ABSTRACT
ARTS (Sept4_i2) is a pro-apoptotic mitochondrial tumor suppressor protein which binds to and causes degradation of XIAP (X-linked inhibitor of apoptosis). We recently showed that ARTS brings XIAP into close proximity to Bcl-2, creating a complex which enables degradation of both these major anti-apoptotic proteins and promotes apoptosis. The possible therapeutic implications are discussed here.
ARTS (Apoptosis Related protein in TGF-beta Signaling pathway)(Sept4_i2) was initially discovered as a pro-apoptotic protein in the TGF-beta signaling pathway but was shown to function in many other apoptotic pathways, including those triggered by staurosporine, arabinoside, etoposide, UV etc. ARTS induces apoptosis through direct binding and antagonizing XIAP. ARTS not only binds directly to XIAP but it also promotes its proteasome-mediated degradation in apoptotic cells.Citation1,Citation2 Support for the function of ARTS as a tumor suppressor protein came from studies both in human patients and in mice. ARTS expression is lost or significantly reduced through epigenetic silencing in more than 70% of tested Acute Lymphoblastic Leukemia (ALL) patients,Citation3 50% of lymphoma patientsCitation4, and more than 70% of hepatocellular carcinoma patients (HCC) (Pham and Steller unpublished results). Evidence for a role of ARTS as a physiological XIAP-antagonist and tumor suppressor came from inactivation of the mouse Sept4/ARTS gene. Sept4/ARTS-Null mice show accelerated spontaneous tumor development, elevated XIAP levels and elevated stem cell numbers which exhibit increased resistance to cell deathCitation4. These data demonstrate the important physiological role of ARTS in regulating apoptosis and as a tumor suppressor protein in vivo.
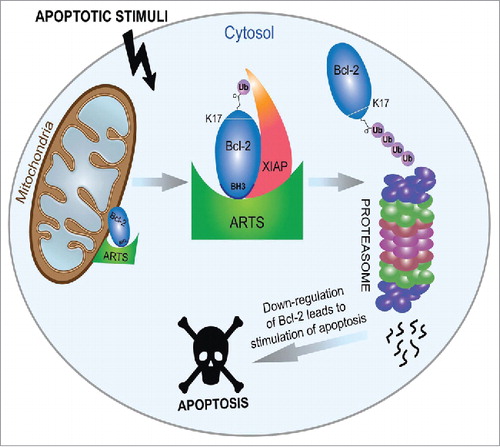
High levels of ARTS are sufficient to induce apoptosis. In response to apoptotic stimuli, transcriptional activation of ARTS results in higher levels of ARTS which bind to XIAP within minutes following induction of apoptosis. This leads to rapid release of active caspases from their complex with XIAP (S. Larisch unpublished results and.Citation5 This de-repression of caspases occurs upstream of MOMP (Mitochondrial Outer Membrane Permeabilization) and precedes the release of cytochrome and Smac/Diablo by several hours.Citation5 Unlike the process of opening of the mitochondrial pores, which is caspase dependent and is required for the release of cytochrome c and Smac/Diablo from the inter membrane space of the mitochondria, the translocation of ARTS from the outer membrane of mitochondria is caspase independent and can occur rapidly. Within minutes following apoptotic induction ARTS can translocate to the cytosol to bind to XIAP and cause de-repression of active caspases.Citation5 The function of ARTS is required for proper, on time MOMP and release of proteins in the mitochondrial inter-membrane space. Accordingly, Sept4/ARTS-Null MEFs (Mouse Embryonal Fibroblasts) exhibit a significant delay in the release of cytochrome c and Smac/Diablo from the mitochondria following apoptotic inductionCitation5. Therefore, we suggest that this pre-MOMP ARTS-dependent release of active caspases enables cleavage of caspase substrates such as Bid that can now promotes MOMP. This also provides an explanation for many reports demonstrating a requirement for caspases to enable release of Smac/Diablo and MOMP (for example,Citation6 We have previously shown that ARTS acts as an XIAP antagonist).Citation1,Citation2 In addition, we identified a distinct mechanism by which ARTS promotes apoptosis through antagonizing Bcl-2 (). In living cells, ARTS is in a complex with Bcl-2 at the outer membrane of mitochondria, and XIAP and Bcl-2 do not bind to each other. However, upon induction of apoptosis ARTS serves as a scaffold to bring XIAP into close proximity with Bcl-2. This now allows XIAP to act as an E3-ligase and degrade Bcl-2 through the ubiquitin-proteasome system.Citation7 Lysine 17 in Bcl-2 is the major acceptor for ARTS and XIAP-mediated ubiquitylation (). In vivo ubiquitylation assays preformed in both XIAP and Sept4/ARTS null MEFs showed a significant inhibition of ubiquitylation of Bcl-2 upon induction of apoptosis. Moreover, mutating Lysine 17 to Alanine in Bcl-2 as well as mutating all 4 Lysines in Bcl-2 caused increased protein stability and more potent anti-apoptotic function.Citation7 The BH3 domain in Bcl-2 is known to mainly interact with BH3 domains of other Bcl-2 members. Interestingly, we showed that ARTS binds directly also to the BH3 domain of Bcl-2, and a mutant Bcl-2 protein lacking its BH3 domain had reduced binding to ARTS and significant decrease in its ability to undergo ubiquitylation.Citation7 Using a peptide array method, we found that ARTS binds to the same Bcl-2-BH3 binding pocket as the pro-apoptotic Bax protein. This suggests that Bcl-2 counteracts ARTS in living cells in a similar way to its interaction with Bax. In addition, Sept4/ARTS null MEFs and ARTS KD (knocked down cells) exhibit high levels of Bcl-2, suggesting that ARTS functions as a novel Bcl-2 antagonist. Interestingly, the BH3-mimetic ABT-199 can attenuate the inter-action between Bcl-2 and ARTS, indicating that the specific BH3-binding motif to which ABT-199 binds in Bcl-2 is also important for the interaction with ARTS. This activity of ABT-199 is expected to reduce degradation of Bcl-2, which has the potential to cause increased resistance towards apoptosis. However, because ABT-199 causes cell killing it is clear that this compound potently inactivates the anti-apoptotic activity of Bcl-2, even if present at somewhat elevated levels.
Figure 1. Regulation of Bcl-2 stability by ARTS and XIAP. ARTS brings XIAP and Bcl-2 into a ternary complex leading to ubiquitylation and degradation of Bcl-2 by XIAP to promote apoptosis. In living cells both ARTS and Bcl-2 are localized at the outer membrane of the mitochondria. Upon induction of apoptosis ARTS and Bcl-2 accumulate in the cytosol. ARTS binds directly to both XIAP and to the BH3 domain in Bcl-2 enabling the formation of ternary complex. Thus, ARTS serves as an adaptor protein bringing XIAP containing an E3-ligase activity in close proximity to Bcl-2. This induces the ubiquitylation of lysine 17 (K17) in Bcl-2 and its degradation by the proteasome. Down regulation of Bcl-2 levels promotes a series of cellular events leading to caspase activation and apoptosis (adapted from).Citation7
Bcl-2 is over-expressed in hematological malignancies and solid tumors.Citation8 Likewise, XIAP is over-expressed in many cancers.Citation9 High XIAP expression has been correlated with resistance to chemotherapy and radiotherapy and to poor prognosis. Therefore, intense efforts have been made to target these two major anti-apoptotic proteins for cancer therapy. Moreover, simultaneous inhibition of both XIAP and Bcl-2 enhances killing of cancer cells in a nonlinear way and improves chemotherapy.Citation10 This suggests that ARTS-mimetic small molecules that could degrade both XIAP and Bcl-2 will be potentially very effective cancer therapeutics.
References
- Gottfried Y, Rotem A, Lotan R, Steller H, Larisch S. The mitochondrial ARTS protein promotes apoptosis through targeting XIAP. EMBO J. 2004;23(7):1627–35. doi:10.1038/sj.emboj.7600155. PMID:15029247.
- Garrison JB, Correa RG, Gerlic M, Yip KW, Krieg A, Tamble CM, Shi R, Welsh K, Duggineni S, Huang Z, et al. ARTS and Siah collaborate in a pathway for XIAP degradation. Mol Cell. 2011;41(1):107–16. doi:10.1016/j.molcel.2010.12.002. PMID:21185211.
- Elhasid R, Sahar D, Merling A, Zivony Y, Rotem A, Ben-Arush M, Izraeli S, Bercovich D, Larisch S. Mitochondrial pro-apoptotic ARTS protein is lost in the majority of acute lymphoblastic leukemia patients. Oncogene. 2004;23(32):5468–75. doi:10.1038/sj.onc.1207725. PMID:15122323.
- Garcia-Fernandez M, Kissel H, Brown S, Gorenc T, Schile AJ, Rafii S, Larisch S, Steller H. Sept4/ARTS is required for stem cell apoptosis and tumor suppression. Genes Dev. 2010;24(20):2282–93. doi:10.1101/gad.1970110. PMID:20952537.
- Edison N, Zuri D, Maniv I, Bornstein B, Lev T, Gottfried Y, Kemeny S, Garcia-Fernandez M, Kagan J, Larisch S. The IAP-antagonist ARTS initiates caspase activation upstream of cytochrome C and SMAC/Diablo. Cell Death Differ. 2012;19(2):356–68. doi:10.1038/cdd.2011.112. PMID:21869827.
- Adrain C, Creagh EM, Martin SJ. Apoptosis-associated release of Smac/DIABLO from mitochondria requires active caspases and is blocked by Bcl-2. EMBO J. 2001;20(23):6627–36. doi:10.1093/emboj/20.23.6627. PMID:11726499.
- Edison N, Curtz Y, Paland N, Mamriev D, Chorubczyk N, Haviv-Reingewertz T, Kfir N, Morgenstern D, Kupervaser M, Kagan J, et al. Degradation of Bcl-2 by XIAP and ARTS Promotes Apoptosis. Cell Reports. 2017;21(2):442–54. doi:10.1016/j.celrep.2017.09.052. PMID:29020630.
- Kelly PN, Strasser A The role of Bcl-2 and its pro-survival relatives in tumourigenesis and cancer therapy. Cell Death Differ. 2011;18(9):1414–24. doi:10.1038/cdd.2011.17. PMID:21415859.
- Tamm I, Kornblau SM, Segall H, Krajewski S, Welsh K, Kitada S, Scudiero DA, Tudor G, Qui YH, Monks A, et al. Expression and prognostic significance of IAP-family genes in human cancers and myeloid leukemias. Clin Cancer Res. 2000;6(5):1796–803. PMID:10815900.
- Skommer J, Das SC, Nair A, Brittain T, Raychaudhuri S Nonlinear regulation of commitment to apoptosis by simultaneous inhibition of Bcl-2 and XIAP in leukemia and lymphoma cells. Apoptosis. 2011;16(6):619–26. doi:10.1007/s10495-011-0593-1. PMID:21442307.
