Abstract
Hemoglobin Tacoma (Hb Tacoma) results from the substitution of serine for arginine at position 30 in the β-globin chain resulting in instability in vitro, and has been identified with gel electrophoresis, isoelectric focusing and high-performance liquid chromatography (HPLC), but the role of capillary electrophoresis (CE) has never been reported. Whole blood samples were received from 4 patients for HbA1c testing at McMaster University Medical Centre (MUMC) in Hamilton, Canada, and initially analyzed via CE with Sebia Capillarys 2 Flex Piercing instrument using the Hb A1c platform. Specimens were then run on the hemoglobin variant platform and HPLC with additional studies including Hemoglobin H (Hb H) body staining, instability testing and β-globin gene sequencing. Hemoglobin concentrations were within normal reference intervals and mean corpuscular volume (MCV) was normal in most cases. Capillary electropherograms produced on the Hb A1c platform demonstrated a small double peak at the 271–273 mark area running past Hb A2 in all samples. On the variant hemoglobin program, Hb A2 percentage was mildly elevated, and a variant hemoglobin (Hb X) peak at 127–128 marks was identified and quantified at 35–37%, yielding a ratio of Hb A: Hb X of 1.7 to 1. Genetic confirmation was performed in 2 of the 4 cases. This series supports that Hb Tacoma heterozygosity is associated with in vitro instability without significant phenotypic consequences. We report identification of Hb Tacoma using CE and propose that CE with Hb A1c and variant hemoglobin platforms is an effective screening tool for Hb Tacoma.
PUBLIC INTEREST STATEMENT
This case series highlights the unique properties and capillary electropherogram pattern of Hemoglobin Tacoma, a β-globin variant. This variant hemoglobin may be identified on capillary electrophoresis screening for diabetes mellitus and could have implications for the accuracy of Hemoglobin A1c monitoring.
1. Introduction
Hb Tacoma was first isolated from a young American woman of European descent in 1965, following documentation of an elevated Hb A2 percentage and presence of an additional trace band running near Hemoglobin A (Hb A) on alkaline electrophoresis (Baur et al., Citation1965). Subsequent molecular characterization revealed substitution of serine for arginine at position 30 in the β-globin chain (Brimhall et al., Citation1969). Physiochemical properties of Hb Tacoma include instability when subjected to heat and alcohol, and formation of Heinz bodies (Deacon-Smith & Lee-Potter, Citation1978), and it has been predominantly identified in persons with Finnish ancestry, but cases have also been reported in individuals of Swedish, Japanese, African, Arabian and Thai ethnicities (Deacon-Smith & Lee-Potter, Citation1978; El-Kalla & Matthews, Citation1997; Harano et al., Citation1985; Honig et al., Citation1980; Landin et al., Citation2000; Landin & Jeppsson, Citation1993; Sangkitporn et al., Citation2009). Heterozygotes are generally asymptomatic but may have mild anemia secondary to a small degree of in vivo instability of this variant (Brimhall et al., Citation1969; Deacon-Smith & Lee-Potter, Citation1978). Single case reports of compound heterozygosity with other β-globin mutations describe variable disease, such as a severe thalassemic phenotype in concert with β0 thalassemia (El-Kalla & Matthews, Citation1997), but mainly normal phenotypes in compound heterozygotes for Hemoglobin S (Honig et al., Citation1980) and β+ thalassemia respectively (Landin et al., Citation2000).
A variety of techniques have previously been employed to identify Hb Tacoma, ranging from gel electrophoresis (Baur et al., Citation1965) to isoelectric focusing (Landin & Jeppsson, Citation1993) and high-performance liquid chromatography (Landin et al., Citation2000). Many cases have been diagnosed following the identification of an elevated Hb A2 percentage with these methods. A further publication (Lenters-Westra et al., Citation2017) reported Hb Tacoma migration in zone 11 of the Capillarys 2 system with a hemoglobin variant platform, but to the best of our knowledge, the utility of the Capillarys 2 A1c platform in uniquely identifying Hb Tacoma in persons undergoing hemoglobin A1c testing has not previously been described.
2. Materials and methods
Whole blood samples were received from 4 patients for Hb A1c testing at MUMC, a tertiary academic hospital in Hamilton, Canada, and initially analyzed via CE using the HbA1c platform. Thereafter, all specimens were run on the CE hemoglobin variant platform and HPLC, with additional studies including Hb H body staining, instability testing and β-globin gene sequencing performed on a case-by-case basis.
2.1. Capillary electrophoresis
The Sebia Capillarys 2 Flex Piercing instrument (Sebia, Évry-Cedex, France) is a fully automated, high-throughput device that employs both hemoglobin variant (Hb[E]) and hemoglobin A1c (Hb A1c) programs for analysis. Both programs require their own set of hemolyzing and buffer solutions provided by Sebia, and the device interchanges between platforms with relative ease.
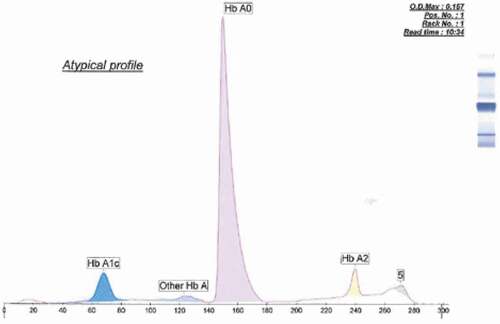
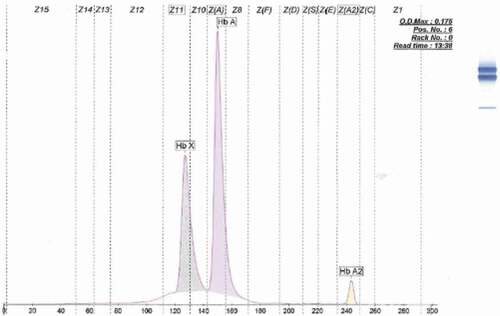
Samples were introduced into the analyzer, and subsequent steps were performed automatically, according to manufacturer guidelines. Samples were identified by barcodes, mixed via inversion, then hemolysed. Electrophoresis was performed in an alkaline buffer (pH 9.4). Detection of hemoglobins occurred at the cathodic end of the capillary where optical density (OD) was measured at 415 nm wavelength. Electropherograms were plotted with each peak representing separate hemoglobins in delineated zones, with Hb A appearing at 150 marks and Hb A2 at 243 marks. Each hemoglobin was quantified on an electropherogram and compared to the manufacturer’s normal ranges. Electropherograms with an OD less than 1.00 on the Hb A1c platform and less than 1.20 on the Hb[E] platform were considered invalid for interpretation, and the CE procedure was repeated and the samples were manipulated until target OD was achieved.
2.2. High-performance liquid chromatography
A Bio-rad Variant II Turbo analyzer (Bio-rad, Hercules, California) using the Beta Thalassemia Dual program and reagents kit was utilized to confirm the presence of variant hemoglobin within the samples. Samples were analyzed according to manufacturer guidelines.
2.3. Hemoglobin H staining
Sample from Patient 2 was prepared by adding six drops of patient whole blood to a glass test tube along with one drop of Brilliant Cresyl Blue (BCB)(EMD Chemicals Inc, Gibbstown New Jersey), then incubated for 1–2 hours at 37°C in a dry heating block. Blood films were prepared from the sample then stored at 37°C until analyzed microscopically. Each slide was reviewed on a Nikon Eclipse 50i microscope under 50× objective lens for 10–15 fields of view. The presence of Hb H bodies was confirmed with the 100× objective.
2.4. Unstable hemoglobin testing
The presence of unstable hemoglobin in Patient 2 was assessed via heat and isopropanol denaturation. Washed packed red blood cells from the sample were combined with an equal volume of water and half volume of carbon tetrachloride, then mixed and centrifuged to create a hemolysate. In the heat denaturation procedure, the hemolysate was combined with a potassium phosphate buffer (pH 7.0), and incubated at 60°C for timed intervals (0, 4, 6, 8, 10, 15, 20, 60 minutes). The OD, indicating the amount of degradation, was measured at 540 nm wavelength for each interval and the percentage of denaturation was manually calculated and plotted. The graphical pattern was examined and compared to known controls. Isopropanol instability was performed by mixing the hemolysate with a Tris-Hydroxymethyl Amino-Methane (THAM) buffered isopropanol solution (pH 7.4), then incubated at 37°C. The OD was measured at one-minute intervals for 15 minutes. The total change in optical density was then calculated and compared to the reference interval.
2.5. Genetic testing
The presence of HBB 30, AGG>AGT was confirmed using polymerase chain reaction (PCR) and direct Sanger-method nucleotide sequence analysis of the β-globin gene in Patients 1 and 2.
3. Results
3.1. Clinical history
Patient 1, a 46-year-old female of Irish and Finnish ancestry with diabetes mellitus, had been referred for routine Hb A1c monitoring when the variant hemoglobin was observed. She had no personal history of anemia or hemolysis, but her family history was significant for anemia in her mother, who had required blood transfusions previously for unclear reasons. Patient 2, a 45-year-old male with German and Finnish ancestry was referred for Hb A1c screening for diabetes mellitus. His comorbidities included diet-controlled dyslipidemia, borderline hypertension and osteoarthritis as well as pyloric stenosis requiring surgical repair as an infant. He had no personal history of anemia or hemolysis, nor did his two full siblings, parents or daughter. Samples from Patient 3, a 74-year-old male, and Patient 4, an 11-year-old female, were received from in-patient units at regional hospitals and no additional clinical information other than age, gender and admitting diagnosis was available. Hb A1c testing had been requested in the setting of an acute myocardial infarction for Patient 3, and for suspected child abuse in Patient 4.
3.2. Red blood cell indices
Hemoglobin concentrations were within normal reference intervals with normal MCV for 3 of the 4 cases, as displayed in , but Patient 3 was anemic at the time of CBC collection.
Table 1. Demographic and laboratory findings associated with Hb Tacoma in four individual cases at MUMC
3.3. Capillary electrophoresis
Electropherograms produced on the Hb A1c platform demonstrated variation in A1c percentage between samples, as shown in . A small double peak at the 271–273 mark area (as displayed in ) running past Hb A2 was present in all four samples, suggesting the presence of a variant hemoglobin. On the Hb[E] program, Hb A2 percentage was mildly elevated (i.e. between 3% and 4%) in all cases. As revealed in , a variant hemoglobin peak at 127–128 marks (Hb X) was identified in all four samples and quantified at 35–37% yielding a ratio of Hb A: Hb X was approximately 1.7:1.
3.4. High-performance liquid chromatography
Column chromatograms were notable for flattening of the Hb A peak running at approximately 1.7 minutes retention time, suggesting co-migration of variant hemoglobin along with Hb A.
3.5. Additional investigations
Hemoglobin H and instability testing were performed in Patient 2, and revealed the presence of Hb H bodies as well as abnormal heat and alcohol instability testing which is summarized in . The characteristic mutation HBB 30, AGG>AGT was confirmed in Patient 1 and Patient 2, but not in the remaining patients as the variant hemoglobin was identified incidentally on Hb A1c screening, and we did not have clinician orders to proceed with genetic studies.
4. Discussion
This case series highlights that CE with an Hb A1c platform in addition to the previously described hemoglobin variant platform (Lenters-Westra et al., Citation2017) is an effective screening tool for identifying the presence of Hb Tacoma given this unique electropherogram pattern. In addition, as Hb A1c quantification utilizing this platform becomes more widely used for screening and monitoring of diabetes, increased numbers of individuals with Hb Tacoma may be incidentally. Of further importance, the relative instability of Hb Tacoma can cause decreased erythrocyte life span, making it difficult to accurately assess Hb A1c levels. Care should be taken when interpreting Hb A1c as part of a diabetic screening tool as the relative percentage of Hb A1c may be falsely decreased.
Hemoglobin Vaasa (another unstable β-globin variant resulting from substitution of glutamine for glutamic acid at position 40) has been shown to have a nearly identical chromatogram pattern to Hb Tacoma on HPLC (Riou et al., Citation2018) and can only be distinguished through genetic analysis. Although we did not analyze any samples with confirmed Hb Vaasa, it has been shown to run in a similar position to Hb Tacoma on CE Hb[E] platform (Ag et al., Citation1977). We are not aware of any studies identifying Hb Vaasa on the CE A1c program and whether there could also be misidentification of Hb Tacoma for Hb Vaasa on this platform. Therefore, genetic analysis is still recommended.
Acknowledgements
We wish to acknowledge the contributions of the Red Cell Disorders Group and Provincial DNA Group at McMaster University to the collection, analysis and interpretation of the data.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
Notes on contributors
Hayley Merkeley
Dr. Merkeley is a Clinical Assistant Professor in the Department of Medicine at the University of British Columbia in Vancouver, Canada with an interest in red cell disorders including hemoglobinopathies. Dr. Verhovsek is an Assistant Professor in the Departments of Medicine as well as Pathology and Molecular Medicine at McMaster University in Hamilton, Canada. We wish to acknowledge the contributions of the Red Cell Disorders Group and Provincial DNA Group at McMaster University to the collection, analysis and interpretation of the data.
References
- Ag, K., Ten Pas, A., JB, W., Cope, N., Bolch, K., & Huisman, T. H. J. (1977). HB Vaasa or α2β2 (39(C5)CLN→GLU), A mildly unstable variant found in a Finnish family. Hemoglobin, 1(3), 292–7. https://doi.org/https://doi.org/10.3109/03630267709003413
- Baur, E. W., Motulsky, A. G., & Tacoma, H. (1965). A β-chain variant associated with increased Hb A2. Humangenetik, 1(7), 621–634.
- Brimhall, B., Jones, R. T., Baur, E. W., & Motulsky, A. G. (1969). Structural characterization of hemoglobin Tacoma. Biochemistry, 8(5), 2125–2129. https://doi.org/https://doi.org/10.1021/bi00833a051
- Deacon-Smith, R. A., & Lee-Potter, J. P. (1978). An unstable haemoglobin, Hb Tacoma β30 (B12) argser, deteted at birth by the demonstration of red cell inclusions. Journal of Clinical Pathology, 31(9), 883–887. https://doi.org/https://doi.org/10.1136/jcp.31.9.883
- El-Kalla, S., & Matthews, A. R. (1997). A Significant β -Thalassemia Heterogeneity in the United Arab Emirates. Hemoglobin, 21(3), 237–247. https://doi.org/https://doi.org/10.3109/03630269708997384
- Harano, K., Harano, T., Ueda, S., Mori, H., Hb Tacoma, S. S., Slightly Unstable, A., & Tsunematsu, T. (1985). Hemoglobin variant found in Japan. Hemoglobin, 9(6), 635–639. https://doi.org/https://doi.org/10.3109/03630268508997047
- Honig, G. R., Mason, R. G., Shamsuddin, M., Vida, L. N., Rao, K. R. P., & Patel, A. R. (1980). Two new sickle cell syndromes: HbS, Hb Camden, and alpha-thalassemia; and HbS in combination with Hb Tacoma. Blood, 55(4), 655–660. https://doi.org/https://doi.org/10.1182/blood.V55.4.655.655
- Landin, B., Alvelius, G., Rai, D. K., & Elinder, G. (2000). Compound Heterozygosity for Hb Tacoma and β+ Thalassemia. Hemoglobin, 24(3), 253–257. https://doi.org/https://doi.org/10.3109/03630260008997534
- Landin, B., & Jeppsson, J. O. (1993). Rare beta-chain hemoglobin variants found is Swedish patients during HbA1C analysis. Hemoglobin, 17(4), 303–318. https://doi.org/https://doi.org/10.3109/03630269308997484
- Lenters-Westra, E., Strunk, A., Campbell, P., & Slingerland, R. (2017). Can the Afinion HbA1c point-of-care instrument be an alternative method for the Tosoh G8 in the case of Hb-Tacoma? Scandinavian Journal of Clinical and Laboratory Investigation, 77(1), 2–7. https://doi.org/https://doi.org/10.1080/00365513.2016.1183261
- Riou, J., Szuberski, J., and Godart, C., Oliveira, J., Hoyer, D., & Bardakdjian-Michau, J. (2018). Precision of CAPILLARYS 2 for the detection of hemoglobin variants based on their migration positions. ASCP, 149(2), 172–180. https://doi.org/https://doi.org/10.1093/ajcp/aqx148
- Sangkitporn, S. K., Eksiri, L., Sangnoi, A., Duangruang, S., Dumbua, A., Rattanakittisophon, K., & Sangkitporn, S. (2009). Identification of β -globin gene mutations in Thailand using an automated fluorescence-based DNA sequencer. The International Journal of Laboratory Hematology, 31(5), 521–527. https://doi.org/https://doi.org/10.1111/j.1751-553X.2008.01072.x