ABSTRACT
How immunological cues trigger cancer cell-intrinsic signaling pathways for their entering dormancy remains an enigma. In out recent studies, we found that IFN-β induces tumor-repopulating cells (TRC) into dormancy by activating Indoleamine-pyrrole 2,3-dioxygenase (IDO)-Kynurenine-aryl hydrocarbon receptor (AhR)-cyclin-dependent kinase inhibitor 1B (p27) pathway, while blocking this pathway leads dormant TRCs to apoptosis by switching to STAT3-cellular tumor antigen p53 (p53) pathway.
Persistent clinical observations imply that cancers do not always grow continuously, and during certain periods of time they enter a dormancy-like steady state. This state of dormancy might occur in early microscopic tumor nodules, remnants of primary tumors following treatments, and micrometastases in distant organs.Citation1 In addition, considering the heterogenicity of tumor growth, the dormancy might even exist in growing tumor masses. Currently, tumor dormancy can be divided into 2 models: (a) tumor mass dormancy due to the balance of tumor cell proliferation and death mediated by poor vascularization, immune cell killing, or chemo-radiotherapy and (b) cellular dormancy by either solitary cells or a small group of cells entering quiescence. However, cellular dormancy is distinct from quiescence, since dormancy is often related to undifferentiated stem cells with downregulated metabolic processes and protein translation. By contrast, quiescence would be a stage for differentiated cells during cell cycle arrest but with active metabolism and protein translation, such as pigment manufacturing cells or brain cells. Such stem-related dormancy is likely inherited from primordial cells, since dormancy might be a hardwired program for ancestral cells against nutrient deficiency and other hash microenvironments. In this regard, stem cell-like tumor cell dormancy can be functionally defined as G0/G1 arrest, being neither apoptotic nor senescent, consuming less glucose, decreasing the reaction to stimulators as well as re-growing once inducers of dormancy are removed.Citation2,Citation9 Due to the limitations of high cellular volumes that restrict extensive mechanistic studies on stem cell-like tumor cells, and also due to the scarcity of mouse models that recapitulate the complex process of tumor cell dormancy, tumor cell dormancy remains an enigma.
Immunologic tumor dormancy has been observed in clinical post-transplantation patients. However, how the immune system is involved in maintaining tumor cells in dormancy remains unclear. In our recent study, we demonstrated that the most important antiviral cytokine IFN-β induces stem cell-like tumor-repopulating cells (TRC) to enter dormancy.Citation3 The addition of 5 or 10 ng/ml IFN-β to TRCs cultured in 90 Pa 3D soft fibrin gels resulted in the effective dormancy of TRCs, as evidenced by unexpanded spheroids, G0/G1 arrest, and the appearance of a NR2F1+Ki67– or DEC2+Ki67– dormant phenotype. We also confirmed this phenomenon in B16 melanoma-bearing mice by intratumoral injection of 250 ng/day IFN-β for 3 days. These data clearly show that IFN-β induces TRCs into dormancy. However, the question remains, what is the physio-pathological relevance of this phenomenon? Is IFN-β expressed in tumor tissues? Using a bioinformatic approach, we found that IFN-β was expressed in various types of cancers, including colon, stomach, breast, lung, pancreatic, and urothelial cancers and melanoma. Intriguingly, IFN-βhi and IFN-βlo expression was linked to the proportion of NR2F1+Ki67– dormant and NR2F1–Ki67+ proliferating cells, respectively.Citation3 So, how is IFN-β induced in tumor tissues? Viral infection is the most effective inducer of IFN-β expression, and around 15% human cancers are initiated by viral infection.Citation4 However, most tumor types are not caused by viral infection. In this case, other signaling pathway(s) might be used to stimulate IFN-β production in tumor microenvironments. Tumor cells face a formidable microenvironment full of hypoxia, nutrient deficiency, low pH value, abnormal blood vessels and irregular matrices, leading to abundant tumor cells suffering apoptosis or necrosis. This manner of cell death results in the direct release of genomic and mitochondrial DNA fragments or their indirect release in the microparticle form to the extracellular space, which may activate the cGAS-STING pathway for IFN-β induction.Citation5,Citation6 In addition, the apoptotic release of RNAs might activate the retinoic acid-inducible gene I (RIG-I) pathway for IFN-β induction.Citation7 Therefore, the presence of IFN-β in tumor microenvironments might be a ubiquitous biological event. But, how does IFN-β induce TRC dormancy?
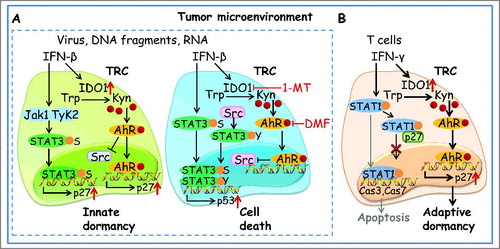
Tryptophan can be metabolized to serotonin and melatonin through the tryptophan hydroxylase pathway and to niacin through the indoleamine-pyrrole -2,3 dioxygenase (IDO) pathway. Upon IDO catalysis, tryptophan is converted to the intermediate metabolite kynurenine, an endogenous ligand of the aryl hydrocarbon receptor (AhR).Citation8 Intriguingly, we found that TRCs had a higher IDO-Kynurenine (Kyn)-AhR baseline, compared to their differentiated counterparts, and IFN-β treatment further enhanced the IDO-kyn-AhR pathway, leading to AhR translocating to the nucleus to exert its transcription factor function.Citation3 Both ChIP-qPCR and luciferase assays showed that AhR bound Cdkn1b, leading to cyclin-dependent kinase inhibitor 1B (p27) expression, thus mediating cell cycle arrest. In line with this mechanism, our previous study showed that IFN-γ also activated this pathway to induce TRCs into dormancy.Citation9 However, IFN-β seemed to have a stronger ability to induce TRC dormancy than IFN-γ. Further study showed that STAT3 in TRCs was intrinsically phosphorylated at the tyrosine residue by Src kinase, however IFN-β-activated AhR inhibited Src activity, thus preventing tyrosine phosphorylation. However, IFN-β signaling, probably through the Jak1/Tyrosine Kinase pathway, caused STAT3 serine phosphorylation. Serine phosphorylation mediated STAT3 translocation into the nucleus, where STAT3 bound to Cdkn1b, further promoting p27 expression.
The elucidation of this mechanism prompted us to block the IDO-Kyn-AhR pathway to presumably awaken dormant TRCs. Surprisingly, the blockade of this pathway led to dormant TRC apoptosis rather than revival. This unusual result prompted us to investigate further. Blocking Kyn-AhR relieved the inhibition on Src kinase, leading to STAT3 serine and tyrosine dual phosphorylation in IFN-β-treated TRCs. This dually phosphorylated STAT3 entered the nucleus where it bound to the Tp53 promoter rather than Cdkn1b, leading to cellular tumor antigen p53 (p53) overexpression. This upregulation of p53 in turn disrupted the pentose phosphate pathway, leading to excessive reactive oxygen species (ROS) production and dormant TRC death. In addition, blocking the IDO-Kyn-AhR pathway also led to the death of dormant TRCs induced by IFN-γ.Citation9 However, the induction of this type of death is distinct from the above p53 pathway. IFN-γ can activate caspases 3 and 7 via STAT1 signaling; however, in TRCs, most IFN-γ signaling is biased to IDO-Kyn-AhR and weak signaling went to STAT1. Under the condition of IDO-Kyn-AhR blockade, IFN-γ signaling re-switched to STAT1, leading to the activation of caspases 3 and 7 as well as dormant TRC death. Based on our recent findings, we propose a model depicting how immune factors from both adaptive and innate immune cells can act as dormancy inducers that force stem cell-like TRCs into dormancy and how to abrogate such dormant tumor cells through different pathways ()
Figure 1 . Regulation of TRC dormancy by members of the interferon family. A, IFN-β induced tumor-repopulating cells (TRC) into dormancy by activating both indoleamine-pyrrole 2,3-dioxygenase (IDO)- Kynurenine (Kyn)- aryl hydrocarbon receptor (AhR)-p27 and STAT3-p27 pathways, while blocking IDO-AhR pathway resulted in TRC apoptosis by switching to STAT3-p53 signaling pathway. B, IFN-γ mediated TRC dormancy by regulating IDO-Kyn-AhR-p27 pathway. Blockade of IDO-AhR promoted dormant TRC into apoptosis by activating STAT1-caspase pathway. Tyk2, tyrosine kinase 2; Trp, tryptophan.
In summary, despite the goal of attacking dormant tumor cells to eradicate cancer, unfortunately, no treatment targeting tumor cell dormancy is available so far. The revelation of the importance of the IDO/Kyn/AhR cascade in IFN-γ/IFN-β induced TRC dormancy may open a new venue for dormancy-targeting cancer immunotherapy.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Additional information
Funding
References
- Holmgren L, O'Reilly MS, Folkman J. Dormancy of micrometastases: balanced proliferation and apoptosis in the presence of angiogenesis suppression. Nat Med. 1995;1(2):149–53.
- Sosa MS, Bragado P, and Aguirre-Ghiso JA. Mechanisms of disseminated cancer cell dormancy: an awakening field. Nat Rev Cancer. 2014;14(9):611–22
- Liu Y, Lv J, Liu J, Liang X, Jin X, Xie J, Zhang L, Chen D, Fskesund R, Tang K, et al. STAT3/p53 pathway activation disrupts IFN-b-induced dormancy 160 in tumor-repopulating cells. J Clin Invest. 2018;128(3):1057-1073. doi: 10.1172/JCI96329. PMID: 29431732.
- De Paoli P, and Carbone A. Carcinogenic viruses and solid cancers without sufficient evidence of causal association. Int J cancer. 2013;133(7):1517–29.
- Sun L, Wu J, Du F, Chen X, and Chen ZJ. Cyclic GMP-AMP synthase is a cytosolic DNA sensor that activates the type I interferon pathway. Science. 2013;339(6121):786–91
- Zhang H, Tang K, Zhang Y, Ma R, Ma J, Li Y, Luo S, Liang X, Ji T, Gu Z, et al. Cell-free tumor microparticle vaccines stimulate dendritic cells via cGAS/STING signaling. Cancer Immunol Res. 2015;3(2):196–205.
- Malathi K, Dong B, Gale M, Jr., and Silverman RH. Small self-RNA generated by RNase L amplifies antiviral innate immunity. Nature. 2007;448(7155):816–9.
- Liu Y LX, Dong W, Lv J, Fang Y, Fiskesund R, Xie J, Liu J, Yin X, Jin X, et al. Tumor repopulating cells induce PD-1 expression in CD8+ T cells by transferring kynurenine and AhR activation. Cancer Cell. 2018;33:480–94
- Liu Y, Liang X, Yin X, Lv J, Tang K, Ma J, Ji T, Zhang H, Dong W, Jin X, et al. Blockade of IDO-kynurenine-AhR metabolic circuitry abrogates IFN-γ-induced immunologic dormancy of tumor-repopulating cells. Nat Commun. 2017;8:15207.
