Abstract
With modern treatment, an increasing proportion of patients with inflammatory bowel disease (IBD) are achieving deep, sustained remission. However, as control of inflammation has become more effective, the general health needs of patients become more evident. Therefore, we assessed the metabolic health and trends in body mass index (BMI) of patients over the past decade. 181 patients with IBD were included (102 with Crohn’s disease; 79 with ulcerative colitis), each attending the same IBD clinic (median follow up 18 years). A significant trend for rising BMI was found for Crohn’s disease (p < 0.001) which appeared to be independent of the use of biologic drugs. In addition, the proportion of patients with abnormalities of serum lipids was higher than expected for these young patients, median age 46 (38–55 interquartile range). These serum data, together with a higher proportion of smokers and higher BMI trends among those with Crohn’s disease compared with ulcerative colitis, illustrate the need for metabolic health awareness. Crohn’s disease, once strongly associated with nutritional deficit, is now characterized by rising BMI and the emergence of metabolic disorders. Whether this reflects the interaction between inflammatory and cytokine cascades or is solely related to similar trends in the background population is uncertain, but it appears to be independent of the use of biologic drugs. Regardless, the trends observed over the past decades suggest that the metabolic health of patients with IBD will require greater attention when planning management strategies at sub-specialty clinics.
PUBLIC INTEREST STATEMENT
Previously, patients with inflammatory bowel disease (IBD) were often underweight and malnourished. This appears to no longer be the case for the majority of patients in part due to improved care pathways and better control of inflammation and associated complications. Our study has demonstrated a significant rise in body mass index (BMI) over a ten year period for patients attending a specialty outpatient clinic for care of patients with IBD. Together with a high proportion of abnormal metabolic blood markers, these findings suggest greater attention must be given to the metabolic health of patients with IBD where complications of overweight and metabolic health abnormalities are of increasing concern. In addition, co-existence of inflammatory and metabolic conditions may be influenced by host gut microbes which affect the signalling mechanisms that are common to the development of both types of disorders.
1. Introduction
There has been a striking increase in non-communicable chronic diseases in socioeconomically developed countries in recent decades. Examples primarily include chronic inflammatory and metabolic disorders. While the risk factors and triggers are varied, multiple and complex, it is noteworthy that inflammatory and metabolic processes are no longer considered as exclusively and mutually distinct entities. Emerging evidence has identified several intersection points for inflammatory and metabolic signalling cascades Shanahan and Sheehan, Citation2016.
The introduction of effective immunomodulatory or biologic therapy for chronic inflammatory disorders within the past two decades has achieved deep remission of inflammation but left concomitant metabolic disturbances unchecked. This has resulted in a changing phenotype for many disorders, and perhaps represents a challenge for medical specialisation. Moreover, the profile of body weight and body mass index (BMI) of patients at modern outpatient clinics raises the question as to whether biologic therapies might have contributed to or accelerated metabolic disturbances.
To begin to address this, we have focussed on inflammatory bowel disease (IBD), including both Crohn’s disease and ulcerative colitis, as this condition has traditionally been linked with under-nutrition and theoretically would be least likely to be associated with overweight or obesity Baumgart and Sandborn, Citation2012, Dong et al., Citation2015. The traditional image of an underweight, malnourished patient with IBD undergoing enteral or parenteral nutrition has been largely superseded by the robust, and often overweight, patient. In a systematic review, compared with controls, a lower BMI was seen in just 37% of patients with Crohn’s disease and 20% with ulcerative colitis Bryant et al., Citation2013. Conversely, an obese BMI is now seen in up to 33% of all IBD cohorts Seminerio et al., Citation2015,Flores et al., Citation2015,Steed et al., Citation2009.
This longitudinal study aimed to investigate and establish the trend for BMI change among patients with IBD suggested by an increasing prevalence of obesity in cross-sectional studies. In addition, further objectives include whether there is a relationship between BMI trend and therapeutic intervention, in particular biologic drugs. Finally, the proportion of patients with IBD that have evidence of abnormal metabolic health was determined. Regardless of any cause-effect relation between the changing phenotype and use of biologics, a more disquieting question is whether there is a substantial proportion of patients which might be undertreated or neglected in regard to their metabolic health.
2. Materials and methods
2.1. Study population
All patients attending the single senior clinician-led specialty clinic dedicated exclusively to IBD were evaluated over a six month period. There are approximately 1,500 patients registered for this clinic, of which approximately 600 have an established diagnosis of Crohn’s disease. This is the sole source of IBD care of these patients, and those with at least ten years of continual follow-up were eligible for analysis. To avoid bias, the case histories of 602 consecutive patients attending the IBD clinic were reviewed. Of these, 421 were excluded due to having less than 10 years of sufficient follow-up data. In the final study population, 181 patients were evaluated.
We identified four subgroups of patients with IBD: (1) those treated primarily with biologic therapies, (2) those who underwent surgery, (3) those treated with both biologic therapy and surgery, and (4) those who received neither. We defined treatment with a biologic drug as having received at least three doses as part of an induction course of treatment. Other specific IBD treatment modalities, such as aminosalicylates and steroids, were not assessed in final analysis as these therapies were given to all patients at various points throughout their treatment course, except those who had proven allergies to 5-aminosalicylic acid.
Demographic information, medical history, clinical examination findings including anthropometric measurements as well as health insurance details were obtained from written and typed data filed in the medical case notes. Blood analysis for metabolic parameters, lipid and glucose profiles, were available from electronic blood reporting systems used in the hospital for all patients.
2.2. Metabolic health definitions
BMI is still the most widely available anthropometric measurement recorded as a marker of nutritional status, likely due to ease of use in clinical practice to measure and monitor changes in weight Dong et al., Citation2015, Nuttall, Citation2015. BMI classifies subjects into underweight (<18.5 kg/m2), normal weight (18.5–24.9 kg/m2), overweight (25–29.9 kg/m2) and obese (>30 kg/m2) categories. It is predictive of nutritional status in IBD, particularly amongst malnourished patients Mijac et al., Citation2010, and a higher BMI is associated with several pathologies including cancers, arthritis, obstructive sleep apnoea, biliary pathology and non-alcoholic fatty liver disease (NAFLD) Dong et al., Citation2015. However, BMI interpretation is limited as it is a poor indicator of body fat percentage and is unable to discriminate between visceral and subcutaneous fat stores Nuttall, Citation2015. Visceral fat has an increased risk of cardio-metabolic disease compared with subcutaneous fat Sato et al., Citation2018, Swanson et al., Citation2018. At every clinic appointment, BMI is calculated for each patient by measuring current weight and height.
As well as overweight and obesity, as defined by BMI, other metabolic parameters are recognized risk factors for type 2 diabetes, cardiovascular disease and associated complications and are collectively termed the metabolic syndrome Alberti et al., Citation2009, Michalak et al., Citation2016. Metabolic syndrome diagnosis requires 3 of the 5 following criteria; elevated waist circumference (≥94 cm in males, ≥80 cm in females), elevated triglycerides (≥1.7 mmol/L), reduced high density lipoprotein (HDL) (<1.0 mmol/L in males, <1.3 mmol/L in females), elevated blood pressure (>130 mm/Hg systolic and/or >85 mmHg diastolic) and elevated fasting glucose (>5.6 mmol/L) Alberti et al., Citation2009.
Overweight, obesity and the metabolic syndrome are pro-inflammatory states which may influence IBD activity Seminerio et al., Citation2015,Flores et al., Citation2015,Steed et al., Citation2009, Chan et al., Citation2013, Harper et al., Citation2013 and hospitalisation Fitzmorris et al., Citation2015. With this in mind, these measurements were recorded and analysed to determine changing patterns of metabolic health in this cohort of patients.
2.3. Ethical approval
This study was approved by the Cork university affiliated hospitals’ clinical research ethics review board. Informed consent was sought from patients on attendance at the outpatient clinic.
2.4. Statistical analysis
Data were analysed using IBM SPSS Version 24.0 and Microsoft Office Excel. Variables are reported as frequencies for categorical variables and as mean and standard deviation, or median and interquartile range, for continuous variables. Depending on the level of measurement, subjects were compared using t-test, repeat measures ANOVA, Kruskal-Wallis and chi-square analysis. Results with p < 0.05 were deemed statistically significant.
3. Results
3.1. Demographic characteristics
A total of 102 patients with Crohn’s disease and 79 patients with ulcerative colitis were analysed. The median time since diagnosis, which is also the median follow-up, was 18 years. This was similar for both those with Crohn’s disease (median follow-up 19 years) and ulcerative colitis (median follow-up 17 years).The characteristics of the study population are summarized in , of which 97 were female, and the median age was 46 years with an age range of 21–82 years.
Table 1. Clinical characteristics of the study population with IBD. Categorical variables are reported as frequencies, and continuous variables as mean±SD, and median and IQR
3.2. Change in body mass index over time
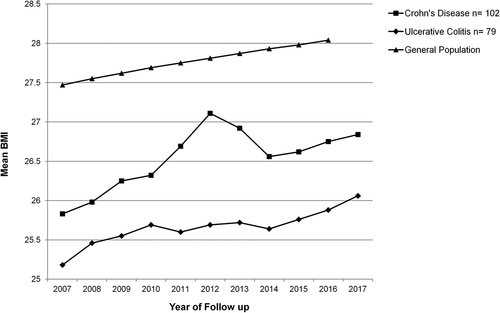
A trend for rising mean BMI for patients with Crohn’s disease was observed, which was statistically significant (p < 0.001). By comparison, for those with ulcerative colitis, the change in BMI did not reach statistical significance (p = 0.158) (). Similar trends were observed for various age groups among the study population. Change in BMI for the general population, for the same age range as the study group, is also shown for comparison.
Figure 1. Mean BMI of patients with IBD over a 10 year period, grouped by IBD subtype. Mean BMI of the general age-matched Irish population is shown for comparison .Abarca-Gómez et al., Citation2017
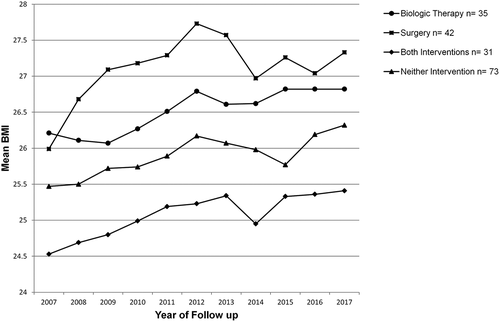
Of the subgroups, patients who underwent either biologic therapy or surgery alone had higher mean BMI’s than the other subgroups (). While a trend for rising BMI was observed (), this was only statistically significant for the surgery subgroup (for the surgery subgroup: p = 0.031, biologic subgroup: p = 0.422, both biologic and surgery subgroup: p = 0.252, neither subgroup: p = 0.070).
Figure 2. Mean BMI of patients with IBD over 10 years of follow-up. Patients are subgrouped according to treatment received
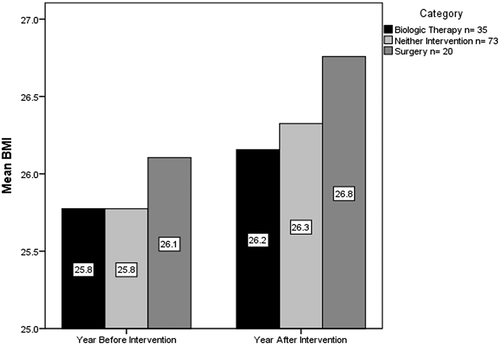
Further analysis of each subgroup addressed the relationship between BMI and biologic therapy. The mean BMI the year before and year after commencing a biologic drug or undergoing surgery were compared with controls who received neither intervention (). There was a statistically significant increase in mean BMI the year after compared with the year before for the biologic subgroup (p < 0.0005), and the neither intervention control subgroup (p = 0.015), but not the surgery subgroup (p = 0.214), as would be expected. This suggests that BMI is rising among patients with IBD regardless of biologic drug treatment.
3.3. Change in metabolic parameters over time
Of the study population, 10.5% of patients had a documented history of hypertension, 7.7% had documented dyslipidemia with 5% on cholesterol lowering agents, and 4.4% documented hyperglycaemia. The most recent metabolic serum analyses (within the previous 2 years) are summarized in . The number of patients with abnormal metabolic profiles is of particular concern considering this is a young cohort of patients, median age 46 years. The pattern of lipid abnormalities observed is similar to that seen among the Irish population Agar et al., Citation2019.
Table 2. Metabolic parameters as documented in past history and categories of recent serum lipid and glucose analysis of the study population with IBD. Variables are reported as frequencies
The most recent available lipid profile for each patient (n = 95) was compared to previous serum values from the beginning of this review period (ten years previous). Those not on cholesterol lowering therapy had a significant increase in mean total cholesterol levels (n = 78, mean 4.88 mmol/L to 5.12 mmol/L, p = 0.015), and LDL levels (n = 23, mean 2.63 mmol/L to 2.93 mmol/L, p = 0.007). Those on cholesterol lowering medications (n = 7) had reduced mean total cholesterol levels (mean 4.8 mmol/L to 4.46 mmol/L, p = 0.365), triglyceride levels (mean 1.65 mmol/L to 0.93 mmol/L, p = 0.024), and increased HDL levels (mean 1.33 mmol/L to 1.71 mmol/L, p = 0.047).
4. Discussion
The results show a significant trend for a rise in BMI over the review period for those with Crohn’s disease, with all mean BMI above the normal reference range of 18.5–25 kg/m2. A similar trend, significant for the surgery subgroup, was observed in subgroup analysis. Importantly, this rise in BMI does not appear to be driven by the use of biologic drugs . It is noteworthy that patients with ulcerative colitis had lower mean BMI for each year, in addition to a less pronounced, non-significant trend for a rise in BMI, than those with Crohn’s disease . As well as recording a rise in BMI, a substantial proportion of this young cohort of patients demonstrate abnormal metabolic health which now becomes a clinical treatment target in addition to inflammatory disease remission. The higher proportion of current and former smokers among those with Crohn’s disease (37.2% of patients with Crohn’s disease, 15.2% with ulcerative colitis, ) further emphasizes the metabolic health needs of these patients. Modification of these cardiovascular disease (CVD) risk factors will potentially help to reduce the increased burden of CVD risk associated with rising age Dhingra and Vasan, Citation2012.
Our findings are in keeping with the modern scenario of a patient with IBD, where under-nutrition and underweight are becoming rarer and replaced by the malnutrition of obesity and the metabolic syndrome Bryant et al., Citation2013, Moran et al., Citation2016. This is at odds with the previous impression of a typical underweight IBD phenotype which may have been explained by reduced dietary intake, malabsorption, increased energy expenditure from inflammation and decreased respiratory quotient Dong et al., Citation2015. A recent meta-analysis suggested that the BMI of patients with IBD is lower than that of healthy controls Dong et al., Citation2015. Interestingly, the mean BMI from almost all studies included in these analyses fell within the normal, or above normal, BMI reference range. Only two individual studies demonstrated an underweight BMI. They were an Egyptian study of 20 patients with active ulcerative colitis (mean BMI 17.2 ± 3 kg/m2) Mohamed-Hussein et al., Citation2007 and an Italian study of 5 patients with active Crohn’s disease (mean BMI 17.2 ± 1.9 kg/m2) Dong et al., Citation2015, Mingrone et al., Citation1998. Our longitudinal study supports such cross-sectional data, where underweight patients with IBD are becoming less prevalent.
The rise in mean BMI among patients attending our IBD clinic, is in keeping with that observed amongst the general population Flores et al., Citation2015, Swanson et al., Citation2018. In Ireland, the prevalence of overweight and obesity are reported to be 44% and 34% respectively Dee et al., Citation2015, Barrett et al., Citation2011. Possibilities for this change among patients with IBD include improved medical therapies and surgical interventions that allow for sustained remission of disease, reduced energy expenditure due to inflammation and changes in dietary habits.
In addition, interactions of host inflammatory, metabolic and microbial signalling cascades may play a significant role Shanahan and Sheehan, Citation2016, Zietek and Rath, Citation2016. For example, mice lacking expression of toll-like receptor 5 (TLR5) produced by intestinal epithelial cells develop low-grade inflammation, are prone to colitis and the metabolic syndrome as well as alterations in gut microbial composition Chassaing et al., Citation2014. Indeed, gut inflammation induced by the absence of TLR5, in mice, appears to be dependent on the presence of gut microbiota Singh et al., Citation2015. In further murine studies, a deficiency of intracellular multiprotein sensor NLRP6 inflammasome is linked to disturbed metabolic function and colitogenic microbiota Shanahan and Sheehan, Citation2016, Levy et al., Citation2015. Additionally, activation of the NLRP3 inflammasome, and associated interleukin production, in both human and murine studies, is associated with metabolic disease De Nardo and Latz, Citation2011. Tumor necrosis factor (TNF) and interleukin (IL)-6, as inflammatory cytokines, have been demonstrated, respectively, to impair and enhance glucagon like peptide (GLP)-1 production with resultant alterations in glucose and insulin metabolism in mice Zietek and Rath, Citation2016. Further associations include altered lipid absorption due to intestinal epithelial immune activation in the presence of microbiota and absence of B-cell lymphocytes and immunoglobulin A (IgA) Shulzhenko et al., Citation2011. It seems likely, therefore, that inflammatory and metabolic disorders are not mutually exclusive and, influenced by gut microbial composition, may co-exist in certain patients.
The advent of biologic therapy used to regulate immune and inflammatory pathways in IBD may then be suggested to result in an offset in metabolic health due to recognized cross points between these pathways and metabolic and microbial signals Shanahan and Sheehan, Citation2016. While we show an increase in BMI in the year after biologic commencement, a similar increase is shown among control patients not receiving such therapies . Accordingly, the trend for a rise in BMI may be explained by factors such as dietary advice to account for weight loss. Intolerance of certain foods or lack of education on requirements in the pre-biologic and active disease phase may result in dietary habits not being adjusted once remission is achieved and lead to undesirable weight gain and metabolic instability. Weight gain associated with biologic drugs has been suggested in a paediatric population Haas et al., Citation2017, but a cause-effect relationship between therapeutic intervention in IBD and obesity and metabolic disturbance remain undetermined.
A further possibility for the elevated BMI in our treatment subgroups is that increased weight and obesity leads to inflammation associated with IBD Goncalves et al., Citation2015, and results in escalation of therapies. Obesity increases acute phase reactants and pro inflammatory cytokines such as IL-1B, IL-6, IL-8, TNF-alpha and CRP and, therefore, treatments for IBD may be less effective in obese populations because of this pro-inflammatory state Dong et al., Citation2015, Swanson et al., Citation2018, Moran et al., Citation2016. Recently, a Danish female cohort identified both low and high BMI as risk factors for Crohn’s disease Mendall et al., Citation2018. They suggest that the low BMI group represent a pre-clinical manifestation of IBD and that high BMI may be a risk factor for exacerbation.
Conversely, a high BMI observed in IBD may represent a less severe phenotype Flores et al., Citation2015. This may describe the subgroup that received neither biologic therapy nor surgical intervention, and explain the higher BMI seen in this subgroup. These BMI and metabolic differences among our subgroups suggest the possibility of distinct IBD phenotypes related to metabolic health, and further alludes to links between these conditions. There is the malnourished, underweight patient who represents a more aggressive form of IBD with extensive complications Dong et al., Citation2015, and then the overweight, or obese, patient with IBD. In the overweight category, altered aetiology due to obesity-related inflammatory cytokines, a less severe phenotype and a failure to adjust other metabolic and lifestyle factors in response to therapy may describe further distinct categories.
This study has several limitations as it does not account for other anthropometric measures of body composition due to lack of available data for comparison. BMI was available for all patients and offers reliable value, in this instance, as the analysis was the change in BMI over time for the same cohort of patients. In addition, the study does not account for other treatment modalities, in particular aminosalicylates, thiopurines, and steroids prescribed. This is because these treatments were used in all patients at various time-points except those with proven allergies. Steroids, in particular, may be associated with weight gain but prescriptions are generally of short term duration and mean BMI over the study period may, in part, control for temporary fluctuations in BMI.
Co-existence of inflammatory and metabolic disease in patients with IBD is becoming increasingly common. Management of these conditions may no longer be considered as distinct categories, particularly with emerging data on cross-points in respective signalling cascades, influenced by the microbiota Shanahan and Sheehan, Citation2016. Greater vigilance for weight gain and metabolic syndrome, similar to the now routine smoking cessation advice, among patients with IBD is required. In future, markers of metabolic abnormalities, such as BMI and serum indices, may offer a dual role in IBD management, serving as both risk factor for IBD and systemic disease Moran et al., Citation2016, Mendall et al., Citation2018 as well as the more recognized marker of IBD severity.
Disclosure of interest
The authors report no conflict of interest.
Additional information
Funding
Notes on contributors
John O’Grady
The data analysis in this study is possible through ongoing collaborative research underway at APC Microbiome Ireland and Cork University Hospital, University College Cork in Ireland. In particular, current research focuses on the non-drug management of patients with chronic gastrointestinal conditions including factors such as diet, metabolic health, co-morbid conditions and side-effects of medical interventions. The goal of such research is to improve both patient care and health outcomes.
References
- Abarca-Gómez, L., Abdeen, Z. A., Hamid, Z. A., et al. (2017). Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: A pooled analysis of 2416 population-based measurement studies in 128.9 million children, adolescents, and adults. The Lancet, 390, 2627–11. https://doi.org/https://doi.org/10.1016/S0140-6736(17)32129-3
- Agar, R., Markham, C., Prendergast, M., Canning, R., Maher, E., Finn, C., . . . Maher, V. (2019). A snapshot of lipid levels in the republic of ireland in 2017. Irish Journal of Medical Science, 188(1), 241–247. https://doi.org/https://doi.org/10.1007/s11845-018-1820-3
- Alberti, K. G. M. M., Eckel, R. H., Grundy, S. M., Zimmet, P. Z., Cleeman, J. I., Donato, K. A., . . . Smith, S. C. (2009). Harmonizing the metabolic syndrome. A joint interim statement of the international diabetes federation task force on epidemiology and prevention; national heart, lung, and blood institute; american heart association; world heart federation; international atherosclerosis society; and international association for the study of obesity. Circulation, 120(16), 1640–1645. https://doi.org/https://doi.org/10.1161/CIRCULATIONAHA.109.192644
- Barrett, A., Burke, H., Cronin, H., Hickey, A., Kamiya, Y., Kenny, R. A., . . . Morgan, K. (May. 2011). Fifty plus in ireland 2011: First results from the irish longitudinal study on ageing (TILDA).Trinity College Dublin.
- Baumgart, D. C., & Sandborn, W. J. (2012). Crohn’s disease. The Lancet, 380(9853), 1590–1605. https://doi.org/https://doi.org/10.1016/S0140-6736(12)60026-9
- Bryant, R. V., Trott, M. J., Bartholomeusz, F. D., & Andrews, J. M. (2013). Systematic review: Body composition in adults with inflammatory bowel disease. Alimentary Pharmacology & Therapeutics, 38(3), 213–225. https://doi.org/https://doi.org/10.1111/apt.12372
- Chan, S. S., Luben, R., Olsen, A., Tjonneland, A., Kaaks, R., Teucher, B., . . . Hart, A. R. (2013). Body mass index and the risk for crohn’s disease and ulcerative colitis: Data from a european prospective cohort study (The IBD in EPIC study). The American Journal of Gastroenterology, 108(4), 575–582. https://doi.org/https://doi.org/10.1038/ajg.2012.453
- Chassaing, B., Ley, R. E., & Gewirtz, A. T. (2014). Intestinal epithelial cell toll-like receptor 5 regulates the intestinal microbiota to prevent low-grade inflammation and metabolic syndrome in mice. Gastroenterol, 147(6), 1363–77.e17. https://doi.org/https://doi.org/10.1053/j.gastro.2014.08.033
- De Nardo, D., & Latz, E. (2011). NLRP3 inflammasomes link inflammation and metabolic disease. Trends in Immunology, 32(8), 373–379. https://doi.org/https://doi.org/10.1016/j.it.2011.05.004
- Dee, A., Callinan, A., Doherty, E., et al. (2015). Overweight and obesity on the island of Ireland: An estimation of costs. BMJ Open, 5(3), e006189. https://doi.org/https://doi.org/10.1136/bmjopen-2014-006189
- Dhingra, R., & Vasan, R. S. (2012). Age as a cardiovascular risk factor. The Medical Clinics of North America, 96(1), 87–91. https://doi.org/https://doi.org/10.1016/j.mcna.2011.11.003
- Dong, J., Chen, Y., Tang, Y., et al. (2015). Body mass index is associated with inflammatory bowel disease: A systematic review and meta-analysis. PLoS One, 10(12), e0144872. https://doi.org/https://doi.org/10.1371/journal.pone.0144872
- Fitzmorris, P. S., Colantonio, L. D., Torrazza Perez, E., Smith, I., Kakati, D. D., & Malik, T. A. (2015). Impact of metabolic syndrome on the hospitalization rate of Crohn’s disease patients seen at a tertiary care center: A retrospective cohort study. Digestion, 91(3), 257–262. https://doi.org/https://doi.org/10.1159/000380763
- Fitzmorris, P. S., Colantonio, L. D., Torrazza Perez, E., Smith, I., Kakati, D. D., & Malik, T. A. (2015). Obesity in inflammatory bowel disease: A marker of less severe disease. Digestive Diseases and Sciences, 60(8), 2436–2445. https://doi.org/https://doi.org/10.1007/s10620-015-3629-5
- Goncalves, P., Magro, F., & Martel, F. (2015). Metabolic inflammation in inflammatory bowel disease: Crosstalk between adipose tissue and bowel. Inflammatory Bowel Diseases, 21(2), 453–467. https://doi.org/https://doi.org/10.1097/MIB.0000000000000209
- Haas, L., Chevalier, R., Major, B. T., Enders, F., Kumar, S., & Tung, J. (2017). Biologic agents are associated with excessive weight gain in children with inflammatory bowel disease. Digestive Diseases and Sciences, 62(11), 3110–3116. https://doi.org/https://doi.org/10.1007/s10620-017-4745-1
- Harper, J. W., Sinanan, M. N., & Zisman, T. L. (2013). Increased body mass index is associated with earlier time to loss of response to infliximab in patients with inflammatory bowel disease. Inflammatory Bowel Diseases, 19(10), 2118–2124. https://doi.org/https://doi.org/10.1097/MIB.0b013e31829cf401
- Levy, M., Thaiss, C. A., Zeevi, D., Dohnalova, L., Zilberman-Schapira, G., Mahdi, J. A., . . . Elinav, E. (2015). Microbiota-modulated metabolites shape the intestinal microenvironment by regulating NLRP6 inflammasome signaling. Cell, 163(6), 1428–1443. https://doi.org/https://doi.org/10.1016/j.cell.2015.10.048
- Mendall, M., Harpsoe, M. C., Kumar, D., Andersson, M., & Jess, T. (2018). Relation of body mass index to risk of developing inflammatory bowel disease amongst women in the danish national birth cohort. PLoS One, 13(1), e0190600. https://doi.org/https://doi.org/10.1371/journal.pone.0190600
- Michalak, A., Mosinska, P., & Fichna, J. (2016). Common links between metabolic syndrome and inflammatory bowel disease: Current overview and future perspectives. Pharmacological Reports : PR, 68(4), 837–846. https://doi.org/https://doi.org/10.1016/j.pharep.2016.04.016
- Mijac, D. D., Jankovic, G. L., Jorga, J., & Krstic, M. N. (2010). Nutritional status in patients with active inflammatory bowel disease: Prevalence of malnutrition and methods for routine nutritional assessment. European Journal of Internal Medicine, 21(4), 315–319. https://doi.org/https://doi.org/10.1016/j.ejim.2010.04.012
- Mingrone, G., Benedetti, G., Capristo, E., De Gaetano, A., Greco, A. V., Tataranni, P. A., & Gasbarrini, G. (1998). Twenty-four-hour energy balance in Crohn disease patients: Metabolic implications of steroid treatment. The American Journal of Clinical Nutrition, 67(1), 118–123. https://doi.org/https://doi.org/10.1093/ajcn/67.1.118
- Mohamed-Hussein, A. A., Mohamed, N. A., & Ibrahim, M. E. (2007). Changes in pulmonary function in patients with ulcerative colitis. Respiratory Medicine, 101(5), 977–982. https://doi.org/https://doi.org/10.1016/j.rmed.2006.09.005
- Moran, C., Sheehan, D., & Shanahan, F. (2016). The changing phenotype of inflammatory bowel disease. Gastroenterology Research and Practice, 2016, 9. https://doi.org/https://doi.org/10.1155/2016/1619053
- Nuttall, F. Q. (2015). Body mass index: obesity, BMI, and health: A critical review. Nutrition Today, 50(3), 117–128. https://doi.org/https://doi.org/10.1097/NT.0000000000000092
- Sato, F., Maeda, N., Yamada, T., et al. (2018). Association of epicardial, visceral, and subcutaneous fat with cardiometabolic diseases. Circulation Journal : Official Journal of the Japanese Circulation Society, 82(2), 502–508. https://doi.org/https://doi.org/10.1253/circj.CJ-17-0820
- Seminerio, J. L., Koutroubakis, I. E., Ramos-Rivers, C., et al. (2015). Impact of obesity on the management and clinical course of patients with inflammatory bowel disease. Inflammatory Bowel Diseases, 21(12), 2857–2863. https://doi.org/https://doi.org/10.1097/MIB.0000000000000560
- Shanahan, F., & Sheehan, D. (2016). Microbial contributions to chronic inflammation and metabolic disease. Current Opinion in Clinical Nutrition and Metabolic Care, 19(4), 257–262. https://doi.org/https://doi.org/10.1097/MCO.0000000000000282
- Shulzhenko, N., Morgun, A., Hsiao, W., et al. (2011). Crosstalk between B lymphocytes, microbiota and the intestinal epithelium governs immunity versus metabolism in the gut. Nature Medicine, 17(12), 1585–1593. https://doi.org/https://doi.org/10.1038/nm.2505
- Singh, V., Yeoh, B. S., Carvalho, F., Gewirtz, A. T., & Vijay-Kumar, M. (2015). Proneness of TLR5 deficient mice to develop colitis is microbiota dependent. Gut Microbes, 6(4), 279–283. https://doi.org/https://doi.org/10.1080/19490976.2015.1060390
- Steed, H., Walsh, S., & Reynolds, N. (2009). A brief report of the epidemiology of obesity in the inflammatory bowel disease population of tayside, scotland. Obesity Facts, 2(6), 370–372. https://doi.org/https://doi.org/10.1159/000262276
- Swanson, S. M., Harper, J., & Zisman, T. L. (2018). Obesity and inflammatory bowel disease: Diagnostic and therapeutic implications. Current Opinion in Gastroenterology, 34(2), 112–119. https://doi.org/https://doi.org/10.1097/MOG.0000000000000422
- Zietek, T., & Rath, E. (2016). Inflammation meets metabolic disease: gut feeling mediated by GLP-1. Frontiers in Immunology, 7, 154. https://doi.org/https://doi.org/10.3389/fimmu.2016.00154