Abstract
Lifelong changes may be expected after sustaining a traumatic brain injury (TBI). Research on relevant treatment options in the chronic phase of TBI is lacking. An innovative, home-based intervention program was developed in the US and showed to be effective among US veterans who had sustained a TBI. However, the cross-cultural applicability and effectiveness are unknown. The aim of the present study is to evaluate the feasibility in a Norwegian population before a future definitive randomized controlled trial (RCT). Six participants with severe TBI in metropolitan Oslo, Norway, were recruited and received the intervention. Primary feasibility objectives were to evaluate (i) recruitment and screening procedures, (ii) baseline and follow-up assessments, (iii) intervention delivery, (iv) acceptability, and (v) order of primary and secondary outcome measures. No adverse effects of the intervention were uncovered. Baseline assessment was found to be too long. Intervention delivery was feasible and acceptability high. Outcome measures were reviewed and amendments were deemed necessary. An individually tailored, goal-focused intervention program was deemed feasible in a population of severe TBI and the preliminary results seem promising. The feasibility trial led to important amendments to inclusion criteria, baseline assessment and outcome measures that were adapted before the RCT study commenced. The RCT-study started recruitment in June 2018.
PUBLIC INTEREST STATEMENT
Traumatic brain injury is a leading cause of disability worldwide. Studies have shown that people might experience several challenges even years after their injury. However, few studies have evaluated relevant treatment options for the individuals and their families after they have returned to their homes and daily living. This study aimed to evaluate whether a home-based rehabilitation program tailored to each individual’s activity challenges was feasible in the context of a universal access health-care system in Norway. The study showed that this eight-session treatment program was feasible, but that some adjustments in regards to measurements and inclusion criteria were necessary. With these amendments, a full-scale trial was initiated, aiming to recruit 120 individuals and include a comparison group to evaluate effectiveness.
Competing Interests
The authors declare no competing interest.
1. Introduction
1.1. Background
Traumatic brain injury (TBI) is a leading cause of death and disability worldwide (Langlois, Rutland-Brown, & Wald, Citation2006; Tagliaferri, Compagnone, Korsic, Servadei, & Kraus, Citation2006), and often leads to persistent difficulties with cognitive, emotional and vocational functioning, as well as reduced community integration and quality of life (Andelic et al., Citation2009; Brooks, Campsie, Symington, Beattie, & McKinlay, Citation1986; Dikmen, Machamer, Powell, & Temkin, Citation2003; Forslund et al., Citation2014; Hoofien, Gilboa, Vakil, & Donovick, Citation2001, Jourdan et al., Citation2018; Olver, Ponsford, & Curran, Citation1996; Ponsford, Draper, & Schonberger, Citation2008; Ruttan, Martin, Liu, Colella, & Green, Citation2008). One of the groups with the highest prevalence of TBI is young adults (Barker-Collo, Wilde, & Feigin, Citation2009; Fail, Xu, Wald, & Coronado, Citation2010; Langlois, Kegler, Butler, & Gotsch, Citation2003) who may live with TBI-related sequelae for decades or throughout life. This entails both severe alterations of the lives of survivors and their families, and incurs high societal costs. For some, TBI should thus be viewed as a chronic disease process rather than a single event. Also, while many individuals experience improved function, others seem to decline in function over time (Corrigan & Hammond, Citation2013; Masel & DeWitt, Citation2010; Pretz & Dams-O’Connor, Citation2013).
Recent studies have suggested that health-care services offered in the chronic phase of TBI are often related to physical functioning, while needs related to cognitive, emotional and vocational difficulties are more often unmet (Andelic, Soberg, Berntsen, Sigurdardottir, & Roe, Citation2014; Heinemann, Sokol, Garvin, & Bode, Citation2002; Jennekens, de Casterle, & Dobbels, Citation2010; Koskinen, Citation1998; Olver et al., Citation1996; Prang, Ruseckaite, & Collie, Citation2012; van Walsem et al., Citation2020). This discrepancy between perceived needs and delivery of health-care services suggests that effort should be made to better tailor rehabilitation services in the chronic phase of TBI. This also involves bridging the gap between the rehabilitation services being offered by specialized health care and community-based services. Further, rehabilitation in this phase may entail incorporating aspects that receive less attention during the acute and subacute phases, such as the patient’s living environment, access to social support, motivation and community reintegration (Gagnon, Lin, & Stergiou-Kita, Citation2016; Sherer et al., Citation2015).
High quality controlled studies evaluating treatment strategies in the chronic phase of TBI should inform treatment planning, but few such studies exist (Ponsford, Harrington, Olver, & Roper, Citation2006; Powell, Heslin, & Greenwood, Citation2002). One exception is a recent treatment intervention study performed by Winter et al. (Citation2016), which included 81 military veterans with mild to severe TBI. Applying an innovative in-home-program with an individualized approach to each participant, the authors targeted current TBI-related problem areas, as well as daily functioning and community integration. The intervention was delivered in collaboration with family members, and consisted of eight intervention sessions delivered over a 4-month period. The treatment group was compared to a control group that received their usual care in the Veterans Affairs medical rehabilitation service. The intervention group showed significantly higher community re-integration and less difficulty in managing targeted outcome areas compared to the control group. Despite these encouraging results, the authors emphasized the need for replication in a civilian population. Further, 70% of the participants had a diagnosis of mild TBI, and the intervention program should be evaluated in a population with moderate and severe TBI. In addition, service delivery might be different in a public health-care system with universal access, like the one in Norway.
A future definitive randomized controlled study (RCT) aiming to include these perspectives has been planned in Norway, and the protocol has been translated into Norwegian in close collaboration with Winter and her colleagues. The intervention will include eliciting Target Outcome areas, that is, current TBI-related problems in everyday life, which participants nominate in their own words at the baseline assessment, in addition to rating the difficulty in handling the problem. This approach seems especially suitable considering that TBI is expected to cause a broad range of possible problems, allowing the intervention to be tailored to the individual’s needs and assessing changes in the severity of the problem. The intervention will address the nominated Target Outcome areas using a SMART-goal approach, which entails establishing goals that should be Specific, Measurable, Achievable, Realistic/Relevant and Timed (Bovend’Eerdt, Botell, & Wade, Citation2009). Goal Attainment Scaling (Malec, Citation1999) will accompany each goal, and therapists will collaborate with participants and family members to develop evidence-based strategies to ameliorate the specific problem area. Further, the Target Outcome-approach allows for assessment of changes in severity pre- and post-treatment to assess the effectiveness of the intervention in light of the heterogeneous nature of long-term sequelae after TBI.
In line with the recommendations of the Medical Research Council (Craig et al., Citation2008), a feasibility trial was performed. The primary objectives of this feasibility trial were to evaluate the screening and recruitment procedures, baseline and follow-up assessments, intervention delivery, acceptability and order of outcome measures in order to inform the future definitive RCT.
2. Methods
2.1. Trial design
The feasibility trial applied a one group pre-post design, including a baseline assessment (T1) and follow-up assessment immediately after the intervention (T2) as well as 8 months after the end of the intervention (T3). The study was approved by the Data Protection Office at Oslo University Hospital (OUH), Norway (2017/10390).
2.2. Procedures
This feasibility study mirrored assessment procedures planned for the future RCT in order to evaluate the protocol. Baseline data (T1) were collected through consultations with both participants and family members. A neuropsychological screening battery was used at baseline for descriptive purposes. The intervention sessions were performed between T1 and T2. Consultations with participants and family members were repeated for outcome assessment at T2 and T3. Table lists all outcome measures planned for the future definitive RCT, with a focus on the use of measures with satisfactory psychometric properties.
Table 1. All measures used at baseline (T1) and outcome (T2) assessments
2.3. Participants
Nineteen eligible participants, who sustained a severe TBI in 2009–2010 in the Oslo area, were identified from participants in the multicenter study previously conducted at OUH (Andelic et al., Citation2012). All participants were invited to participate by letter that included informed consent forms. A scripted telephone interview was performed to screen for inclusion- and exclusion criteria, and assess willingness to participate. The initial inclusion criteria were: (i) TBI diagnosis established in the acute phase, with radiologically verified intracranial injury, (ii) age 16–80 years at the time of injury, (iii) minimum 2 years since time of injury, (iv) ongoing self-reported TBI-related cognitive, emotional and/or physical problems, and/or reduced physical and mental health, and/or difficulties with participation in activities with family, friends and/or in the community, (v) living at home, and (vi) having a family member that could participate during the intervention sessions. Exclusion criteria were: (i) ongoing severe psychiatric disorders, (ii) comorbid neurological illness that could confound outcome, (iii) inability to participate in goal-setting process, (iv) inability to provide informed consent, and (v) insufficient understanding of the Norwegian language to understand intervention instructions and to complete the assessment protocol. Eligible participants were invited to complete T1 assessment at the outpatient clinic at OUH. All eligible participants and participating family members returned the written informed consent forms at T1.
2.4. Intervention
2.4.1. Framework
The intervention consisted of six in-home visits and two telephone contacts, and was delivered over a period of 4 months. Four therapists were responsible for intervention delivery. The therapists included one psychologist and one physician (junior therapists), and one neuropsychologist and one physiotherapist (senior therapists with >10 years’ experience from neuro-rehabilitation). The intervention delivery to individual participants was performed by two collaborating therapists, in order to ensure uniform treatment delivery and to increase learning. In most cases, senior and junior therapists were paired together, in order to increase reliability in the future definitive RCT-study. Therapists and study PI and co-PI (authors CR and ML) met once every or every second week for consensus discussions and supervision. A major focus in these consensus meetings was to ensure that the professional background of the therapist did not lead to lack of adherence to protocol, and to ensure common procedures for establishment of treatment plans. The TBI expertise in these meetings was considered to be high. All participants were either medical doctors, psychologists or physiotherapists. Four of the consensus participants have Ph.D.’s in the field of acquired brain injury and all participants expect the junior therapists (authors IMHB and MVF) have extensive experience from neurorehabilitation.
2.4.2. Content
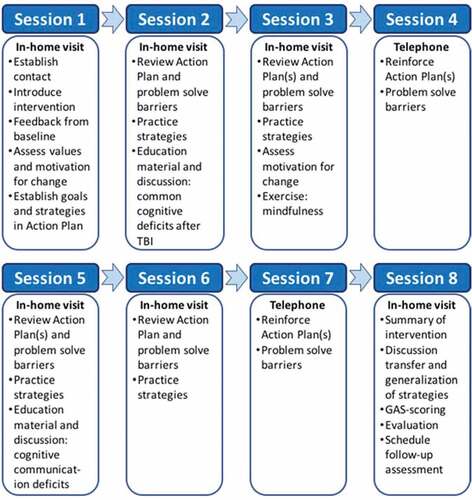
During the in-home visits, therapists collaborated with the participant and family member to identify relevant goals (usually related to the Target Outcomes nominated at baseline). A SMART-goal approach was adopted (Bovend’Eerdt et al., Citation2009). For each established SMART-goal, an accompanying Goal Attainment Scaling (GAS; Malec Citation1999) was developed to establish a quantifiable measure of goal achievement. The expected level of goal achievement was set to “0”, while higher levels of goal achievement than expected were set to “+1” and “+2”, and lower levels than expected were set to “-1” and “-2”. Next, an Action Plan was established, which included strategies to be used by the participant to achieve his or her SMART-goals. In addition to profitable strategies suggested by participants and family members, the therapist would suggest evidence-based strategies (Beck, Citation1995; Cicerone et al., Citation2011; Gracey et al., Citation2008; Haskins, Cicerone, & Trexler, Citation2012; Lejuez, Hopko, Acierno, Daughters, & Pagoto, Citation2011; Myles, Citation2004; Ponsford et al., Citation2014; Ruff, Citation2013; Tate et al., Citation2014; Togher et al., Citation2014; Velikonja et al., Citation2014; Yeates, Gracey, & Mcgrath, Citation2015), including environmental modifications and compensatory strategies. Strategy training was a main focus throughout the intervention, in addition to identification of obstacles to adaptive use of strategies and discussion regarding generalizability and transferability of strategies and new skills. Goal attainment and acceptability were evaluated during the last in-home visit (session 8). Figure shows an overview of the intervention sessions.
One area of interest in the feasibility trial was to explore the degree to which cooperation with local health professionals was relevant and feasible. Participants were asked to name a current health-care provider at T1, and all agreed that this person could be contacted for collaboration throughout the intervention. In cases where other relevant collaborators were discerned during the intervention, therapists had the opportunity to contact these if the participant consented and the contact seemed relevant.
2.5. Feasibility
The following methodological approaches were used to assess the primary objectives of the feasibility trial:
Screening and recruitment procedures were evaluated by assessing the scripted telephone interview, consent rate and time to recruit.
The T1, T2 and T3 assessments were examined for time consumption and participant burden, including ease of filling out questionnaires and burden of the neuropsychological screening battery.
Intervention delivery was evaluated based on consensus meetings, and included discussion about the appropriateness of the intervention procedures, ease of establishing SMART-goals and GAS and how the collaboration with family members and local health professionals worked in practice. Therapist burden was assessed by looking at time spent per intervention session and travel time to each appointment.
The number of sessions attended by both participants and family members was recorded. Further, the acceptability of the intervention was assessed by scores on the Acceptability scale. At T3, participants were asked about their willingness to partake in future research studies.
The order of primary and secondary outcome measures was evaluated by looking at the consistency between Target Outcome areas reported at baseline, and the goal-setting process, as well as the burden to complete outcome assessments.
3. Results
3.1. Participants
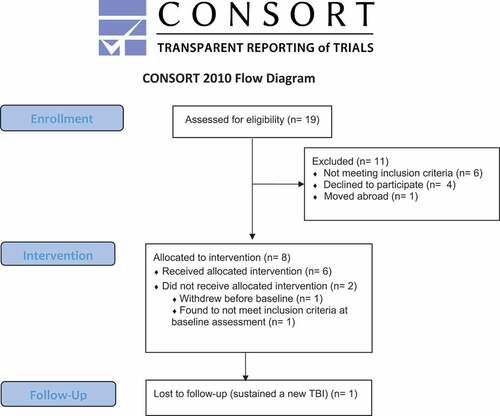
Figure displays a flow chart for the feasibility trial. Participants' age ranged from 35 to 78 years, and 5/6 were males. Three of the participants were injured in falls, while three were injured in transport-related accidents. Lowest GCS during acute care was 3, 6, 7, 7, 8 and 8 for the participants. Time since injury ranged from 91 to 104 months (approximately 7.5–8.5 years). Minimum level of education was high school (12 years). One participant was retired and one participant received disability pension. The other participants had 40% to 100% paid employment. Three were married and three were single. Participant characteristics were evaluated at baseline assessment. Table provides information about the global outcome, neuropsychological functioning and the Target Outcomes nominated by participants.
Table 2. Baseline characteristics of six participants
3.2. Feasibility
3.2.1. Objective 1: recruitment and screening procedures
The recruitment phase took place in December 2017-February 2018. The same therapist screened all 19 participants, and the prepared screening form was deemed satisfactory. The consent rate was at 40% for this sample. Half of the eligible participants were not able to appoint a family member for participation, because they were single, living far away from other relatives and did not want to include friends in the study as this was seen as too high of a burden on the friendship. This was surprising, given that Winter et al. (Citation2016) reported that only 7% of their patients were not able to include a family member. However, they recruited participants in the densely populated Philadelphia metropolitan region, and networks might be more available than in more rural Norway. We thus decided to evaluate feasibility both for patients with and without family members included. The three married participants nominated their spouses as a participating family member. All family members nominated by participants consented to participate.
3.2.2. Objective 2: baseline and follow-up assessments
Scripts to ensure reliable delivery were evaluated and judged satisfactory with only minor revisions. The baseline assessment took between 4 and 5 h, with a mean of 4.5 h and tired the participants. The burden of the neuropsychological battery was found to be high. Some of the tests were deemed redundant in that they mainly provided measures of the same cognitive functions. T2 and T3 evaluations took between 1 and 2 h for all participants, which was considered acceptable.
3.2.3. Objective 3: intervention delivery
Intervention delivery was conducted from February to June 2018. Therapists gave feedback that the intervention seemed suitable for the patient group and that both participants and family members contributed in a meaningful way to establish goals, discuss strategies and challenges to goal achievement. Five participants were able to nominate SMART-goals. However, the oldest participant displayed difficulties with collaborating in the goal-setting process, and therapists described possible signs of a progressive neurological disorder. The manual advised that the maximum number of SMART-goals should be seven, but the actual number of SMART-goals established was three for all participants. Although the manualized approach to intervention delivery was seen as ensuring treatment fidelity, therapists reported that the manual also allowed for individual adjustments that were deemed both necessary and advisable in the context of rehabilitation for the patient group. Further, therapists described the need to be more guiding in the goal establishment process for participants with more severe cognitive deficits. Table displays the SMART-goals and GAS-score outcomes from session 8.
Table 3. SMART-goals and GAS-score outcomes for each participant
In-home visits ranged from 100 to 150 min, while phone sessions ranged from 40 to 90 min. The total travel time for in-home visits ranged from 40 to 120 min. Some strategies entailed therapists being in contact with participants outside the direct contact during planned sessions, e.g., for one participant, the therapist called the participant approximately once a week to enable training on note-taking during telephone calls. None of the participants received follow-up from local health personnel at the time of inclusion. For three participants, therapists made contact with relevant health-care professionals involved in the participant’s community care. One participant sustained a new TBI right before the last intervention session, which was postponed and shortened to avoid unnecessary burden for the participant, and therapists had closer contact with the family member for guidance in handling the sub-acute phase after injury to ensure proper follow up. For two participants, therapists were in contact with their labor and welfare coordinators to discuss further strategies for work training and provide necessary information about TBI.
3.2.4. Objective 4: acceptability
Participants attended 100% of all sessions; one participant did, however, postpone the last intervention session for 6 months due to unrelated health issues. Family members attended 100% of the in-home visits. Four versions of the Acceptability scale were applied; one Participant Form, one Family Member Form, one Therapist Form for the participant and one Therapist Form for the family member (see Table ). On the Acceptability scale (ranged 0–4), higher scores reflect higher acceptability. On the Participant Form, the acceptability items of “felt bored or uninterested” and “preferred the ‘old way’ of doing activities” showed the highest scores among all participants (all scored 4, reversed), whereas the single item with the lowest score among participants was “opportunity to give feedback on therapist suggestions” (score range 3 to 4). The mean acceptability score for the six participants on the Participant Form was 3.58 out of the maximum score of 4. On the Therapist Form, the therapists scored the participants highest on “expressed the need for more information”. The therapists scored two participants to 1 on an item related to their ability to communicate effectively with the therapist. The mean acceptability score on the Therapist Form for participants was 3.38. Mean acceptability score on the Family Member Form was 3.70, and mean score on the Therapist Form for family members was 3.57. Both family members and therapists displayed the lowest scores on the item related to the family member providing feedback to suggestions made by the therapist. The five participants who completed T3 assessment all answered yes to a question regarding if they would have participated in a similar study at a later point if asked.
3.2.5. Objective 5: order of primary and secondary outcome measures
Target Outcome severity was intended as the primary outcome measure in the future definitive RCT. For the four participants who completed their T2 assessment immediately after the end of the intervention, seven Target Outcome severity scores indicated less difficulty managing the Target Outcome, three indicated increased difficulties and two indicated no change (see Table ). At T3, 3 severity scores were improved compared to T2, 7 were unchanged, while 2 scores were worse than at T2 and 3 scores were reverted to baseline levels. However, reduced awareness and response shift was found to be possible confounders. For example, for one participant that displayed reduced awareness, the selected Target Outcomes was rated as “a little problematic” at T1. Family member scoring of the same Target Outcomes gave indications that these low scores might be due to a lack of awareness. In addition, participants with increasing self-awareness during intervention might have reported more “appropriate” scoring of Target Outcome severity at T2 and T3 (as opposed to at T1), which then could make comparison with the T1 reporting difficult. Further, as participants were allowed to nominate SMART-goals that were unrelated to Target Outcomes from T1, this outcome measure did not seem well tailored to capture meaningful changes related to the intervention. For example, one participant reported frustration that he could not report back the significant change he had experienced with his difficulties with anger management, as he had not initially nominated this as a Target Outcome at T1.
Table 4. Outcome scores at baseline (T1) and 4-month (T2) and 12-month (T3) assessments
Table 5. SF-36 subscale scores for all participants at T1, and T3
However, most participants reported fewer problems with handling their targeted problem areas at follow-ups, with the biggest (mainly positive) change occurring from T1 to T2. Table and Table displays scores on outcome measures for all participants. TBI-related and depressive symptoms as well as participation tended to show favorable outcomes at T2, but tended to revert at T3. Functional competency, quality of life and Target Outcomes, on the other hand, appeared to depict a positive change that kept up at T3. As previously stated, the intention of this trial was not to evaluate the effectiveness of the intervention due to small sample size. However, it will be important to evaluate both immediate effectiveness and how the changes are maintained over time in the future definitive RCT.
3.3. Harms
No harms or unintended effects were reported.
4. Discussion
The intervention was found to be overall feasible in a population of severe TBI. Nevertheless, we discovered several elements in need of amendments.
4.1. Recruitment and screening procedures
The screening form was considered satisfactory for the future RCT. The consent rate in this sample was 40%, which is in line with the percentage reporting unmet needs for rehabilitation in the chronic phase of TBI (Andelic et al., Citation2009).
Screening revealed that several participants were unable to nominate a family member for participation to the study. Further, these individuals reported high motivation for participation and stated a clear need for rehabilitation. The feasibility trial enabled evaluating the intervention delivery for these participants as well. Therapists reported that the intervention delivery was feasible without a family member. Moreover, participants without family members showed comparable goal attainment and acceptability scores to those who had family members participating in this sample. Based on these results, a consensus was reached that the intervention is feasible without the family member participation, and that future participants without family members should be included. At the same time, family member participation was found beneficial, so inclusion of family members is recommended if available in the future definitive RCT.
Due to an increased risk for neurodegenerative disorders confounding outcome with higher age, an age limit was discussed and deemed appropriate. An upper limit of 72 years was thus set for the future definitive RCT, an age which corresponds with the retirement age in Norway. Furthermore, the lower age limit was redefined as to ensure that the TBI occurred after the age of 16, thus excluding pediatric TBIs.
4.2. Baseline and follow-up assessments
Baseline assessment posed a burden on participants and needed reduction. The IQ-estimate was considered less important than providing information regarding specific cognitive deficits, as this is relevant to tailored treatment planning. Also, several neuropsychological measures seemed to address the same functional areas. This battery was thus abbreviated, removing four of the nine tests (Vocabulary, Block Design, Coding and Symbol Search from the WAIS-IV). Similarities and Matrix reasoning were kept in order to have a general idea of level of abstract thinking. A measure of attention was deemed lacking and relevant for the population, and the Digit Span from the WAIS-IV was added.
A decision was made that the SF-36 should be replaced by Quality of Life after Brain Injury Overall Scale (QOLIBRI-OS; von Steinbuechel et al., Citation2012) and EQ-5D (Brooks, Citation1996). These measures are both shorter and easier to complete for the participants, which further decreases the participant burden. Moreover, these instruments have the added benefit of providing a diagnosis-specific measure of the quality of life; they have been more newly developed and are considered to have good validity and reliability (Janssen et al., Citation2013; von Steinbuechel, Citation2014; von Steinbuechel et al., Citation2016).
4.3. Intervention delivery
The translated and adapted manual was deemed satisfactory with minor revisions. The manual allows for individualized sessions, but includes suggested scripts that are optional. This approach was deemed clinically sound, as the level of cognitive function among the participants varied widely. Therapist burden is considered high in this study, taking into account time to travel, time spent during home visits and time spent planning sessions, contacting local professionals and participating in consensus meetings for supervision. Furthermore, the burden related to travel time will increase in the future definitive RCT as the geographical area covered in the current study was restricted to <1-h travel each way. The geographical area supported in the RCT will be larger, with travel times up to 4 h each way, and the feasibility trial was considered helpful in logistics planning in preparation for the RCT. Consensus meetings and group discussions of clinical challenges were deemed useful and will be continued in the RCT, in order to uphold shared clinical understandings of intervention content and maintain common prioritizations during goal setting. An interesting finding was that the prioritized goals by patients in this sample were mainly related to difficulties with cognitive, emotional and social functioning, areas shown in previous studies to be prominent after TBI, but receive less attention than, e.g.,, physical difficulties (Andelic et al., Citation2014). This suggests that the intervention is suitable for targeting some of the unmet needs reported in the literature in this population. To our knowledge, no comparable interventions exist in Norway. The intervention is feasible, but is also costly, as travelling time to participants results in a time-consuming intervention. Given that the future RCT provides proof of efficacy, a cost-effectiveness analysis will be performed.
4.4. Acceptability
Acceptability was high and comparable to scores in the Winter study (Winter et al Citation2016). Items with lower scores for both participants and family members were the ability to give feedback to therapist suggestions. This result might reflect a dilemma therapists had in balancing the need to be sensitive to feedback, while also structuring the intervention sessions in accordance with the manual and predefined time limit. Although therapists are encouraged to continue to be sensitive to this issue, no major changes are suggested. Lengthening intervention sessions further is not recommended, as intervention sessions >120 min were reported by therapists to be too tiring for participants.
4.5. Order of primary and secondary outcome measures
Target Outcome severity was evaluated for appropriateness as a primary outcome measure in the future definitive RCT. However, the feasibility trial demonstrated some uncertainties as to the appropriateness of retaining this measure as the sole primary outcome measure. Firstly, participants varied in how they reported Target Outcomes, i.e., both the broadness of the problem areas and evaluation of their severity. This led to some difficulties in comparing scores both within and across participants. The range of the severity scale (0–4) was considered restrictive, possibly failing to detect nuances in difficulty. Further, as described above, reduced awareness of deficits provided an additional issue during both baseline and outcome assessments. Making the decision to remove family member participation as an inclusion criterion (see above) entails that family member scores might not be provided for all participants in the future definitive RCT. Overall, it seemed prudent to replace Target Outcome severity as a primary measure, while retaining it as secondary outcome.
Reduced quality of life and participation are commonly reported problem areas in the chronic phase of TBI. Early rehabilitation seldom targets these areas, but interventions delivered in the chronic phase should entail targeting these important areas. Thus, measures of quality of life (QOLIBRI and EQ-5D) and participation (PART-O) were chosen as primary outcomes for the future definitive RCT. These are included as common data elements (CDE) recommendations for TBI outcomes and are considered methodologically strong (Maas, Harrison-Felix, & Menon et al., Citation2010; Wilde et al., Citation2010).
During analysis of feasibility data, researchers were alerted to a possible bias in assessment of mental health, as only depressive symptomatology was being assessed, not anxiety. After TBI, the risk of depression is higher than in the average population, but so is the risk for anxiety-related disorders (Sigurdardottir, Andelic, Roe, & Schanke, Citation2013). Symptoms of anxiety were also detected during intervention delivery for several participants, and the Generalized Anxiety Disorder seven-item (GAD-7; Spitzer, Kroenke, Williams, & Lowe, Citation2006) was thus added to the protocol. This 7-item questionnaire is unlikely to increase participant burden noticeably.
4.6. Limitations
The current feasibility trial has several limitations. Firstly, it only included six participants. Secondly, the sample for this trial was rather selective, and generalizability might thus be limited.
5. Conclusion
The present home-based rehabilitation program was feasible with civilian persons having sustained a TBI in Norway. Participants reported high acceptability and the process of setting SMART-goals and Goal Attainment Scaling was deemed suitable, feasible and acceptable. The feasibility trial led to important amendments to inclusion criteria, baseline assessment and outcome measures that were adapted before the RCT study commenced. The RCT study started recruitment in June 2018.
Protocol
Protocol for the future definitive RCT study has been submitted to the journal Trials. The protocol is registered at ClinicalTrials.gov NCT03545594. Protocol for the feasibility study is available by contacting the first author.
Authors’ Contributions
CR, ML, and SS are senior researchers in the project and developed the design of the feasibility trial based on LW’s research and with her collaboration. IMHB is a doctoral fellow, while MVF, SH and IK are post-doctoral fellows in this project and they have piloted all procedures and contributed to the final study design and methods. All authors helped draft the manuscript and consent to publication. All authors read and approved the final manuscript.
Ethical Approval
The study has been presented to the Norwegian Regional Committee for Medical and Health Research Ethics (REK) (REK number 2017/1081) and approved by the Data Protection Office at OUH (2017/10390). The project will be conducted according to the ethical guidelines of the Helsinki declaration. Information about the study will be presented to the participants in written and oral form. Written informed consent will be obtained, and the right to withdraw from the project at any time without any explanation necessary will be emphasized. All participants will be assigned an identification number, and all the questionnaires and datasets will be anonymized. Only the project team will have access to the document that links study identifiers with participant names.
Additional information
Funding
Notes on contributors
Ida Maria H. Borgen
The current trial is part of a larger collaborative research project conducted by the Department of Physical Medicine and Rehabilitation at Oslo University Hospital (OUH) and Sunnaas Rehabilitation Hospital. The authors contribute to several multidisciplinary research groups, including “Rehabilitation after trauma” at OUH that aims to generate knowledge about neurotrauma, treatment and rehabilitation, with a main focus on traumatic brain injury (TBI), and Research Centre for Habilitation and Rehabilitation Models & Services funded by the Norwegian Research Council to strengthen research in rehabilitation services. The project is further conducted in collaboration with Laraine Winter, who developed the intervention program and evaluated its effectiveness in Philadelphia, USA. The larger research project will contribute to the knowledge on how rehabilitation services could be provided by specialist health care institutions in Norway for patients with chronic TBI, and might inform more generally on provision of ambulatory rehabilitation services for comparable patient groups.
References
- Andelic, N., Anke, A., Skandsen, T., Sigurdardottir, S., Sandhaug, M., Ader, T., & Roe, C. (2012). Incidence of hospital-admitted severe traumatic brain injury and in-hospital fatality in Norway: A national cohort study. Neuroepidemiology, 38(4), 259–19. doi:10.1159/000338032
- Andelic, N., Hammergren, N., Bautz‐Holter, E., Sveen, U., Brunborg, C., & Røe, C. (2009). Functional outcome and health‐related quality of life 10 years after moderate‐to‐severe traumatic brain injury. Acta Neurologica Scandinavica, 120(1), 16–23. doi:10.1111/j.1600-0404.2008.01116.x
- Andelic, N., Soberg, H. L., Berntsen, S., Sigurdardottir, S., & Roe, C. (2014, Nov). Self-perceived health care needs and delivery of health care services 5 years after moderate-to-severe traumatic brain injury. PM&R, 6(11), 1013–1021. doi:10.1016/j.pmrj.2014.05.005
- Barker-Collo, S., Wilde, J. H., & Feigin, V. L. (2009). Trends in head injury incidence in New Zealand: A hospital-based study from 1997/1998 to 2003/2004. Neuroepidemiology, (2009)(32), 32–39. doi:10.1159/000170090
- Beck, J. S. (1995). Cognitive therapy: basics and beyond. New York, NY: Guilford Press.
- Bovend’Eerdt, T. J., Botell, R. E., & Wade, D. T. (2009). Writing SMART rehabilitation goals and achieving goal attainment scaling: A practical guide. Clinical Rehabilitation, 23(4), 352–361. doi:10.1177/0269215508101741
- Brooks, N., Campsie, L., Symington, C., Beattie, A., & McKinlay, W. (1986). The five year outcome of severe blunt head injury: A relative’s view. Journal of Neurology, Neurosurgery and Psychiatry, 49, 764–770. doi:10.1136/jnnp.49.7.764
- Brooks, R. (1996). EuroQol: The current state of play. Health Policy (Amsterdam, Netherlands), 37(1), 53–72. doi:10.1016/0168-8510(96)00822-6
- Cicerone, K. D., Langenbahn, D. M., Braden, C., Malec, J. F., Kalmar, K., Fraas, M., … Azulay, J. (2011). Evidence-based cognitive rehabilitation: Updated review of the literature from 2003 through 2008. Archives of Physical Medicine and Rehabilitation, 92(4), 519–530. doi:10.1016/j.apmr.2010.11.015
- Corrigan, J. D., & Hammond, F. M. (2013, Jun). Traumatic brain injury as a chronic health condition. Archives of Physical Medicine and Rehabilitation, 94(6), 1199–1201. doi:10.1016/j.apmr.2013.01.023
- Craig, P., Dieppe, P., Macintyre, S., Michie, S., Nazareth, I., & Petticrew, M. (2008). Developing and evaluating complex interventions: The new medical research council guidance. BMJ, 337, a1655. doi:10.1136/bmj.a1655
- Delis, D. C., Kaplan, E., & Kramer, J. H. (2001). Delis-Kaplan Executive function system: Examiners manual. San Antonio: The Psychological Corporation.
- Delis, D. C., Kramer, J. H., Kaplan, E., & Ober, B. A. (2000). California verbal learning test (2nd ed.). San Antonio: Harcourt Assessment.
- Dikmen, S. S., Machamer, J. E., Powell, J. M., & Temkin, N. R. (2003). Outcome 3 to 5 years after moderate to severe traumatic brain injury1. Archives of Physical Medicine and Rehabilitation, 84(10), 1449–1457. doi:10.1016/S0003-9993(03)00287-9
- Elmståhl, S., Malmberg, B., & Annerstedt, L. (1996). Caregiver’s burden of patients 3 years after stroke assessed by a novel caregiver burden scale. Archives of Physical Medicine and Rehabilitation, 77, 177–182. doi:10.1016/S0003-9993(96)90164-1
- Fail, M., Xu, L., Wald, M. M., & Coronado, V. G. (2010). Traumatic brain injury in the United States: Emergency department visits, hospitalization and deaths 2002–2006. Atlanta, GA: Centers for Disease Control and Prevention, National Center for Injury Prevention and Control. Retrieved from https://stacks.cdc.gov/view/cdc/5571
- Forslund, M. V., Arango-Lasprilla, J. C., Roe, C., Perrin, P. B., Sigurdardottir, S., & Andelic, N. (2014). Multi-level modelling of employment probability trajectories and employment stability at 1, 2 and 5 years after traumatic brain injury. Brain Injury, 28(7), 980–986. doi:10.3109/02699052.2014.888770
- Gagnon, A., Lin, J., & Stergiou-Kita, M. (2016, Mar). Family members facilitating community re-integration and return to productivity following traumatic brain injury – motivations, roles and challenges. Disability and Rehabilitation, 38(5), 433–441. doi:10.3109/09638288.2015.1044035
- Gracey, F., Palmer, S., Rous, B., Psaila, K., Shaw, K., O’Dell, J., … Mohamed, S. (2008). “Feeling part of things”: Personal construction of self after brain injury. Neuropsychological Rehabilitation, 18(5–6), 627–650. doi:10.1080/09602010802041238
- Haskins, E. C., Cicerone, K. D., & Trexler, L. E. (2012). Cognitive rehabilitation manual: Translating evidence-based recommendations into practice. Reston, VA: ACRM Publishing.
- Heinemann, A. W., Sokol, K., Garvin, L., & Bode, R. K. (2002). Measuring unmet needs and services among persons with traumatic brain injury. Archives of Physical Medicine and Rehabilitation, 83(8), 1052–1059. doi:10.1053/apmr.2002.34283
- Hoofien, D., Gilboa, A., Vakil, E., & Donovick, P. J. (2001). Traumatic brain injury (TBI) 10-20 years later: A comprehensive outcome study of psychiatric symptomatology, cognitive abilities and psychosocial functioning. Brain Injury, 15(3), 189–209. doi:10.1080/026990501300005659
- Janssen, M. F., Pickard, A. S., Golicki, D., Gudex, C., Niewada, M., Scalone, L., … Busschbach, J. (2013, Sep). Measurement properties of the EQ-5D-5L compared to the EQ-5D-3L across eight patient groups: A multi-country study. Quality of Life Research, 22(7), 1717–1727. doi:10.1007/s11136-012-0322-4
- Jennekens, N., de Casterle, B. D., & Dobbels, F. (2010). A systematic review of care needs of people with traumatic brain injury (TBI) on a cognitive, emotional and behavioural level. Journal of Clinical Nursing, 19(9–10), 1198–1206. doi:10.1111/jcn.2010.19.issue-9-10
- Jennett, B., & Bond, M. (1975). Assessment of outcome after severe brain damage. Lancet, 1, 480–484. doi:10.1016/S0140-6736(75)92830-5
- Jourdan, C., Azouvi, P., Genêt, F., Selly, N., Josseran, L., & Schnitzler, A. (2018). Disability and health consequences of traumatic brain injury: National prevalence. American Journal of Physical Medicine & Rehabilitation, 97(5), 323–331. doi:10.1097/PHM.0000000000000848
- King, N. S., Crawford, S., Wenden, F. J., Moss, N. E., & Wade, D. T. (1995). The rivermead post concussion symptoms questionnaire: A measure of symptoms commonly experienced after head injury and its reliability. Journal of Neurology, 242(9), 587–592. doi:10.1007/BF00868811
- Koskinen, S. (1998). Quality of life 10 years after a very severe traumatic brain injury (TBI): The perspective of the injured and the closest relative. Brain Injury, 12(8), 631–648. doi:10.1080/026990598122205
- Kroenke, K., Spitzer, R. L., & Williams, J. B. (2001). The PHQ-9: Validity of a brief depression severity measure. Journal of General Internal Medicine, 16(9), 606–613. doi:10.1046/j.1525-1497.2001.016009606.x
- Langlois, J. A., Kegler, S. R., Butler, J. A., & Gotsch, K. (2003). Traumatic brain injury-related hospital discharges: Results from a 14-state surveillance system, 1997. Mortality and Morbidity Weekly Report Surveillance Summaries, 52, 1–18.
- Langlois, J. A., Rutland-Brown, W., & Wald, M. M. (2006). The epidemiology and impact of traumatic brain injury: A brief overview. Journal of Head Trauma Rehabilitation, 21(5), 375–378. doi:10.1097/00001199-200609000-00001
- Lejuez, C. W., Hopko, D. R., Acierno, R., Daughters, S. B., & Pagoto, S. L. (2011). Ten year revision of the brief behavioral activation treatment for depression: Revised treatment manual. Behavior Modification, 35(2), 111–161. doi:10.1177/0145445510390929
- Maas, A. I., Harrison-Felix, C. L., Menon, D., Adelson, P. D., Balkin, T., Bullock, R., … & Robertson, C. (2010). Common data elements for traumatic brain injury: Recommendations from the interagency working group on demographics and clinical assessment. Archives of Physical Medicine and Rehabilitation, 91(11), 1641–1649. doi:10.1016/j.apmr.2010.07.232
- Malec, J. F. (1999). Goal attainment scaling in rehabilitation. Neuropsychological Rehabilitation, 9(3/4), 253–275. doi:10.1080/096020199389365
- Masel, B. E., & DeWitt, D. S. (2010, Aug). Traumatic brain injury: A disease process, not an event. Journal of Neurotrauma, 27(8), 1529–1540. doi:10.1089/neu.2010.1358
- Myles, S. M. (2004). Understanding and treating loss of sense of self following brain injury: A behavior analytic approach. International Journal of Psychology and Psychological Therapy, 4(3), 487–504.
- Olver, J. H., Ponsford, J. L., & Curran, C. A. (1996). Outcome following traumatic brain injury: A comparison between 2 and 5 years after injury. Brain Injury, 10(11), 841–848. doi:10.1080/026990596123945
- Ponsford, J., Bayley, M., Wiseman-Hakes, C., Togher, L., Velikonja, D., McIntyre, A., ., … Tate, R. (2014). INCOG recommendations for management of cognition following TBI, part II: Attention and information processing speed. Journal of Head Trauma Rehabilitation, 29(4), 321–337. doi:10.1097/HTR.0000000000000072
- Ponsford, J., Draper, K., & Schonberger, M. (2008). Functional outcome 10 years after traumatic brain injury: Its relationship with demographic, injury severity, and cognitive and emotional status. Journal of the International Neuropsychological Society, 14(2), 233–242. doi:10.1017/S1355617708080272
- Ponsford, J., Harrington, H., Olver, J., & Roper, M. (2006). Evaluation of a community-based model of rehabilitation following traumatic brain injury. Neuropsychological Rehabilitation, 16(3), 315–328. doi:10.1080/09602010500176534
- Powell, J., Heslin, J., & Greenwood, R. (2002). Community based rehabilitation after severe traumatic brain injury: A randomised controlled trial. Journal of Neurology, Neurosurgery & Psychiatry, 72(2), 193–202. doi:10.1136/jnnp.72.2.193
- Prang, K. H., Ruseckaite, R., & Collie, A. (2012). Healthcare and disability service utilization in the 5-year period following transport-related traumatic brain injury. Brain Injury, 26(13–14), 1611. doi:10.3109/02699052.2012.698790
- Pretz, C. R., & Dams-O’Connor, K. (2013, Dec). Longitudinal description of the glasgow outcome scale-extended for individuals in the traumatic brain injury model systems national database: A National Institute on Disability and Rehabilitation Research traumatic brain injury model systems study. Archives of Physical Medicine and Rehabilitation, 94(12), 2486–2493. doi:10.1016/j.apmr.2013.06.021
- Prigatano, G. P., Fordyce, D., Zeiner, H., Roueche, J., Pepping, M., & Wood, B. (1986). Neuropsychological rehabilitation after brain injury. Baltimore, MD: John Hopkins University press.
- Roth, R. M., Isquith, P. K., & Gioia, G. A. (2005). Behavior rating inventory of executive function-adult version (BRIEF-A). Lutz, FL: Psychological Assessment Resources.
- Ruff, R. (2013). Selecting the appropriate psychotherapies for individuals with traumatic brain injury: What works and what does not? NeuroRehabilitation, 32(4), 771–779. doi:10.3233/NRE-130901
- Ruttan, L., Martin, K., Liu, A., Colella, B., & Green, R. E. (2008). Long-term cognitive outcome in moderate to severe traumatic brain injury: A meta-analysis examining timed and untimed tests at 1 and 4.5 or more years after injury. Archives of Physical Medicine and Rehabilitation, 89(12), 69–76. doi:10.1016/j.apmr.2008.07.007
- Sherer, M., Sander, A. M., Maestas, K. L., Pastorek, N. J., Nick, T. G., & Li, J. (2015, Apr). Accuracy of self-reported length of coma and posttraumatic amnesia in persons with medically verified traumatic brain injury. Archives of Physical Medicine and Rehabilitation, 96(4), 652–658. doi:10.1016/j.apmr.2014.10.024
- Sigurdardottir, S., Andelic, N., Roe, C., & Schanke, A. K. (2013). Depressive symptoms and psychological distress during the first five years after traumatic brain injury: Relationship with psychosocial stressors, fatigue and pain. Journal of Rehabilitation Medicine, 45(8), 808–814. doi:10.2340/16501977-1156
- Spitzer, R. L., Kroenke, K., Williams, J. B., & Lowe, B. (2006). A brief measure for assessing generalized anxiety disorder: The GAD-7. Archives of Internal Medicine, 166(10), 1092–1097. doi:10.1001/archinte.166.10.1092
- Tagliaferri, F., Compagnone, C., Korsic, M., Servadei, F., & Kraus, J. (2006, 1). A systematic review of brain injury epidemiology in Europe. Acta Neurochirurgica, 148(3), 255–268. doi:10.1007/s00701-005-0651-y
- Tate, R., Kennedy, M., Ponsford, J., Douglas, J., Velikonja, D., Bayley, M., & Stergiou-Kita, M. (2014). INCOG recommendations for management of cognition following TBI part III: Executive function and self-awareness. Journal of Head Trauma Rehabilitation, 29(4), 338–352. doi:10.1097/HTR.0000000000000068
- Togher, L., Wiseman-Hakes, C., Douglas, J., Stergiou-Kita, M., Ponsford, J., Teasell, R., … Turkstra, L. S. (2014). INCOG recommendations for management of cognition following TBI, part IV: Cognitive communication. Journal of Head Trauma Rehabilitation, 29(4), 353–368. doi:10.1097/HTR.0000000000000071
- Turner-Stokes, L., & Siegert, R. J. The needs and provision complexity scale: Measuring met and unmet needs in the community for patients with complex neurological disabilities. Poster Presentation. 7th World Congress in Neurorehabilitation; Melbourne. May 2012 Neurorehabilitation and Neural Repair 2012; 26(6):695-804 (Poster 49)
- van Walsem, M. R., Howe, E. I., Perrin, P. B., Sigurdardottir, S., Røe, C., Sveen, U., … Andelic, N. (2020, Jan). Trajectories of self-reported competency up to 10 years following moderate-to-severe traumatic brain injury. Brain Injury, 14, 1–8. doi:10.1080/02699052.2019.1704061
- Velikonja, D., Tate, R., Ponsford, J., McIntyre, A., Janzen, S., & Bayley, M. (2014). INCOG recommendations for management of cognition following TBI, part V: Memory. Journal of Head Trauma Rehabilitation, 29(4), 369–386. doi:10.1097/HTR.0000000000000069
- von Steinbuechel, N. (2014, Dec 1). Lessons from the Qolibri Overall Scale (QOLIBRI-OS) for its use worldwide. European Health Psychologist, 16(S), 642.
- von Steinbuechel, N., Covic, A., Polinder, S., Kohlmann, T., Cepulyte, U., Poinstingl, H., … Formisano, R. (2016). Assessment of health-related quality of life after TBI: Comparison of a disease-specific (Qolibri) with a generic (sf-36) instrument. Behavioural Neurology, 2016. doi:10.1155/2016/7928014
- Von Steinbuechel, N., Wilson, L., Gibbons, H., Muehlan, H., Schmidt, H., Schmidt, S., Sasse, N., Koskinen, S., Sarajuuri, J., Höfer, S. and Bullinger, M. (2012). QOLIBRI overall scale: a brief index of health-related quality of life after traumatic brain injury. Journal of Neurology, Neurosurgery & Psychiatry, 83(11), 1041–1047.
- Ware, J. E., Jr, Sherbourne, C. D., & The, M. O. S. (1992, Jun). 36-item short-form health survey (SF-36): I. Conceptual framework and item selection. Medical Care, 1, 473–483. doi:10.1097/00005650-199206000-00002
- Wechsler, D. (2008). Wechsler Adult Intelligence Scale (Fourth ed.). San Antionio: Pearson.
- Whiteneck, G. G., Dijkers, M. P., Heinemann, A. W., Bogner, J. A., Bushnik, T., Cicerone, K. D., Corrigan, J. D., Hart, T., Malec, J. F. and Millis, S. R. (2011). Development of the Participation Assessment with Recombined Tools–Objective for use after traumatic brain injury. Archives of physical medicine and rehabilitation, 92(4), 542–551.
- Wilde, E. A., Whiteneck, G. G., Bogner, J., Bushnik, T., Cifu, D. X., Dikmen, S., … French L (2010, Nov). Recommendations for the use of common outcome measures in traumatic brain injury research. Archives of Physical Medicine and Rehabilitation, 91(11), 1650–1660. doi:10.1016/j.apmr.2010.06.033
- Winter, L., Moriarty, H. J., Robinson, K., Piersol, C. V., Vause-Earland, T., Newhart, B., … Iacovone DB. (2016). Efficacy and acceptability of a home-based, family-inclusive intervention for veterans with TBI: A randomized controlled trial. Brain Injury, 16, 1–15.
- Yeates, G. N., Gracey, F., & Mcgrath, J. C. (2015). A biopsychosocial deconstruction of “personality change” following acquired brain injury. Neuropsychological Rehabilitation, 18(5–6), 566–589. doi:10.1080/09602010802151532