr, Resnick, Pineda, & Haghighi, Citation1985; Petersson, Citation1983) and is caused by the thinning of the articular surface of the humeral head and glenoid (Tempelhof, Rupp, & Seil, Citation1999). Pain, decreased mobility and function are all symptoms of shoulder OA and RCT that cause patients decreased quality of life (van Kampen et al., Citation2014). Many patients turn to surgery for symptomatic relief; however, the risk of re-injury or complications are high (Henry et al., Citation2015). Furthermore, the economic burden of shoulder pain is largely attributed to operative treatment (Meislin, Sperling, & Stitik, Citation2005). With an aging population and rising healthcare costs, there exists a need for an effective non-operative treatment modality for chronic shoulder pain.
Regenerative medicine has gained traction within recent years to be a safe and conservative procedure to treat a variety of musculoskeletal conditions. The pioneering regenerative treatment was dextrose prolotherapy, which is an irritant solution that stimulates growth factor secretion and soft tissue healing when injected to treat musculoskeletal conditions (Kim, Stitik, Foye, Greenwald, & Campagnolo, Citation2004). Studies have shown positive effects of dextrose prolotherapy on OA. (Fortney et al., Citation2012; Rabago et al., Citation2013) In addition, a double-blind placebo study found that dextrose prolotherapy outperformed a control group in terms of pain and quality of life in patients with rotator cuff tendinopathy (Bertrand, Reeves, Bennett, Bicknell, & Cheng, Citation2016).
Human bone marrow offers a promising new therapy for the treatment of chronic shoulder pain. This includes Whole Bone Marrow (WBM) therapy, where the bone marrow is aspirated and reinjected without manipulation, or as Bone Marrow Concentrate (BMC) therapy, where the bone marrow is centrifuged and cleared of red blood cells. Human bone marrow contains many growth factors and important cytokines as well as platelets and mesenchymal stem cells (MSCs) (Sampson, Botto-van Bemden, & Aufiero, Citation2013). This unique combination of cells creates an environment rich in healing and regenerative capacity. Specifically, MSCs release trophic factors and differentiate into cartilage, bone or muscle that facilitate tissue regeneration while also providing lasting anti-inflammatory effects (Fu et al., Citation2017). In addition, studies have shown that platelets are able to regulate the early inflammatory response involved in healing and recruit growth factors to begin the healing process at the site of injury (Sampson et al., Citation2013). These properties make BMC and WBM attractive treatment modalities for cartilage and tissue regeneration.
Animal studies have shown that BMC combined with platelet-rich plasma (PRP) enhanced the proliferation and migration of tendon derived stem cells in animal rotator cuff models (Kim, Park, Park, Kim, & Song, Citation2017). Based on these studies and current research, surgeons began using BMC injections in surgical rotator cuff repairs (Gomes, Da Silva, Silla, Abreu, & Pellanda, Citation2012). These patients outperformed the historical data of the same procedure without BMC injections (Gomes et al., Citation2012). Following these positive results, physicians began to treat shoulder injuries with BMC treatments alone. Specifically, a trial of 102 patients who underwent BMC injection for glenohumeral OA or rotator cuff injury reported a decrease in pain, an increase in functionality, and a mean 48.8% overall improvement (Centeno, Al-Sayegh, Bashir, Goodyear, & Freeman, Citation2015). A case series using WBM in knee, hip, and ankle OA patients demonstrated symptomatic relief (Hauser & Orlofsky, Citation2013). The authors in this study attributed the relief to the microenvironment of the cells not the process of concentration. Our group has had a long history of using BMC, and then experimented using WBM.
The objective of our study is to demonstrate the therapeutic effects of one and two BMC or WBM injections in the treatment of chronic shoulder pain associated with RCT and glenohumeral OA. We have not compared BMC to WBM as these results will be reported in a separate study that include all musculoskeletal conditions that we treat.
2. Methods
2.1. Patients
This is a study that reports private clinical practice outcomes, in which variables were determined prospectively and data was analyzed retrospectively. Patients included in this study underwent one or two BMC or WBM treatments for shoulder RCT or OA at a solo practitioner private practice. These procedures were performed from July 2016 to September 2018. Patients who were treated from June 2016 to August 2017 were injected with BMC and patients who were treated from September 2017 to September 2018 were injected with WBM. There was no patient in the two-injection group that received both BMC and WBM treatments. The diagnosis of shoulder RCT or OA was based on radiographic findings, however the severity of pathology was not taken into account. All treatments were prescribed on an individual basis, as recommended by a physician. Written informed consent was obtained prior to each treatment.
As stated in our previous publication (Shaw, Darrow, & Derian, Citation2018), if a patient at our clinic undergoes multiple injections, we advise them to receive injections approximately fourteen days apart. However scheduling conflicts often cause greater injection intervals. The reasoning behind this fourteen-day interval is that this is the time period when there is growth factor secretion from various cell types that participate in the late phases of wound healing (Barrientos et al., Citation2008; Enoch & Price, Citation2004). Patients were instructed not to use anti-inflammatory drugs during treatment, as they diminish tissue regeneration and impair MSC’s therapeutic abilities (Müller, Raabe, Addicks, Wenisch, & Arnhold, Citation2011). In addition, patients were instructed not to perform any strenuous exercise, and instructed to perform a series of shoulder stretches each day to improve joint mobility and reduce the possibility of adhesive capsulitis. For patients who underwent bilateral shoulder treatment, each shoulder was given a separate survey and thus considered separately for statistical analysis. This study was constructed to follow all ethical guidelines directed by the Declaration of Helsinki.
2.2. Procedure
Patients were in the prone position and sterilized with 10% Povidone-Iodine on the skin above the posterior superior iliac spine (PSIS). Next, 4% Chlorhexidine Gluconate (Hibiclens) was administered with sterile gauze in a circular motion starting at the PSIS. Patients were then anesthetized with 10 cc of 1% lidocaine and 2 cc of 8.4% sodium bicarbonate, injected locally on and around the patient’s posterior superior iliac spine. After local anesthesia was achieved, a fenestrated 11 gauge, 4-inch disposable needle was drilled to penetrate the PSIS and extract bone marrow. A 20-cc syringe prepared with 1 cc of heparin (1,000 USP Units/cc) was used to extract bone marrow for a total yield of 19 cc. To maximize stem cell yield and avoid an excess of peripheral blood, the needle was rotated slowly within the ilium cavity and penetrated deeper as required. If the patient received a BMC injection, the aspirated bone marrow was spun in a centrifuge, and the upper portion without visible red cells was isolated from the centrifuged bone marrow. About 1-cc of ropivacaine was added to 5-cc of centrifuged BMC to ensure that the area was less painful after injection. Ropivacaine has shown limited toxicity to MSCs (Breu, Eckl, Zink, Kujat, & Angele, Citation2013). If the patient received WBM treatment the bone marrow was aspirated and then injected with a filter attachment to prevent clotting. The BMC or WBM was injected by the physician into the glenohumeral joint and labrum under ultrasound guidance with a 2 inch 25 gauge needle, and then into the supraspinatus, infraspinatus, teres minor, and subdeltoid bursa depending on the patient’s pain and pathology determined by our physician.
2.3. Outcomes
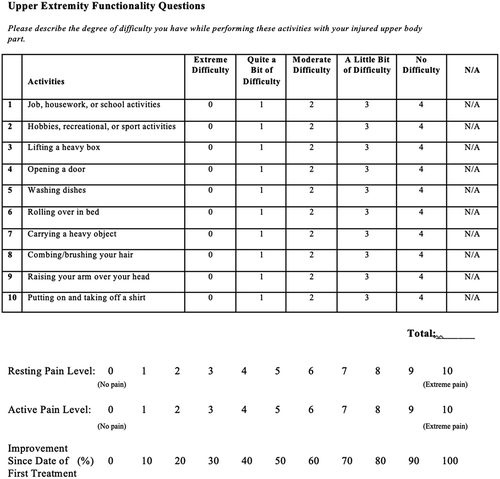
The outcomes of interest for this study were changes to pain (resting and active), overall improvement (percentage scale), and a joint function questionnaire. Data were collected at baseline, preceding each treatment, at 1 month, at 3 months, at 6 months, and annually after treatment (Figure ). The functionality portion of the questionnaire, which assessed degree of difficulty in performing daily activities, was based on 10 of 20 activities assessed in the Upper Extremity Functional Index, (Chesworth et al., Citation2014) but also included a “not applicable (N/A)” response option. This scale has shown to be a valid and reliable functionality questionnaire for upper extremity limbs (Chesworth et al., Citation2014). The numerical pain scale (NPS) to assess resting and active pain used a scale of 0 (no pain) to 10 (extreme pain) (Childs, Piva, & Fritz, Citation2005). Lastly, the form included a subjective measure of how much overall improvement the patient experienced following treatment on a scale of 0% to 100%.
2.4. Statistical analysis
Baseline and post-intervention data were compared using means and standard deviations. Each follow-up response was compared to its corresponding baseline response using the Wilcoxon signed-rank test. Post-interventional data between treatment groups were compared using the Wilcoxon sum-rank test. Responses per shoulder were assumed independent for analytic purposes. Statistical significance was set at P equal to or less than .05 and statistical analysis was performed using R.
2.5. Results
In total, 47 patients participated in this study with 3 of whom had bilateral shoulders treated. All bilateral shoulder patients received two injections in each shoulder. Patients who had two BMC or WBM injections received them a mean 22.33 days apart. 29 shoulders received WBM injections and 21 shoulders received BMC injections. Out of the 50 shoulders treated, there were 32 cases of osteoarthritis and 18 cases of rotator cuff tears. When these groups were compared there was no statistical differences between pathology groups. Patient outcomes can be found in Table –.
Table 1. Patient characteristics
Table 2. Baseline and post-treatment scores of patients who received 1 BMC/WBM treatment for shoulder osteoarthritis and rotator cuff tears. N = 20 Shoulders
Table 3. Baseline and post-treatment scores of patients who received two BMC/WBM treatments for shoulder osteoarthritis and rotator cuff tears. N = 30 Shoulders
Table 4. Differences between 1 and 2 treatment groups
Table 5. Differences between rotator cuff tear and osteoarthritis groups
Both Groups 1 and 2 showed statistical significance in terms of pain and functionality when compared to baseline. Group 1 experienced a 33.33% decrease in resting pain (P = .027) and a 28.13% decrease in active pain (P = .004). These patients also experienced a mean 42.25% total overall improvement and a 15.81% increase in functionality score (P = .028). Group 2 experienced a 47.31% decrease in resting pain (P < .001) and 42.92% decrease in active pain (P < .001). These patients also experienced a 50.17% total overall improvement and a 36.89% increase in functionality score (P < .001). Patients in group 2 experienced substantial improvement in active pain (P = .019) when compared to patients who only received one injection.
3. Discussion
Our study demonstrated that a single injection of BMC or WBM in the treatment of shoulder OA and rotator cuff tears significantly improved resting pain, active pain and functionality score. Patients that received two injections of BMC or WBM reported greater mean improvement that were also statistically significant when compared to baseline. When injection groups were compared, the two treatment group demonstrated statistically significant improvements in active pain when compared to the one injection group. In addition, there were significant findings across all measure variables when analyzing based on OA and RCT pathologies. The results of our study show that BMC and WBM are efficacious in the treatment of RCT as well as OA with clinically significant benefits to patients after one treatment.
Cartilage lesions have limited regenerative capacity due to the low mitotic ability of chondrocytes as well as limited, or lack of, blood, lymphatic, and nerve supply, which creates a challenge for physicians (Herin et al., Citation2012). The poor healing of partial or full RCTs results in the local formation of fibrous or fibrocartilaginous tissue that degenerates over time, commonly evolving into OA (Chillemi & Franceschini, Citation2013). OA affects the underlying bone and destruction of joint cartilage resulting in pain and loss of function. Traditionally, RCTs are treated surgically; however, studies have shown that up to 79% of patients re-tear their rotator cuff and 12% of patients experience adverse effects following arthroplasty for the treatment of glenohumeral OA (Aldinger, Raiss, Rickert, & Loew, Citation2010; van Kampen et al., Citation2014). In severe cases, prosthetic replacement of the affected joint is required. Conservative treatments include physical therapy, rest, pain management or steroid injections; however, long term benefit is dependent on the degree of injury and evidence for long term efficacy has not been fully characterized.
Due to the intrinsic characteristics of chondral lesions, tissue engineering and regenerative medicine have been explored to overcome the challenges of poor healing. According to the literature, autologous MSCs resolve these barriers due to their capacity to proliferate and differentiate into a variety of cells, including chondrocytes, without evoking a pathologic inflammatory response (Gao et al., Citation2016). The regenerative capacity of MSCs is due to their ability to secrete chemokines, cytokines, growth factors, and a variety of other proteins. These bioactive molecules stimulate angiogenesis and endogenous repair as well as limit inflammation and preventing apoptosis, all of which are important in healing and regeneration (Veronesi et al., Citation2013).
Bone marrow is a rich source of MSCs that is both easily accessible as well as dispensable (Caplan, Citation1991). As such, investigators have begun to use bone marrow derived-MSCs with an increased frequency to treat a myriad of musculoskeletal issues, with over 150 clinical trials currently registered (at clinicaltrials.gov). Studies have demonstrated that MSCs are also found locally in synovial joints with the potential for self-renewal and differentiation like those of bone marrow (Murphy, Fink, Hunziker, & Barry, Citation2003). These local MSCs play a role in the maintenance and repair of local cartilage, which are important for homeostasis and integrity of the joint (Park et al., Citation2012). Given the intrinsic properties of chondral lesions, these local MSCs are unable to respond to trauma and lack the ability of functional repair. Bone marrow derived MSCs do not exhibit this flaw as the cells are obviously capable of responding to bone fractures (Rubio-Azpeitia & Andia, Citation2014).
This area of research continues to be investigated and there remains significant debate about the exact mechanism of action of MSCs. However, preliminary results are promising for the treatment of RCTs and OA. Based on defective cellular responses observed in chondral lesions and those of OA, it could be postulated that stimulating these damaged cells directly may increase innate regenerative ability or be used prophylactically. In addition to the MSCs, the platelets found in bone marrow could also contributed to the therapeutic effect. Platelets have been shown to enhance the proliferation, migration, stemness and preservation of MSC immune-modulatory properties (Rubio-Azpeitia & Andia, Citation2014). Further studies should be performed to determine the potential effects seen in WBM vs BMC as well as exploring dose-dependent responses with multiple treatments over time. Other treatment modalities that may stimulate these defective cells include granulocyte-colony stimulating factor, erythropoietin, endothelial-derived growth factors, platelet-derived growth factor or even hyperbaric oxygen therapy.
The results from this study are limited due to the absence of a control group, short follow-up times, and possible self-report bias from the subjective response variables. The absence of treatment randomization, nucleated cell counts, and small sample size limit the external validity. In addition, the conclusions of this study are limited by not accounting for all variables that may have influenced patient outcomes, such as accounting for age, BMI, and severity of pathology between groups. Additional studies that compared patients who received one injection to patients who received multiple injections are needed to validate these results.
4. Conclusion
Our study demonstrated that one or two injections of BMC or WBM were sufficient to produce statistically significant improvements in reducing pain and improving functionality in patients with OA and RCT of the shoulder. These results contribute to the growing body of literature showing the clinical benefits associated with BMC/WBM and their regenerative capacities. Further research is warranted to compare the efficacy of WBM vs BMC injections.
Additional information
Funding
Notes on contributors
Brent Shaw
The authors of this study are apart of a private clinical practice called the Darrow Stem Cell Institute, which treats degenerative musculoskeletal conditions and sports injuries with regenerative medicine therapies. Our research department is dedicated to educate both the medical community and our patients about the efficacy of regenerative medicine with our clinical practice outcomes. This will be our 6th publication in the treatment of different musculoskeletal conditions with Platelet-Rich Plasma and Bone Marrow-Derived Therapies.
References
- Aldinger, P. R., Raiss, P., Rickert, M., & Loew, M. (2010). Complications in shoulder arthroplasty: An analysis of 485 cases. International Orthopaedics, 34(4), 517–10. doi:10.1007/s00264-009-0780-7
- Barrientos, S., Stojadinovic, O., Golinko, M. S., Brem, H., & Tomic-Canic, M. (2008). Growth factors and cytokines in wound healing. Wound Repair and Regeneration, 16, 585–601. doi:10.1111/j.1524-475X.2008.00410.x
- Bertrand, H., Reeves, K. D., Bennett, C. J., Bicknell, S., & Cheng, A. L. (2016). Dextrose prolotherapy versus control injections in painful rotator cuff tendinopathy. In Archives of physical medicine and rehabilitation (Vol. 97, pp. 17–25). doi:10.1016/j.apmr.2015.08.412
- Breu, A., Eckl, S., Zink, W., Kujat, R., & Angele, P. (2013). Cytotoxicity of local anesthetics on human mesenchymal stem cells in vitro. Arthroscopy - Journal of Arthroscopic and Related Surgery, 29(10), 1676–1684. doi:10.1016/j.arthro.2013.06.018
- Caplan, A. I. (1991). Mesenchymal stem cells. Journal of Orthopaedic Research, 9(5), 641–650. doi:10.1002/jor.1100090504
- Centeno, C. J., Al-Sayegh, H., Bashir, J., Goodyear, S. H., & Freeman, M. D. (2015). A prospective multi-Site registry study of a specific protocol of autologous bone marrow concentrate for the treatment of shoulder rotator cuff tears and osteoarthritis. Journal of Pain Research, 8, 269–276. doi:10.2147/JPR.S80872
- Chesworth, B. M., Hamilton, C. B., Walton, D. M., Benoit, M., Blake, T. A., Bredy, H., … Yardley, D. (2014). Reliability and validity of two versions of the upper extremity functional index. Physiotherapy Canada, 66(3), 243–253. doi:10.3138/ptc.2013-45
- Childs, J. D., Piva, S. R., & Fritz, J. M. (2005). Responsiveness of the numeric pain rating scale in patients with low back pain. Spine, 30(11), 1331–1334. doi:10.1097/01.brs.0000164099.92112.29
- Chillemi, C., & Franceschini, V. (2013). Shoulder osteoarthritis. Arthritis, 2013, 1–7. doi:10.1155/2013/370231
- Enoch, S., & Price, P. (2004). Cellular, molecular and biochemical differences in the pathophysiology of healing between acute wounds, chronic wounds and wounds in the aged. World Wide Wounds.
- Fortney, L., Zgierska, A., Patterson, J. J., Kijowski, R., Grettie, J., Mundt, M., … Ryan, M. (2012). Hypertonic dextrose injections (Prolotherapy) for knee osteoarthritis: Results of a single-arm uncontrolled study with 1-year follow-up. The Journal of Alternative and Complementary Medicine, 18(4), 408–414. doi:10.1089/acm.2011.0030
- Fu, Y., Both, S. K., Wu, L., Karperien, M., Karbaat, L., & Leijten, J. (2017). Trophic effects of mesenchymal stem cells in tissue regeneration. Tissue Engineering Part B: Reviews, 23(6), 515–528. doi:10.1089/ten.teb.2016.0365
- Gao, F., Chiu, S. M., Motan, D. A. L., Zhang, Z., Chen, L., Ji, H. L., & Lian, Q. (2016). Mesenchymal stem cells and immunomodulation: Current status and future prospects. Cell Death & Disease, 7, e2062-e2062. doi:10.1038/cddis.2015.327
- Gomes, J. L. E., Da Silva, R. C., Silla, L. M. R., Abreu, M. R., & Pellanda, R. (2012). Conventional rotator cuff repair complemented by the aid of mononuclear autologous stem cells. Knee Surgery, Sports Traumatology, Arthroscopy, 20(2), 373–377. doi:10.1007/s00167-011-1607-9
- Hauser, R. A., & Orlofsky, A. (2013). Regenerative injection therapy with whole bone marrow aspirate for degenerative joint disease: A case series. Clinical Medicine Insights: Arthritis and Musculoskeletal Disorders, 6. doi:10.4137/CMAMD.S10951
- Henry, P., Wasserstein, D., Park, S., Dwyer, T., Chahal, J., Slobogean, G., & Schemitsch, E. (2015). Arthroscopic repair for chronic massive rotator cuff tears: A systematic review. Arthroscopy - Journal of Arthroscopic and Related Surgery, 31(12), 2472–2480. doi:10.1016/j.arthro.2015.06.038
- Herin, F., Vézina, M., Thaon, I., Soulat, J. M., & Paris, C. (2012). Predictors of chronic shoulder pain after 5 years in a working population. Pain, 153(11), 2253–2259. doi:10.1016/j.pain.2012.07.024
- Kerr, R., Resnick, D., Pineda, C., & Haghighi, P. (1985). Osteoarthritis of the glenohumeral joint: A radiologic-pathologic study. American Journal of Roentgenology, 144(5), 967–972. doi:10.2214/ajr.144.5.967
- Kim, S. J., Park, J. W., Park, S., Kim, S. J., & Song, D. H. (2017). Effect of bone marrow aspirate concentrate-platelet-rich plasma on tendon-derived stem cells and rotator cuff tendon tear. Cell Transplantation, 26(5), 867–878. doi:10.3727/096368917x694705
- Kim, S. R., Stitik, T. P., Foye, P. M., Greenwald, B. D., & Campagnolo, D. I. (2004). Critical review of prolotherapy for osteoarthritis, low back pain and other musculoskeletal conditions: A physiatric perspective. American Journal of Physical Medicine and Rehabilitation, 83, 379–389. doi:10.1097/01.PHM.0000124443.31707.74
- Mather, R. C., Koenig, L., Acevedo, D., Dall, T. M., Gallo, P., Romeo, A., … Williams, G. (2013). The societal and economic value of rotator cuff repair. Journal of Bone and Joint Surgery - Series A, 95(22), 1993–2000. doi:10.2106/JBJS.L.01495
- Meislin, R. J., Sperling, J. W., & Stitik, T. P. (2005). Persistent shoulder pain: Epidemiology, pathophysiology, and diagnosis. American Journal of Orthopedics (Belle Mead, N.J.), 34(12 Suppl), 5–9.
- Müller, M., Raabe, O., Addicks, K., Wenisch, S., & Arnhold, S. (2011). Effects of non-steroidal anti-inflammatory drugs on proliferation, differentiation and migration in equine mesenchymal stem cells. Cell Biology International, 35, 235–248. doi:10.1042/CBI20090211
- Murphy, J. M., Fink, D. J., Hunziker, E. B., & Barry, F. P. (2003). Stem cell therapy in a caprine model of osteoarthritis. Arthritis and Rheumatism, 48(12), 3464–3474. doi:10.1002/art.11365
- Oh, L. S., Wolf, B. R., Hall, M. P., Levy, B. A., & Marx, R. G. (2007). Indications for rotator cuff repair: A systematic review. Clinical orthopaedics and related research (pp. 52–63). doi:10.1097/BLO.0b013e31802fc175
- Park, D., Spencer, J. A., Koh, B. I., Kobayashi, T., Fujisaki, J., Clemens, T. L., … Scadden, D. T. (2012). Endogenous bone marrow MSCs are dynamic, fate-restricted participants in bone maintenance and regeneration. Cell Stem Cell, 10(3), 259–272. doi:10.1016/j.stem.2012.02.003
- Petersson, C. J. (1983). Degeneration of the gleno-humeral joint: An anatomical study. Acta Orthopaedica, 54(2), 277–283. doi:10.3109/17453678308996570
- Pope, D. P., Croft, P. R., Pritchard, C. M., & Silman, A. J. (1997). Prevalence of shoulder pain in the community: The influence of case definition. Annals of the Rheumatic Diseases, 56(5), 308–312. doi:10.1136/ard.56.5.308
- Rabago, D., Patterson, J. J., Mundt, M., Kijowski, R., Grettie, J., Segal, N. A., & Zgierska, A. (2013). Dextrose prolotherapy for knee osteoarthritis: A randomized controlled trial. Annals of Family Medicine, 11(3), 229–237. doi:10.1370/afm.1504
- Rubio-Azpeitia, E., & Andia, I. (2014). Partnership between platelet-rich plasma and mesenchymal stem cells: In vitro experience. Muscles, Ligaments and Tendons Journal, 4(1), 52–62. doi:10.32098/mltj.01.2014.10
- Sampson, S., Botto-van Bemden, A., & Aufiero, D. (2013). Autologous bone marrow concentrate: Review and application of a novel intra-articular orthobiologic for cartilage disease. The Physician and Sportsmedicine, 41, 7–18. doi:10.3810/psm.2013.09.2022
- Shaw, B., Darrow, M., & Derian, A. (2018). Short-term outcomes in treatment of knee osteoarthritis with 4 bone marrow concentrate injections. Clinical Medicine Insights: Arthritis and Musculoskeletal Disorders, 11. doi:10.1177/1179544118781080
- Tempelhof, S., Rupp, S., & Seil, R. (1999). Age-related prevalence of rotator cuff tears in asymptomatic shoulders. Journal of Shoulder and Elbow Surgery, 8(4), 296–299. doi:10.1016/S1058-2746(99)90148-9
- van Kampen, D. A., van Den Berg, T., van der Woude, H. J., Castelein, R. M., Scholtes, V. A. B., Terwee, C. B., & Willems, W. J. (2014). The diagnostic value of the combination of patient characteristics, history, and clinical shoulder tests for the diagnosis of rotator cuff tear. Journal of Orthopaedic Surgery and Research, 9(1). doi:10.1186/s13018-014-0070-y
- Veronesi, F., Giavaresi, G., Tschon, M., Borsari, V., Aldini, N. N., & Fini, M. (2013). Clinical use of bone marrow, bone marrow concentrate, and expanded bone marrow mesenchymal stem cells in cartilage disease. Stem Cells and Development, 22(2), 181–192. doi:10.1089/scd.2012.0373