 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
In urethane/α-chloralose anesthetized rats, electrical stimulation of cervical vagal afferent fibers inhibited the increases in brown adipose tissue sympathetic nerve activity and brown adipose tissue thermogenesis evoked by cold exposure, by nanoinjection of the GABAA receptor antagonist, bicuculline, in the dorsomedial hypothalamus, and by nanoinjection of N-methyl-D-aspartate in the rostral raphe pallidus. Vagus nerve stimulation-evoked inhibition of brown adipose tissue sympathetic nerve activity was prevented by blockade of ionotropic glutamate receptors in the termination site of vagal afferents in the nucleus of the solitary tract, and by nanoinjection of GABAA receptor antagonists in the rostral raphe pallidus. In conclusion, the brown adipose tissue sympathoinhibitory effect of cervical afferent vagal nerve stimulation is mediated by glutamatergic activation of second-order sensory neurons in the nucleus of the solitary tract and by a GABAergic inhibition of brown adipose tissue sympathetic premotor neurons in the rostral raphe pallidus, but does not require GABAergic inhibition of the brown adipose tissue sympathoexcitatory neurons in the dorsomedial hypothalamus.
Introduction
The levels of brown adipose tissue (BAT) thermogenesis and BAT energy expenditure are regulated by dedicated central neural circuitry that determines BAT sympathetic outflow.Citation1 The activity in the CNS circuits generating BAT sympathetic nerve activity (SNA) is influenced by peripheral afferent signals, principally those from cutaneous thermal afferents,Citation2,3 but also those from other sensors, including arterial chemoreceptors in the carotid sinus nerve, through which hypoxia reduces BAT thermogenesis.Citation4 Although visceral sensory endings with afferent axons in the cervical vagus nerve also influence BAT thermogenic responses to febrileCitation5,6 as well as metabolic stimuli,Citation7-10 our understanding of the roles of vagal afferents in the regulation of BAT and of the central neural pathways through which they regulate BAT SNA are still incomplete.
Largely based on the role of vagal afferent fibers in the regulation of feeding, and on the putative role of the vagus nerve in the successful weight loss following bariatric surgery, studies have begun to assess the potential of vagal nerve stimulation (VNS) as a treatment for obesity.Citation11 Chronic VNS has been accompanied by weight loss,Citation12 and increased metabolism in BAT may contribute to such weight loss.Citation13 However, other studies have failed to observe weight loss with chronic VNSCitation14 and mean uptake of 18F-fluorodeoxyglucose (an index of activation) in human BAT was not increased by VNS.Citation15 Given the potential role for sympathetic activation of BAT in therapeutic VNS for obesity and the potential for ‘off-target’ metabolic and thermoregulatory effects of VNS used for other indications (such as epilepsy, depression, heart failure, etc.) the current study was designed to determine directly the effect of electrical activation of cervical vagal afferents on rat BAT SNA. Electrical stimulation of cervical vagal afferent fibers inhibits BAT SNA and BAT thermogenesis via glutamatergic activation of neurons in the nucleus of the solitary tract (NTS) and a subsequent GABAergic inhibition of the BAT sympathetic premotor neurons in the rostral raphe pallidus area (rRPa).
Materials and methods
All procedures conform to the regulations detailed in the Guide for the Care and Use of Laboratory Animals: Eighth Edition (National Research Council, National Academies Press, 2010) and were approved by the Animal Care and Use Committee of the Oregon Health and Science University.
Male Sprague-Dawley and Wistar rats (344–754 grams, Charles River, Indianapolis, IN, USA) were anesthetized with isoflurane (2–3% in 100% O2) and the femoral artery and vein were cannulated. No differences in responses were noted between rat strains, therefore the results from both strains were considered as a single group. Rats were then transitioned from isoflurane to a combination of urethane (750 mg/kg, iv) and alpha-chloralose (60 mg/kg, iv) anesthesia. The trachea was cannulated and the animals were artificially ventilated with 100% O2 (stroke volume: ∼1 ml/100 g body weight, 50–70 strokes per minute) and paralyzed with d-tubocurarine (0.6 mg/rat, iv, supplemented thereafter with 0.3 mg when spontaneous respiratory activity was observed). At the time of the tracheal cannulation, the cervical vagi were isolated, and either the left or right vagus was secured to a bipolar hook stimulating electrode with Kwik-sil or Kwik-cast (World Precision Instruments, Sarasota, FL). The stimulated vagus nerve was crushed or transected distal to the electrode and, in most cases, the contralateral cervical vagus was also transected. VNS (1 ms pulses; 80–500 µA; 1, 2, 5 or 10 Hz) was delivered in 30 s trains at stimulus intensities of 3–4 times the threshold for complete inhibition of BAT SNA at 10 Hz.
Animals were placed in a stereotaxic frame with the incisor bar set to ∼4 to 6 mm below the interaural line such that bregma and lambda were level, except for experiments targeting the NTS, in which the incisor bar was positioned −12 mm below the interaural line. Thermocouples were positioned (a) in the rectum to monitor body core temperature (TCORE), which, unless otherwise noted, was maintained at 37.0 ± 0.5 °C with a heat lamp and a water-perfused thermal blanket; (b) in the left interscapular BAT pad to monitor BAT temperature (TBAT); and (c) on the skin of the shaved hindquarter to monitor skin temperature (TSKIN) under the thermal blanket. BAT SNA was recorded from a sympathetic nerve bundle dissected from the ventral surface of the right interscapular BAT pad and placed on a bipolar hook electrode under warm mineral oil.Citation16 BAT SNA was differentially amplified (10,000–50,000 times; CyberAmp 380, Axon Instruments, Union City, CA, USA), filtered (1–300 Hz), digitized and recorded onto a hard drive using Spike 2 software (Cambridge Electronic Design, Cambridge, UK).
VNS was applied during increases in BAT SNA produced by a) cooling rats (n = 8) with a water perfused thermal blanket,Citation17 b) nanoinjection of the GABAA receptor antagonists, bicuculline (BIC, 500 µM, 60 nl, n = 7)Citation18 or 2-(3-carboxypropyl)-6-(4-methoxyphenyl)-2,3-dihydropyridazin-3-iminium bromide (GABAzine, 1 mM, 60 nl, n = 7) in the rRPa (with a level skull, coordinates were: −3.0 mm caudal to lambda, midline, −9.5–9.8 mm ventral to the dura, or, with the incisor bar at −12 mm below the interaural line and relative to the calamus scriptorius: +3.0 mm rostral, midline, and −2.8 to −3.2 mm ventral), c) nanoinjection of N-methyl-D-aspartate (NMDA; 0.2 mM, 60 nl) in the rRPa (n = 8),Citation16 or d) nanoinjection of BIC (500 µM, 60 nl) in the dorsal medial hypothalamus (DMH; n = 5; with a level skull, coordinates were: −3.0 to −3.3 mm caudal to bregma, 0.5 to 0.7 mm lateral to the midline, and −7.8 to −8.2 mm ventral to dura).Citation19
Since vagal afferents terminate on second-order sensory neurons in the NTS and use glutamate is their principle excitatory neurotransmitterCitation20 and activation of NTS neurons can inhibit BAT SNA and BAT thermogenesis,Citation21 we tested the hypothesis that glutamatergic activation of neurons in the NTS is necessary for the inhibition of BAT SNA evoked by VNS. During increases in BAT SNA evoked by nanoinjection of NMDA in the rRPa (n = 5), VNS was performed before and after nanoinjections of the excitatory amino acid receptor antagonists, AP5 combined with CNQX (5 mM each, 80–100 nl) in the NTS (relative to the calamus scriptorius, coordinates were: 0.5 mm rostral, 0.5 mm lateral and 0.5 mm ventral). Control nanoinjections (80–100 nl) of saline vehicle into the NTS were also performed in a subset of rats (n = 3).
Data and statistical analyses
All physiological variables and stimulus trigger pulses were digitized (MicroII 1401; Cambridge Electronic Design, Cambridge, UK) to a computer hard drive for analysis (Spike 2, Cambridge Electronic Design). A continuous measure (4 s bins) of BAT SNA amplitude was obtained from the autospectra of sequential 4-s segments of the raw BAT SNA by calculating the root mean square (rms) value (square root of the total power in the 0.1 to 20 Hz band). The minimal level of BAT SNA was taken as the mean BAT SNA amplitude during a 30 second period recorded either when the rat was in a warm condition (TCORE > 37°C) or following administration of the ganglionic blocker, hexamethonium (30 mg/kg, iv). Percentage inhibitions of BAT SNA were calculated using the formula:
All statistics were performed using Systat software (Version 10, Cranes Software International, Chicago, IL, USA). Data are expressed as mean ± SE. Statistical comparisons were done between the 30-second period prior to treatment and the 30-second window at the peak or nadir of the treatment-evoked effect. Statistical significance was assessed using paired T-Tests. Results with p < 0.05 were considered significant.
Results
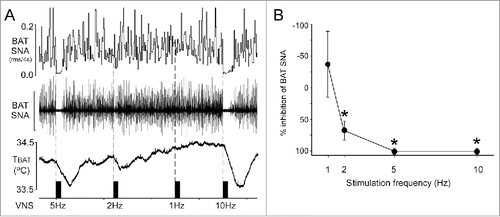
VNS inhibits cold-evoked BAT SNA. To test the hypothesis that activation of vagal afferent fibers inhibits the increase in BAT SNA evoked by cold exposure, the cervical vagus nerve was electrically stimulated during cold exposure. During cold exposure, BAT SNA is characterized by large bursts of activity () reflecting the summed action potentials of postganglionic axons innervating BAT. VNS for 30 seconds at frequencies of 2, 5, and 10 Hz significantly inhibited cold-evoked BAT SNA and decreased TBAT by −0.1 ± 0.0, −0.3 ± 0.1, and −0.3 ± 0.1°C, respectively ().
Figure 1. Electrical stimulation of afferent fibers in the cervical vagus nerve inhibits brown adipose tissue (BAT) sympathetic nerve activity (SNA). (A) Representative example demonstrating that vagal afferent fiber stimulation inhibits cold-evoked BAT SNA and BAT thermogenesis. Scale bar for BAT SNA is 200 µV. (B) Group data (mean ± SE; n = 7) illustrating the frequency response curve of the inhibition of BAT SNA evoked by vagal afferent fiber stimulation. * indicates p < 0.05 compared to the pre-stimulation value of cold-evoked BAT SNA. rms, root mean square; TBAT, BAT temperature.
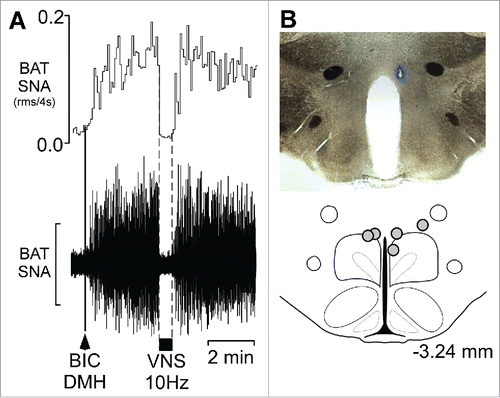
VNS inhibits BAT SNA evoked by disinhibition of neurons in the DMH. Nanoinjection of BIC in the DMH () increased BAT SNA to 1127 ± 259% of control, and VNS delivered during the peak of the BAT activation significantly decreased BAT SNA () to 168 ± 15% of control, a 93 ± 2% inhibition.
Figure 2. Vagal nerve stimulation (VNS) inhibits the increase in BAT SNA evoked by disinhibition of neurons in the dorsomedial hypothalamus (DMH) (A) Representative example demonstrating that VNS (10 Hz, 1 ms pulses, between the dotted lines) inhibits the increase in BAT SNA evoked by nanoinjection (black arrowhead) of bicuculline (BIC) in the DMH. Scale bar for BAT SNA is 50 µV. (B) A photomicrograph of a representative nanoinjection site (blue beads) and schematic representation of the BIC nanoinjection sites (filled circles) plotted on an atlas drawing, adapted from,Citation27 approximately 3.24 mm caudal to bregma that includes the DMH.
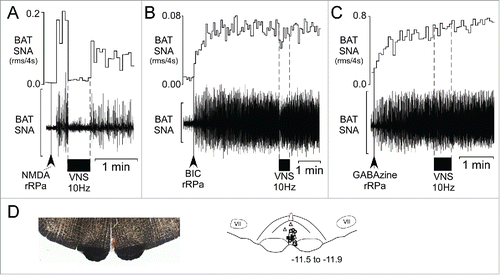
VNS inhibits the increased BAT SNA evoked by nanoinjection of NMDA in the rRPa, but not that evoked by blockade of GABAA receptors in the rRPa. Nanoinjection of NMDA in the rRPa () increased BAT SNA to 2542 ± 569% of control, and subsequent VNS significantly decreased BAT SNA to 662 ± 174% of control, representing a 77 ± 6% inhibition (). Nanoinjections of BIC () and of GABAzine in the rRPa () increased BAT SNA to 2371 ± 726% of control and 2350 ± 814% of control, respectively. However, subsequent VNS did not affect the amplitude of the increased BAT SNA evoked either by BIC (, post-drug BAT SNA: 2226 ± 676% of control, p = 0.53) or by GABAzine (post-drug BAT SNA: 2148 ± 706% of control, p = 0.33).
Figure 3. VNS inhibits the increase in BAT SNA evoked by activation of glutamate receptors in the rostral raphe pallidus (rRPa), but not that following blockade of GABAA receptors in rRPa. (A) Representative example of the VNS–evoked (10 Hz, 1 ms pulses, between the dotted lines) inhibition of the increase in BAT SNA evoked by nanoinjection (black arrowhead) of N-methyl-D-aspartate (NMDA) in the rRPa. (B, C) Representative examples demonstrating that the increases in BAT SNA evoked by nanoinjections (black arrowheads) of the GABAA receptor antagonists, bicuculline (BIC, B) or GABAzine (C), in the rRPa are not inhibited by VNS (10 Hz, 1 ms pulses, between the dotted lines). Scale bar for BAT SNA is 200 µV, 50 µV and 600 µV in A, B, and C, respectively. (D) Photomicrograph (left) of a representative nanoinjection site (red beads) of GABAzine in rRPa, and an atlas drawing (right), adapted from,Citation27 showing the locations of the nanoinjection sites in rRPa for NMDA (triangles, n = 7, the site from one rat was not recovered), BIC (squares, n = 7) and GABAzine (circles, n = 7). Numbers in lower right indicate the approximate distance from bregma.
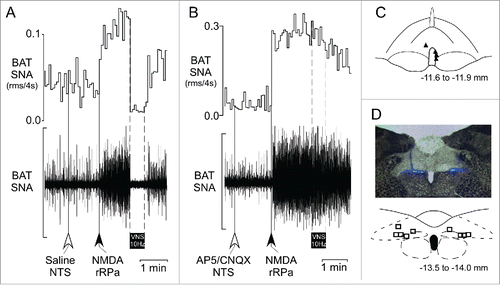
Nanoinjection of glutamate receptor antagonists in the NTS prevents the VNS-evoked inhibition of BAT SNA. Following nanoinjection of saline in the medial NTS (), nanoinjection of NMDA in the rRPa () increased BAT SNA to 1061 ± 240% of control (). Subsequent VNS significantly reduced the amplitude of the BAT SNA evoked by nanoinjection of NMDA in rRPa, to 235 ± 44% of control (; during VNS: 80 ± 12% inhibition of BAT SNA), as in naïve rats (). After nanoinjection of AP5/CNQX in the NTS (), nanoinjection of NMDA in the rRPa increased BAT SNA to 2411 ± 1186% of control (). Subsequent VNS had no effect on the amplitude of the NMDA-evoked level of BAT SNA (, during VNS: 2299 ± 1139% of control, p = 0.38).
Figure 4. Blockade of glutamate receptors in the nucleus of the solitary tract (NTS) prevents the VNS-evoked inhibition of the increase in BAT SNA evoked by glutamate receptor activation in rRPa. (A) Representative example illustrating the VNS-evoked (10 Hz, 1 ms pulses, between the dotted lines) inhibition of the increase in BAT SNA evoked by nanoinjection (black arrow) of NMDA in the rRPa following nanoinjection (white arrow) of saline in the NTS. (B) Representative example demonstrating the absence of a VNS-evoked (10 Hz, 1 ms pulses, between the dotted lines) inhibition of the increase in BAT SNA evoked by nanoinjection (black arrow) of NMDA in the rRPa after nanoinjection (white arrow) of the glutamate receptor antagonists, AP5/CNQX, in the NTS. (C) Schematic representation of the NMDA nanoinjection sites (solid triangles, one site not recovered) in the rRPa plotted on an atlas drawing, adapted from,Citation27 of a brainstem section at 11.6 to 11.9 mm caudal to bregma, which includes the rRPa. (D) A photomicrograph (upper) of a representative nanoinjection site in the NTS (blue beads) and schematic representation (lower) of the bilateral AP5/CNQX injection sites plotted on an atlas drawing, adapted from,Citation27 of a brainstem section at 13.5 to 14.0 mm caudal to bregma, which includes the medial NTS. Scale bar for BAT SNA is 150 µV and 300 µV in A and B, respectively.
Discussion
Stimulation of afferent fibers in the cervical vagus nerve inhibited the increases in BAT SNA evoked by cold, by disinhibition of neurons in DMH, and by activation of NMDA receptors in the rRPa. In contrast, the increase in BAT SNA evoked by blockade of GABAA receptors in the rRPa was resistant to inhibition by VNS. These data demonstrate that GABAA receptor activation in the DMH is not required for the VNS-evoked inhibition of BAT SNA and suggest that the neural pathway involved in this VNS-evoked inhibition of BAT SNA includes a GABAergic input to the BAT sympathetic premotor neurons in the rRPa. Furthermore, blockade of ionotropic glutamate receptors in the NTS prevented the VNS-evoked inhibition of BAT SNA, an observation consistent with the location of vagal afferent terminals in the NTS and the use of glutamate as the primary neurotransmitter by these afferents.Citation20
The location of the GABAergic neurons whose input to the rRPa is responsible for the vagal afferent-induced inhibition of BAT SNA and BAT thermogenesis remains to be discovered. Although a GABAergic input from the NTS to the BAT sympathetic premotor neurons in the rRPa has been suggested to play a role in the BAT sympathoinhibition evoked from the paraventricular hypothalamus in the mouse,Citation22 in the rat, very few NTS neurons are retrogradely labeled from the rRPa,Citation23 consistent with the absence of a direct input to the rRPa from the NTS.
VNS has been purported to activate human BAT thermogenesis,Citation15 although the FDG uptake data reported by Vijgen et al.Citation15 actually shows that mean BAT activity was not significantly different between periods of active VNS and periods when the vagus nerve was not stimulated. Thus, one must question the apparently erroneous interpretation that BAT thermogenesis played a role in the VNS-evoked increase in energy expenditure in the study of Vijgen et al.Citation15 Our observation that BAT SNA is inhibited during VNS has important implications for the use of VNS for weight reduction and may help to explain the equivocal effects of VNS on body weight.Citation14,24 Reductions in BAT activity during VNS would be expected to reduce overall energy expenditure, thereby counteracting any beneficial effect of the treatment on body weight.
Although the current study indicates that electrical stimulation of vagal afferent fibers elicits a potent sympathoinhibition of BAT, it would be inappropriate to conclude that there are no vagal afferent fibers capable of exciting BAT SNA. At lower frequencies of stimulation (<1 Hz), we have observed VNS-evoked excitatory potentials on BAT SNA and low frequency VNS increased BAT SNA in some rats (). Furthermore, activations of subpopulations of vagal afferents, such as those containing CCK receptorsCitation8 or those involved in the early phase of the lipopolysaccharide (LPS)-evoked febrile response can increase thermogenesis,Citation5,6 likely via sympathetic activation of BAT.
Our data indicate that the predominant effect of 2–10 Hz electrical stimulation of afferents in the cervical vagus nerve is sympathoinhibitory for BAT SNA, and thus maintaining such VNS would be expected to reduce BAT thermogenesis and BAT energy expenditure. We have not yet identified any endogenous stimuli that might normally activate the relevant vagal afferent fibers that evoke BAT sympathoinhibition. However, we have demonstrated that vagal afferent fibers contribute to the inhibition of cold-evoked activation of BAT SNA and BAT thermogenesis during maintenance on high fat diet,Citation10 and the activation of these fibers would be expected to contribute to the inhibition of BAT SNA observed during VNS in the current study. Since upregulation of hepatic glucokinase decreases BAT thermogenesis via a vagal afferent mechanism, hepatic vagal afferents may represent one population of vagal afferents capable of inhibiting BAT SNA. Additionally, intravenous ghrelin suppresses norepinephrine release in BAT via a vagal afferent mechanism,Citation25 although this effect may be concentration dependent.Citation26
In conclusion, activation of a population of vagal afferent fibers is capable of completely inhibiting the sympathetic activation of BAT thermogenesis. The VNS-evoked inhibition of BAT SNA requires glutamatergic activation of neurons in the NTS and is likely mediated via a GABAergic input to the BAT sympathetic premotor neurons in the rRPa. These observations have important implications for the now common, therapeutic applications of VNS.
Abbreviations
| AP5 | = | 2-amino-5-phosphonopentanoic acid |
| BAT | = | brown adipose tissue |
| BIC | = | bicuculline |
| CNQX | = | 6-cyano-7-nitroquinoxaline-2,3-dione |
| DMH | = | dorsomedial hypothalamus |
| GABA | = | gamma-aminobutyric acid |
| GABAzine | = | 2-(3-carboxypropyl)-6-(4-methoxyphenyl)-2,3-dihydropyridazin-3-iminium bromide |
| NMDA | = | N-methyl-D-aspartate |
| NTS | = | nucleus of the solitary tract |
| rms | = | root mean square |
| rRPa | = | rostral raphe pallidus area |
| SNA | = | sympathetic nerve activity |
| SE | = | Standard error |
| TBAT | = | brown adipose tissue temperature |
| TCORE | = | body core temperature |
| TSKIN | = | skin temperature |
| VNS | = | vagal nerve stimulation |
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
We are grateful to Rubing Xing for technical assistance.
Funding
This work was supported by the National Institutes of Health [R01-NS091066] (S.F.M.) and the American Diabetes Association [1-13-BS-120] (C.J.M.).
References
- Morrison SF, Madden CJ. Central nervous system regulation of brown adipose tissue. Compr Physiol 2014; 4:1677-713; PMID:25428857; http://dx.doi.org/10.1002/cphy.c140013
- Nakamura, K, Morrison SF. A thermosensory pathway that controls body temperature. Nat Neurosci 2008; 11:62-71; PMID:18084288; http://dx.doi.org/10.1038/nn2027
- Nakamura K, Morrison SF. A thermosensory pathway mediating heat-defense responses. Proc Natl Acad Sci U S A 2010; 107:8848-53; PMID:20421477; http://dx.doi.org/http://dx.doi.org/10.1073/pnas.0913358107
- Madden CJ, Morrison SF. Hypoxic activation of arterial chemoreceptors inhibits sympathetic outflow to brown adipose tissue in rats. J Physiol 2005; 566:559-73; PMID:15878945; http://dx.doi.org/10.1113/jphysiol.2005.086322
- Blatteis CM, Sehic E, Li S. Afferent pathways of pyrogen signaling. Ann N Y Acad Sci 1998; 856:95-107; PMID:9917870
- Romanovsky AA, Ivanov AI, Szekely M. Neural route of pyrogen signaling to the brain. Clin Infect Dis 2000; 31:S162-7; PMID:11113019; http://dx.doi.org/10.1086/317515
- Szekely M. The vagus nerve in thermoregulation and energy metabolism. Auton Neurosci 2000; 85:26-38; PMID:11189024; http://dx.doi.org/10.1016/S1566-0702(00)00217-4
- Blouet C, Schwartz GJ. Duodenal lipid sensing activates vagal afferents to regulate non-shivering brown fat thermogenesis in rats. PLoS One 2012; 7:e51898; PMID:23251649; http://dx.doi.org/10.1371/journal.pone.0051898
- Tsukita S, Yamada T, Uno K, Takahashi K, Kaneko K, Ishigaki Y, Imai J, Hasegawa Y, Sawada S, Ishihara H, et al. Hepatic glucokinase modulates obesity predisposition by regulating BAT thermogenesis via neural signals. Cell Metab 2012; 16:825-32; PMID:23217261; http://dx.doi.org/10.1016/j.cmet.2012.11.006
- Madden CJ, Morrison SF. A high-fat diet impairs cooling-evoked brown adipose tissue activation via a vagal afferent mechanism. Am J Physiol Endocrinol Metab 2016; 311:E287-92; PMID:27354235; http://dx.doi.org/10.1152/ajpendo.00081.2016
- Berthoud HR. The vagus nerve, food intake and obesity. Regul Pept 2008; 149:15-25; PMID:18482776; http://dx.doi.org/10.1016/j.regpep.2007.08.024
- Pardo JV, Sheikh SA, Kuskowski MA, Surerus-Johnson C, Hagen MC, Lee JT, Rittberg BR, Adson DE. Weight loss during chronic, cervical vagus nerve stimulation in depressed patients with obesity: an observation. Int J Obes (Lond) 2007; 31:1756-9; PMID:17563762; http://dx.doi.org/10.1038/sj.ijo.0803666
- Li H, Zhang JB, Xu C, Tang QQ, Shen WX, Zhou JZ, Chen JD, Wang YP. Effects and mechanisms of auricular vagus nerve stimulation on high-fat-diet–induced obese rats. Nutrition 2015; 31:1416-22; PMID:26429664; http://dx.doi.org/10.1016/j.nut.2015.05.007
- Koren MS, Holmes MD. Vagus nerve stimulation does not lead to significant changes in body weight in patients with epilepsy. Epilepsy Behav 2006; 8:246-9; PMID:16343997; http://dx.doi.org/10.1016/j.yebeh.2005.10.001
- Vijgen GH, Bouvy ND, Leenen L, Rijkers K, Cornips E, Majoie M, Brans B, van Marken Lichtenbelt WD. Vagus nerve stimulation increases energy expenditure: relation to brown adipose tissue activity. PLoS One 2013; 8:e77221; PMID:24194874; http://dx.doi.org/10.1371/journal.pone.0077221
- Madden CJ, Morrison SF. Neurons in the paraventricular nucleus of the hypothalamus inhibit sympathetic outflow to brown adipose tissue. Am J Physiol Regul Integr Comp Physiol 2009; 296:R831-43; PMID:19129373; http://dx.doi.org/10.1152/ajpregu.91007.2008
- Nakamura K, Morrison SF. Central efferent pathways mediating skin cooling-evoked sympathetic thermogenesis in brown adipose tissue. Am J Physiol Regul Integr Comp Physiol 2007; 292:R127-36; PMID:16931649; http://dx.doi.org/10.1152/ajpregu.00427.2006
- Morrison SF, Sved AF, Passerin AM. GABA-mediated inhibition of raphe pallidus neurons regulates sympathetic outflow to brown adipose tissue. Am J Physiol 1999; 276:R290-7; PMID:9950904
- Cao WH, Fan W, Morrison SF. Medullary pathways mediating specific sympathetic responses to activation of dorsomedial hypothalamus. Neuroscience 2004; 126:229-40.
- Andresen MC, Kunze DL. Nucleus tractus solitarius–gateway to neural circulatory control. Annu Rev Physiol 1994; 56:93-116; PMID:7912060; http://dx.doi.org/10.1146/annurev.ph.56.030194.000521
- Cao WH, Madden CJ, Morrison SF. Inhibition of brown adipose tissue thermogenesis by neurons in the ventrolateral medulla and in the nucleus tractus solitarius. Am J Physiol Regul Integr Comp Physiol 2010; 299:R277-90; PMID:20410479; http://dx.doi.org/10.1152/ajpregu.00039.2010
- Kong D, Tong Q, Ye C, Koda S, Fuller PM, Krashes MJ, Vong L, Ray RS, Olson DP, Lowell BB. GABAergic RIP-Cre neurons in the arcuate nucleus selectively regulate energy expenditure. Cell 2012; 151:645-57; PMID:23101631; http://dx.doi.org/10.1016/j.cell.2012.09.020
- Tupone D, Madden CJ, Morrison SF. Central activation of the A1 adenosine receptor (A1AR) induces a hypothermic, torpor-like state in the rat. J Neurosci 2013; 33:14512-25; PMID:24005302; http://dx.doi.org/10.1523/JNEUROSCI.1980-13.2013
- Ikramuddin S., Blackstone RP, Brancatisano A, Toouli J, Shah SN, Wolfe BM, Fujioka K, Maher JW, Swain J, Que FG, et al. Effect of reversible intermittent intra-abdominal vagal nerve blockade on morbid obesity: the ReCharge randomized clinical trial. JAMA 2014; 312:915-22; PMID:25182100; http://dx.doi.org/10.1001/jama.2014.10540
- Mano-Otagiri A, Ohata H, Iwasaki-Sekino A, Nemoto T, Shibasaki T. Ghrelin suppresses noradrenaline release in the brown adipose tissue of rats. J Endocrinol 2009; 201:341-9; PMID:19351665; http://dx.doi.org/10.1677/JOE-08-0374
- Habara H, Hayashi Y, Inomata N, Niijima A, Kangawa K. Organ-specific activation of the gastric branch of the efferent vagus nerve by ghrelin in urethane-anesthetized rats. J Pharmacol Sci 2014; 124:31-9; PMID:24366191
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Ed 6. Sydney: Academic; 2007.
