Abstract
This study assessed the impacts of an ointment on treatment efficacy, side effects and global tolerance of most common genital diseases. This observational study enrolled symptomatic patients with LS and psoriasis under maintenance therapy (topical steroids); patients with condyloma acuminata treated with liquid nitrogen and/or imiquimod; patients suffering from vulvodynia under multidisciplinary treatment. The tested ointment was added to the usual treatment. Subjective and objective clinical scores, tolerance and Dermatology Life Quality Index (DLQI) were evaluated. One hundred patients completed the study: 26 LS, 6 psoriasis, 52 CA and 16 vulvodynia. There were significant reductions in global symptom and clinical scores for LS (−80.61% and −59.20%, respectively, mean follow-up 66 days) and psoriasis (−82.88% and −82.80%, respectively, mean follow-up of 51 days). The DLQI decreased by 40.17% for LS and 76.92% for psoriasis. The use of ointment resulted in low post-treatment scores for CA (Symptom score: 1.92/maximum 50, clinical score: 2.46/24 maximum, mean follow-up of 17 days). Tolerance was good for all except two patients (one CA, one vulvodynia). Our results indicated the daily applications of an ointment improved treatments for lichen sclerosus, psoriasis (symptoms, clinical signs and quality of life) and for condyloma acuminata (reduced healing time) and was well tolerated during vulvodynia.
PUBLIC INTEREST STATEMENT
Most treatments of genital diseases are not adapted to this area where the skin is fragile and submitted to frictions and occlusion. They address clinical signs but do not consider clinical symptoms. This often leads to partial remission, decreased quality of life (QOL) and lack of adherence to treatment. The aim of this study was to demonstrate that adding an ointment to classical genital treatments can increase their performances for both symptoms and signs and thus QOL of patients.
And indeed, the emollient improved symptoms by more than 80% within 2 months for women with lichen sclerosus and psoriasis under topical steroid treatment. It also diminished clinical signs by 69% and 82%, respectively, and improved QOL by 40% and 76%. It enabled a quick healing time after treatments of genital warts (liquid nitrogen and/or imiquimod) for both men and women and was well tolerated by patients with very sensitive/painful skin affected by vulvodynia.
1. Introduction
Genital skin is subject to various irritant factors (Czuczwar et al., Citation2016). Emollients are important moisturising agents that play an integral part in the treatment of skin disorders. They act by producing an occlusive film on the skin and prevent water loss. Best practice generally advises the use of emollients after the cleansing routine when the skin has a high water—allowing at least 30 minutes to one hour between topical medications, which prevents dilution and the unknown effects on the stability and absorption of the medications (usually topical steroids; Lawton & Littlewood, Citation2006). When used for vulvar conditions patients may need to use them at least twice a day and after going to the toilet. External genital diseases may benefit from the addition of skin care with an emollient to medical treatment. However, very few studies of this approach have been published.
Lichen sclerosus (LS) is the most common genital skin disease, causing pruritus, burning sensation, pain (soreness), and dyspareunia. One-third of patients reports severe impairment of Quality of Life (QoL) (Van Cranenburgh et al., Citation2017). Vulvar LS is a relapsing disease (relapse rate 50% at 1.3 years, 84% at 4 years post-treatment; Renaud-Vilmer et al., Citation2004). Patients with vulvar LS require lifetime surveillance and topical steroid (TS) treatment. The ultra-potent clobetasol propionate is the most frequently used treatment, but mometasone furoate (MMF) has similar efficacy (Moyal-Barracco & Wendling, Citation2014; Virgili et al., Citation2014). After a 3-month initial attack phase, symptom improvement is incomplete (47.3%) and reversal of clinical signs is rare (21.4%) (Borghi et al., Citation2018). To prevent relapse and scarring, and to help prevent malignant change, a proactive long-term treatment (e.g., twice-weekly application of TS) strategy is recommended (Kirtschig et al., Citation2015; Van der Meijden et al., Citation2017; Virgili, Minghetti, Borghi, Corazza et al., Citation2013a). Complete remission occurs for only 32% of women after 3 years and 58% after 6 years. (Virgili, Minghetti, Borghi, Corazza et al., Citation2013b). This means that a lot of patients remain symptomatic, with some activity of their LS, despite the maintenance therapy. However, it is not recommended to increase the frequency of TS applications, because long-term TS therapy can alter skin barrier function and result in steroid-induced dermatitis. Recent guidelines recommend emollient use to protect the skin barrier and relieve symptoms during and after steroid treatment (Lewis et al., Citation2018).
During psoriasis, 33%–63% of women will develop genital psoriasis (Meeuwis et al., Citation2011). It causes pruritus, pain, burning sensation, and dyspareunia. It typically expresses as flares but may become chronic and impair QoL and sexual health (Ryan et al., Citation2015). Treatment includes the use of long-term, sequential or intermittent application of moderate to potent TSs. However, these steroids are often not potent enough to induce a complete response, and thus to completely relieve patients from their symptoms (pruritus mainly; Czuczwar et al., Citation2016). Patients should avoid trigger factors associated with the Koebner phenomenon. Emollient use is recommended during extra-genital psoriasis to reduce treatment-induced irritation and maintain therapeutic results (Paulsen et al., Citation2005).
The development of condyloma acuminata (CA) remains the most frequent sexually transmitted infection (STI). CA can be located on external genital organs and the perianal region. All treatments for CA are aggressive to the skin, especially liquid nitrogen (LN) and imiquimod. Imiquimod induces some combination of vulvar erythema, inflammation that sometimes includes erosion and ulceration. It seems that irritation parallels efficacy (Werner et al., Citation2016).
Vulvodynia affects 7–10% of women worldwide. 2003 International Society for the Study of Vulvovaginal Diseases (ISSVD) terminology defines vulvodynia as vulvar discomfort (e.g., burning pain) that occurs without visible findings or a specific, clinically identifiable, neurological disorder (Bornstein et al., Citation2016). Treatments for this neuropathic pain syndrome are complex, and a multidisciplinary approach is recommended (Edwards et al., Citation2015). Vulvar care measures include avoiding irritating factors and emollient soap substitute use (Van der Meijden et al., Citation2017). Most patients experience intolerance to emollients possibly related to increased local inflammation or a nocebo effect.
Our objective was to evaluate the complementary effects of the use of an ointment applied during the treatment of the most common genital conditions on clinical parameters and quality-of-life (QoL) characteristics.
2. Materials and methods
This monocentric observational study was performed at the Institut Alfred Fournier (Paris, France). Recruitments for LS and psoriasis concerned only female patients during a specialized vulvar clinic. Men and women were recruited for CA participation during vulvar and andrological visits. Inclusion was based on first-raw visits. The overall sample size goal was 100 patients (80 women, 20 men).
The goal was to evaluate a once to twice daily topical emollient (paraffinum liquidum, petrolatum, paraffinum, tocopheryl acetate; Deumavan® Intimate Hygiene Salve Natural, Kaymogyn GmbH, Freiburg im Breisgau, Germany) application added to the usual care protocol. There were no changes in the frequencies of the TS and imiquimod applications. Patients were instructed to maintain the use of their usual toilet and washing products.
Patients were examined at the initial visit (T0) and a second visit (T1). Investigators scored symptoms (Visual Analog Score (VAS) 0–10), clinical signs (score 0–4), tolerance. The scoring system was modified from Borghi et al. (Edwards et al., Citation2015; Virgili et al., Citation2014). Each patient completed an auto-evaluation questionnaire (adapted to the pathology with seven questions that were similar between pathologies). The responses “totally agree” and “rather agree” were rated as favorable responses. Women with LS, psoriasis, or CA had illustrative photos taken at each consultation. Women with LS or psoriasis completed a dermatological QoL questionnaire (Dermatology Life Quality Index (DLQI)).
2.1. Inclusion/exclusion criteria and objectives
Pregnant or lactating women, women applying cosmetic or hormonal topical treatments, and patients with known hypersensitivity to an ingredient of the tested product were excluded from the study.
For the vulvar LS group (30 expected patients), we selected patients who already completed the active treatment phase with TS; who were under maintenance therapy (usually TS twice weekly), who were still symptomatic despite the treatment; whose examination discovered an LS with moderate activity which did not require an intensification of the TS steroid (LS without signs of surinfection or signs of severity, e.g., leukoplakia, ulceration); and who did not apply any moisturizing cream. LS diagnosis was based on clinical observation of typical clinical signs. Patients were asked not to modify their TS treatment and only to add the ointment once to twice daily. As there was only one changing parameter, we could reasonably attribute modifications of clinical signs and/or symptoms to the ointment. The T1 visit was expected at 30 days after T0. At each visit, information on age, date of diagnosis, topography, and frequency of TS were recorded. Six symptoms (pruritus, burning sensation, dyspareunia, discomfort, sensation of dryness, spontaneous pain) were recorded using a modified Global Symptoms Score (mGSS). Nine clinical signs (pallor/whiteness, dryness, sclerosus/atrophy/synechia, lichenification, hyperkeratosis, erosions, fissures, erythema, purpura) were recorded using a modified Global Clinical Score (mGCS). The numbers (%) of patients with improvement scores ≥75% (mGSS75, mGCS75) and ≥50% (mGSS50, mGCS50) were recorded. An Investigator Global Assessment (IGA) was also performed at T1 (i.e., T1 results compared with baseline). The DLQI scoring was completed at T0 and T1 and auto-evaluation questionnaire (13 questions) at T1. Clinical pictures were taken at T0 and T1 with the consent of the patients.
The primary objective was to determine if there were statistically significant improvements in mGS and mGC scores between T0 and T1 (Mann–Whitney statistical score, p < 0.05). Another objective was to determine if IGA results were 0, 1 or 2 for most patients (i.e., improvement of ≥75%).
Criteria to include patients with vulvar psoriasis were similar to the ones for LS. They were patients who had finished the active treatment phase, who were regularly treated with TS (maintenance therapy) to prevent relapses but who were still symptomatic. Patients applying moisturizing cream were not included (10 expected patients). A psoriasis diagnosis was made on based typical clinical signs. Testing included a vulvovaginal swab to exclude candidiasis if suspected. The T1 evaluation was expected at D30 after T0. Scores (mGSS, IGA, and DLQI) and auto-evaluation questionnaire were like the LS protocol. The clinical signs recorded in the psoriasis GCS (pGCS) were desquamation, dryness, lichenification, erosions, fissures, and erythema. The DLQI scoring was completed at T0 and T1 and auto-evaluation questionnaire (13 questions) at T1. Clinical pictures were taken at T0 and T1 with the consent of the patients.
Patients with exophytic or papular CA on the external genital area, without signs of infection (candidiasis) and without prior treatment within the preceding month, were included (20 expected women, 20 expected men). Application of LN (T1 after 7 days) or imiquimod (T1 after 30 days), or both, was proposed depending on the extent of the CA and prior treatment use. The information collected on CA was CA topography, five symptoms using a CA GSS (mGSS without dyspareunia), and six clinical signs (desquamation, dryness, erosions, fissures, erythema, edema) using a CA GCS. The auto-evaluation questionnaire (15 questions) was completed at T1. The primary objectives for liquid nitrogen were to improve healing and to improve tolerance for treatment with imiquimod. Therefore, the analysis was used to determine if minimal changes in scores were obtained at T1.
The information collected for the vulvodynia group (20 patients) at T0 was the type of vulvodynia (spontaneous, provoked, or both), mGSS, and treatments used. The clinical examination results were, by definition, normal for the patients in this group. The diagnosis was made following the ISSVD definition (Werner et al., Citation2016). The expected T1 was 30 days. The primary objective was to determine tested-product tolerance using the self-evaluation questionnaire (12 questions).
Our study follows the principles of the Declaration of Helsinki and has been performed according to proper ethical standards. All patients signed consent forms for photos.
2.2. Statistical analysis
Statistical analysis was performed using the R software application (Borghi et al., Citation2015). Quantitative scores at D0 and after numbers of days of the application were compared using Wilcoxon test for paired data. The frequencies of positive answers to questions were evaluated using Chi-square tests.
3. Results
One hundred twenty-five patients were included in the study from July 2017–March 2018. Nineteen of these patients were lost to follow-up (8 LS, 3 psoriasis, 4 CA, 4 vulvodynia) and six had a deviation from the study protocol (stopped tested product prematurely: 4 LS, 1 CA, 1 vulvodynia). One hundred patients complied with the protocol and were evaluated (26 LS, 6 psoriasis, 52 CA (33 women, 19 men), 16 vulvodynia) (demographic and clinical data at T0 are presented in Table ).
Table 1. Results for demographic and clinical data at time 0 (T0). Except for the CA group, the mean age was after menopause, with a long duration of symptoms (mean ≥1 year) and significant symptoms (17 to 24/60 maximum). TS, topical steroids; mGSS, modified Global Symptoms Score (pruritus + burning sensations + dyspareunia + discomfort + dryness sensation + spontaneous pain); condyloma acuminata group, 33 women and 19 men, mGSS did not include evaluation of dyspareunia
In the LS group, the patients were followed for a mean time of 60 days (24–120 days).
The results for the analysis of the clinical data are presented in Table . Steroids applied for maintenance therapy were: Clobetasol propionate 0.05% (n = 19), betamethasone dipropionate 0.05% (n = 5), diflucortone valerate 0.1% (n = 1) and betamethasone valerate 0.1% (n = 1). All patients kept the same steroid at the same posology during the study. They all applied the ointment once to twice daily. Except for dyspareunia, spontaneous pain and sclerosus/atrophy/synechia, the improvements of each criterion and of global scores were statistically significant (mGSS −80.61%, mGCS −59.2%). A total of 76.9% (20/26) attained mGSS75 and 23% (6/26) attained mGCS75; 84.6% (22/26) attained mGSS50 and 65.3% (17/26) attained mGCS50. The DLQI questionnaire was completed by 21 patients. The mean score decreased significantly, by 40.17% (Figure ). A total of 73% of patients (19/26) attained an IGA ≥2. Tolerance was rated good by all patients. Representative photographs are shown (Figure , patients a and b).
Table 2. Clinical results for patients with lichen sclerosus. Symptoms diminished by 80.61% (minimum for dyspareunia and maximum for burning sensations). Signs diminished by 59.2% (minimum pallor/whiteness and maximum fissures). Dryness decreased by more than 87% (both symptoms and signs). mGSS, modified Global Symptoms Score; mGCS, modified Global Clinical Score; *p < 0.05; **p < 0.01. All values are first cited as mean values
Figure 1. Dermatology Life Quality Index (DLQI) for patients with lichen sclerosus or psoriasis. Improvement in both groups **p < 0.01.
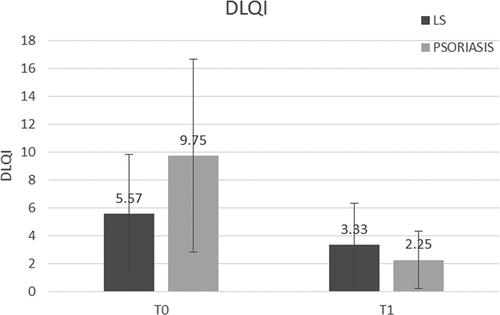
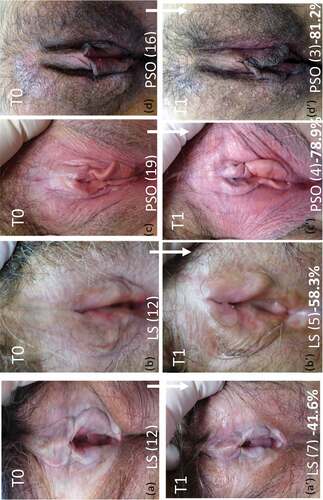
Figure 2. Illustrative photos of patients with lichen sclerosus (LS) and psoriasis, at time 0 (T0) and time 1 (T1). a, b: LS at T0; c, d: psoriasis at T0; a’, b’: LS at T1; c’, d’: psoriasis at T0. GCS (Global Clinical Score), a: 12, a’: 7 (−41.6%); b: 12, b’: 5 (−58.3%); c: 19, c’: 4 (−78.9%); d: 16, d’: 3 (−81.2%).
Patients with psoriasis were followed for a mean time of 51 days (27–83 days). Topical steroids applied were betamethasone dipropionate0.05% + calcipotriol 50 µg/g for one patient, fluticasone propionate 0.05% for one patient and betamethasone dipropionate0.05% for 4 patients. The mean mGSS decreased from 24.33 (range 12–34) to 4.17 (range 0–11) (−82.88%; p < 0.05) and the mean pGCS decreased from 10.70 (range 4–19) to 1.83 (range 0–4) (−82.80%; p < 0.05). The DLQI results are presented in Figure . All patients (6/6) attained an IGA ≥2. Tolerance was rated good by all patients. Representative photographs are shown (Figure , patients c and d).
The 33 female patients with CA were treated using LN (n = 21), imiquimod (n = 9), or LN + imiquimod (n = 3); the 19 men with CA were treated using LN. The mean time to follow-up was 16.94 days (5–50 days; 9.62 days for LN and 41.33 days for imiquimod). At T1 the mean GSS was 1.92 (SD 2.33; range 0–9) and the mean GCS was 2.47 (SD 2.47; range 0–15) in the entire CA group. The mean GSS and GCS values were 1.03 and 2.15, respectively, for women with CA. The mean GSS and GCS values were 3.47 and 3.0, respectively, for men with CA. Tolerance was evaluated as good for all except one female patient who was treated using LN and experienced pruritus. The mean time to follow-up in the group of patients with vulvodynia was 74.6 days (range 28–150 days). The mean mGSS value at T0 was 18.94. Tolerance was rated good by all patients except one; this patient experienced transient tingling.
The results for six questions common for the four-different auto-evaluation questionnaires are presented in Table . Responses to the seventh question (“better than similar product used”) are not included because 42% of the patients with LS, 17% of the patients with psoriasis, 66% of the patients with CA, and 34% of the patients with vulvodynia chose 0 (neither agree nor disagree) when they answered this question. This response was not the case for the other questions.
Table 3. Results for the synthesis of similar questions used between auto-evaluation questionnaires. Minimum 75% of patients totally or rather agree *p < 0.05
4. Discussion
Emollient use is recommended in therapeutic guidelines for some genital diseases, especially for LS and psoriasis treatment. However, very few publications concern this precise topic. Our objective was to quantify the effects of an ointment with a simple formulation and a lipidic phase that would maximize efficacy, tolerance and provide a protective effect. This study included a variety of external genital diseases that affect women (vulva, perianal region) and men (penis, glans, perianal region). This observational study was performed in real-life conditions during vulvar and andrological visits. Because most participants were women, the discussion will focus on vulvar diseases.
The ointment was added to complement proactive treatment with TS for LS and psoriasis (TS application usually twice a week) in women complaining of residual symptoms but without any clinical signs requiring intensifying TS treatment. It was also added to physical (LN) or medical (imiquimod) treatment for CA and as part of the multidisciplinary approach for vulvodynia treatment. In the LS and psoriasis groups, one-third of the patients suffered from urinary incontinence which may aggravate signs and symptoms (Kirtschig et al., Citation2015).
We studied the effects of treatment on three major symptoms (from GSS, pruritus, burning sensations, dyspareunia (Borghi et al., Citation2015, Citation2018)) and added pain, dryness sensation, and discomfort, which are frequently cited by patients (mGSS). The mean mGSS value was greater for the psoriasis group (24.33) than the LS group (17.65). After the use of the ointment, it decreased significantly (−82.88% and −80.61% respectively). For the LS group, the results were statistically significant for all symptoms (pruritus −77.71%, burning sensations −89.24%, discomfort −81%, dryness sensation −97.07%) except for pain (very low initial level of 0.62) and dyspareunia (−50%), consistent with Borghi et al’s (Citation2015) results. They assessed a topical product and a nutritional supplement containing avocado and soybean extracts for the treatment of LS at 12- and 24-week follow-ups. Their protocol differed from ours. No TS was applied before or during the study period. We compared the percentage of patients who had decreases of 50% and 75% of GSS. Borghi et al (Citation2015) found 55% and 35%, respectively, for these two outcomes vs. 80.7% and 65.3%, respectively, in our study population. The difference may be explained by the TS maintenance therapy used in our study population. Murina et al (Citation2017) studied soft foam use for the supportive treatment of LS (n = 43). Twenty-one patients were treated with MMF 0.1% for 4 weeks; then, twice weekly for 4 weeks in combination with a moisturizing cream; 22 patients were treated with MMF for 20 days, then with a soft foam. At 8 weeks, there were statistically significant reductions in the severity of symptoms in both groups. The authors concluded that the tested product could be an effective adjunct treatment for LS via its moisturizing effects and reductions in factors associated with epithelial disruption. A controlled, randomized, double-blind study compared silk briefs “Dermasilk” vs. standard cotton briefs in patients affected by LS (n = 42) (D’Antuono et al., Citation2011). The patients were treated with clobetasol propionate 0.05% plus moisturizer, daily for 6 months. Patients in both groups experienced symptomatic relief of soreness and itching. The number of patients with pruritus decreased significantly at 1 month in the tested group. All these studies suggest that the addition of an emollient or special underwear tends to improve the symptoms of LS patients treated with TS.
Gottlieb et al., (Citation2018) developed a new symptom scoring system (Genital Psoriasis Symptoms Scale) that includes symptoms (itch, pain, discomfort, stinging, burning) and clinical signs (redness, scaling, cracking) (each scored 0–10). Our mGSS differed moderately; stinging was not included, and we added dyspareunia and dryness sensation. To our knowledge, no studies of the symptoms of genital psoriasis and emollient use have been published. Paulsen et al. (Citation2005) compared aloe Vera gel use to a placebo (n = 41) in a study of cutaneous extra-genital psoriasis. The score sum of erythema, infiltration, and desquamation decreased in 72.5% of the aloe Vera-treated sites and in 82.5% of the placebo-treated areas from week 0 to week 4. We also assessed changes in clinical signs in the patients with LS or psoriasis. In the LS group, mGCS decreased significantly by 59.2%. Each item decreased significantly except for sclerosus/atrophy/synechia, which is known to be stable under treatment. A total of 65% of the patients attained mGCS50; 23% attained mGCS75. Borghi et al (Citation2015) obtained 52.4% and 28.6%, respectively, according to their global score (5 of our 9 criteria: erythema, leukoderma (pallor), sclerosus/scarring/atrophy, hyperkeratosis, and purpura/erosion). Murina et al. (Citation2017) used a global severity score (0–3) and also found a statistically significant decrease in vulvar signs. Fissures and erosions were the two clinical signs that showed the most improvement across both groups of patients in D’antuono et al.’s study (Citation2011). Erythema had the greatest improvement in the group of patients who used “Dermasilk”.
Genital LS and psoriasis result in considerable impairment on QoL (Ryan et al., Citation2015; Van den Nieuwenhof et al., Citation2010). A study of Dutch patients with LS found that the mean DLQI score was 11.92 (Van den Nieuwenhof et al., Citation2010). In patients with psoriasis, the DLQI score increased to 8.7 if genital involvement compared to 4 if not (Ryan et al., Citation2015). In our study, the women were under treatment and the initial mean DLQI scores were lower for the LS (5.57) and similar for the psoriasis groups (9.75). During the study, the mean DLQI score decreased by 40.17% in the LS group (statistically significant) and by 76.92% in the psoriasis group.
In our study, the low symptom and clinical scores after treatment (1.92 and 2.47, respectively) suggested that the ointment likely provided soothing and healing effects for the patients with CA (n = 52). The CA GSS was higher for the men (3.47) than for the women (1.03 for all women, 0.90 after LN and 1.25 after imiquimod treatment). These differences might be explained by differences in reported sensations between sexes or by different intensities of LN application between the two examiners, or both.
Tolerance was good for all patients except one patient treated for CA (transient pruritus after LN) and one treated for vulvodynia (transient tingling). Tolerance was the only criteria tested for vulvodynia patients because the complexity of the multidisciplinary approach did not allow a specific efficacy evaluation of the ointment. Treatment consists of a multidisciplinary approach involving topical therapies (emollients, anesthetics, hormonotherapy if necessary), pelvic floor physiotherapy with electromyographic biofeedback, drug treatment of pain with antidepressants (amitriptyline …) or anticonvulsants (pregabaline …) and a psychosexual support (De Belilovsky, Citation2013). Intolerance to topical products, known as the nocebo effect, is frequent. No patients stopped using the ointment because of intolerance or side effects in the present study.
This study had some limitations. Because of the inclusion criteria for psoriasis (i.e., TS maintenance therapy), only six patients were included as we mostly see patients during flares and no previous treatment. Women were most likely to be included in the study population because our clinics offer specialized services, so we could only perform limited extrapolation of results for men with genital LS and psoriasis. This study did not include a control group, but the comparison of clinical symptoms and signs before and after adding an ointment or any other cosmetic product to an unchanged therapeutic protocol is a typical approach used for studies of other general dermatoses (e.g., atopic dermatitis; Van Zuuren et al., Citation2017).
5. Conclusion
Our results illustrated the indications and effects of an ointment added to the usual treatments for the genital diseases LS, psoriasis, CA, and vulvodynia. When added to maintenance therapy with TS for LS and psoriasis treatment, the ointment may improve symptoms, some clinical signs, and QoL characteristics, and thus improve adherence of patients to this very long-term treatment. It may reduce the irritation and side effects of topical genital wart treatments and, again, improve adherence. Although patients suffering from vulvodynia often experience negative reactions to any topical product, tested ointment was very well tolerated during this study. Good tolerance is a prerequisite for the use of any topical product for the treatment of genital diseases.
Acknowledgements
The authors would like to thank Mr. Gaetan Boyer for assistance with the statistical analysis.
Additional information
Funding
Notes on contributors
Clarence de Belilovsky
Authors work for more than 20 years in a medical center with a high degree of specialization in diagnostic and treatments of genital infections, other diseases and sexual counselling. Dr JM Bohbot is an andrologist specialized in genitourinary infections and published a lot on vaginal microbiota. Dr C de Belilovsky is a dermatologist specialized in vulvar diseases. She is a member of the European and International societies of vulvar diseases (ECSVD and ISSVD) and active in many vulvar Continuing Medical Educations. The original idea of this clinical study on different genital diseases was based on their clinical daily experience and their wish to quantify and communicate the results of a simple method to improve global results and patients' satisfaction.
References
- Borghi, A., Corazza, M., Minghetti, S., Toni, G., & Virgili, A. (2015). Avocado and soybean extracts as active principles in the treatment of mild-to-moderate vulvar lichen sclerosus: Results of efficacy and tolerability. Journal of the European Academy of Dermatology and Venereology: JEADV, 29(6), 1225–13. https://doi.org/10.1111/jdv.12617
- Borghi, A., Virgili, A., Minghetti, S., Toni, G., & Corazza, M. (2018). Clearance in vulvar lichen sclerosus: A realistic treatment endpoint or a chimera? Journal of the European Academy of Dermatology and Venereology, 32(1), 96–101. https://doi.org/10.1111/jdv.14516
- Bornstein, J., Goldstein, A. T., Stockdale, C. K., Bergeron, S., Pukall, C., Zolnoun, D., & Coady, D. (2016). 2015 ISSVD, ISSWSH and IPPS consensus terminology and classification of persistent vulvar pain and vulvodynia. Obstetrics and Gynecology, 127(4), 745–751. https://doi.org/10.1097/AOG.0000000000001359
- Czuczwar, P., Stępniak, A., Goren, A., Wrona, W., Paszkowski, T., Pawlaczyk, M., Piekarska-Myślińska, D., Woźniak, S., & Pietrzak, A. (2016). Genital psoriasis: A hidden multidisciplinary problem— A review of literature. Ginekologia Polska, 87(10), 717–721. https://doi.org/10.5603/GP.2016.0074
- D’Antuono, A., Bellavista, S., Negosanti, F., Zauli, S., Baldi, E., & Patrizi, A. (2011). Dermasilk briefs in vulvar lichen sclerosus: An adjuvant tool. Journal of Lower Genital Tract Disease, 15(4), 287–291. https://doi.org/10.1097/LGT.0b013e31821380a0
- De Belilovsky, C. (2013). 2013 vulvodynia update. Gynecologie, Obstetrique & Fertilite, 41(9), 505–510. https://doi.org/10.1016/j.gyobfe.2013.06.008
- Edwards, S. K., Bates, C. M., Lewis, F., Sethi, G., & Grover, D. (2015). 2014 UK national guideline on the management of vulval conditions. International Journal of STD & AIDS, 26(9), 611–624. https://doi.org/10.1177/0956462414554271
- Gottlieb, A. B., Kirby, B., Ryan, C., Naegeli, A. N., Burge, R., Potts Bleakman, A., Anatchkova, M. D., & Yosipovitch, G. (2018). The development of a patient-reported outcome measure for assessment of genital psoriasis symptoms: The Genital Psoriasis Symptoms Scale (GPSS). Dermatology and Therapy, 8(1), 45–56. https://doi.org/10.1007/s13555-017-0213-2
- Kirtschig, G., Becker, K., Gunthert, A., Jasaitiene, D., Cooper, S., Chi, C., Kreuter, A., .. ... Barbagli, G., & Wojnarovska, J. (2015). Evidence-based (S3) Guideline on (anogenital) Lichen sclerosus. Journal of the European Academy of Dermatology and Venereology, 29, e1–e43. https://doi.org/10.1111/jdv.13136
- Lawton, S., & Littlewood, S. (2006). Vulval skin disease: Clinical features, assessment and management. Nursing Standard, 20(42), 57–63. https://doi.org/10.7748/ns2006.06.20.42.57.c6555
- Lewis, F. M., Tatnall, F. M., Velangi, S. S., Bunker, C. B., Kumar, A., Brackenbury, F., Mohd Mustapa, M. F., Exton, L. S., McHenry, P. M., Leslie, T. A., Wakelin, S., Hunasehally, R. Y. P., Cork, M., Johnston, G. A., Chiang, N., Worsnop, F. S., Buckley, D., Petrof, G., Salin, A., Callachand, N., & Salad, A. A. (2018). British association of dermatologists guidelines for the management of lichen sclerosus, 2018*. The British Journal of Dermatology, 178(4), 839–853. https://doi.org/10.1111/bjd.16241
- Meeuwis, K. A., De Hullu, J. A., Massuger, L. F., Van de Kerkhof, P. C., & Van Rossum, M. M. (2011). Genital psoriasis: A systematic literature review on this hidden skin disease. Acta dermato-venereologica, 91(1), 5–11. https://doi.org/10.2340/00015555-0988
- Moyal-Barracco, M., & Wendling, J. (2014). Vulvar dermatosis. Best Practice & Research: Clinical Obstetrics & Gynaecology, 28(7), 946–958. https://doi.org/10.1016/j.bpobgyn.2014.07.005
- Murina, F., Vicariotto, F., Di Francesco, S., & Oneda, S. (2017). Vulvar lichen sclerosus: Could a moisturizing and healing product reduce the prolonged use of topical steroid? Clinical Obstetrics, Gynecology and Reproductive Medicine, 3(6), 1–3. https://doi.org/10.15761/COGRM.1000203
- Paulsen, E., Korsholm, L., & Brandrup, F. (2005). A double-blind, placebo controlled study of a commercial Aloe vera gel in the treatment of slight to moderate psoriasis vulgaris. Journal of the European Academy of Dermatology and Venereology: JEADV, 19(3), 326–331. https://doi.org/10.1111/j.1468-3083.2004.01186.x
- Renaud-Vilmer, C., Cavelier-Balloy, B., Porche, R., & Dubertret, L. (2004). Vulvar Lichen sclerosus: Effect of long-term topical application of a potent steroid on the course of the disease. Archives of Dermatology, 140(6), 709–712. https://doi.org/10.1001/archderm.140.6.709
- Ryan, C., Sadlier, M., De Vol, E., Patel, M., Lloyd, A. A., Day, A., Lally, A., Kirby, B., & Menter, A. (2015). Genital psoriasis is associated with significant impairment in quality of life and sexual functioning. Journal of the American Academy of Dermatology, 72(6), 978–983. https://doi.org/10.1016/j.jaad.2015.02.1127
- Van Cranenburgh, O. D., Nijland, S. B. W., Lindeboom, R., De Korte, J., De Rie, M. A., Ter Stege, J. A., & Prinsen, C. A. C. (2017). Patients with lichen sclerosus experience moderate satisfaction with treatment and impairment of quality of life: Results of a cross-sectional study. The British Journal of Dermatology, 176(6), 1508–1515. https://doi.org/10.1111/bjd.15125
- Van den Nieuwenhof, H. P., Meewis, K. A., Nieboer, T. E., Vergeer, M. C. M., Massuger, L. F. A. G., & De HULLU, J. A. (2010). The effect of vulvar lichen sclerosus on quality of life and sexual functioning. Journal of Psychosomatic Obstetrics & Gynecology, 31(4), 279–284. https://doi.org/10.3109/0167482X.2010.507890
- Van der Meijden, W. I., Boffa, M. J., Ter Harmsel, W. A., Kirtschig, G., Lewis, F. M., Moyal-Barracco, M., Tiplica, G. S., & Sherrard, J. (2017). European guideline for the management of vulval conditions. Journal of the European Academy of Dermatology and Venereology: JEADV, 31(6), 925–941. https://doi.org/10.1111/jdv.14096
- Van Zuuren, E. J., Fedorowicz, Z., Christensen, R., Lavrijsen, A., & Arents, B. W. M. (2017). Emollients and moisturisers for eczema. Cochrane Database of Systematic Reviews, (2),Art.No.:CD012119. https://doi.org/10.1002/14651858.CD012119.pub2
- Virgili, A., Borghi, A., Minghetti, S., & Corazza, M. (2014). First randomized trial on clobetasol propionate and mometasone furoate in the treatment of vulvar lichen sclerosus: Results of efficacy and tolerability. The British Journal of Dermatology, 171(2), 388–396. https://doi.org/10.1111/bjd.12910
- Virgili, A., Minghetti, S., Borghi, A., & Corazza, M. (2013a). Proactive maintenance therapy with a topical corticosteroid for vulvar lichen sclerosus: Preliminary results of a randomized study. The British Journal of Dermatology, 168(6), 1316–1324. https://doi.org/10.1111/bjd.12273
- Virgili, A., Minghetti, S., Borghi, A., & Corazza, M. (2013b). Long-term maintenance therapy for vulvar lichen sclerosus: The results of a randomized study comparing topical vitamin E with an emollient. European Journal of Dermatology: EJD, 23(2), 189–194. https://doi.org/10.1684/ejd.2013.1987
- Werner, R. N., Westfechtel, L., Dressler, C., & Nast, A. (2016). Self-administered interventions for anogenital warts in immunocompetent patients: A systematic review and meta-analysis. Sexually Transmitted Infections, 2016, 1–7. http://dx.doi.org/10.1136/sextrans-2016-052768
