ABSTRACT
We have recently demonstrated that macrophage-specific loss of Protein tyrosine phosphatase non-receptor type 2 (PTPN2) promotes inflammasome activation, resulting in protection from colorectal cancer. Here we place these findings in context with the role of inflammasomes in colorectal carcinoma, and with a recent study indicating that PTPN2-silencing promotes anti-cancer immunotherapy.
Protein tyrosine phosphatase non-receptor type 2 (PTPN2) is a ubiquitously expressed tyrosine phosphatase that regulates several pro-inflammatory pathways, including Interferon (IFN)-γ- induced Janus kinase (JAK)-signal-transducer and activator of transcription (STAT) signaling and mitogen activated protein kinase (MAPK) pathways, as well as growth factor signaling, such as signaling cascades downstream of epidermal growth factor receptor (EGFR) and vascular endothelial growth factor (VEGFR) (reviewed inCitation1). The importance of PTPN2 in regulating inflammatory pathways is highlighted by the fact that PTPN2 dysfunction, as observed in individuals carrying genetic PTPN2 variants, promotes the risk to develop inflammatory disorders including Rheumatoid arthritis, type-I-diabetes, and inflammatory bowel disease (IBD).Citation1 Further, full-body deletion of Ptpn2 in mice results in severe systemic inflammation with pronounced colitis, leading to death few weeks after birth.Citation2 Over the past years, our group investigated the tissue-specific contribution of PTPN2 to this severe phenotype. In a recent study using mice with a myeloid cell-specific deletion of Ptpn2 (Ptpn2-LysMCre mice), we found that Ptpn2 expression in macrophages/monocytes is important to control intestinal inflammation, but at the same time promotes the development of colitis-associated tumors.Citation3
One major pathway affected upon deletion of Ptpn2 in macrophages is the activation of inflammasomes (). Inflammasomes are multi-protein complexes that form upon presence of danger-associated molecular patterns (DAMPS) in the cytosol and mediate activation of the protease caspase-1, which, in turn, mediates the cleavage of pro-interleukin (IL)-1β and pro-IL18 to their active forms. Once activated, IL-1β has potent pro-inflammatory properties, including recruitment of pro-inflammatory phagocytes and promotion of T helper (Th)17 cell differentiation.Citation4 IL-18 on the other hand promotes the induction of IFN-γ producing cells, including Th1 cells, CD8+ cytotoxic T cells, and natural killer (NK) cells.Citation5 Notably, these cells are importantly involved in promoting anti-cancer immunity.
Figure 1. Loss of PTPN2 promotes anti-cancer immunity. PTPN2-depletion in different cell types involved in CRC development results in changes in anti-cancer immunity. PTPN2: Protein tyrosine phosphatase non-receptor type 2, IFN: Interferon.
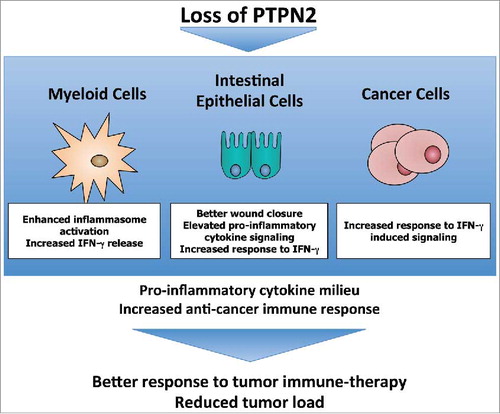
The role of inflammasomes in the development of intestinal inflammation and colorectal cancer is still controversial and several reports with contradictory results have been published. In colitis-associated cancer, inflammation is an important driver of tumorigenesis, hence inflammation-promoting factors, such as deregulated, enhanced inflammasome activation can promote tumor development. However, as pointed out above, inflammasome activation is also important for the recruitment of immune cells and can thereby promote anti-cancer immune responses.
Inflammasome activation is a highly regulated process, and several regulatory steps prevent inadequate inflammasome assembly/activation. Upon activation, inflammasome receptors recruit the adaptor molecule apoptosis-associated speck like protein containing a caspase recruitment domain (ASC), which in turn stabilizes the inflammasome complex, and forms large cytosolic, multimeric complexes, which mediate caspase-1 cleavage. In order to stabilize these complexes, ASC needs to be phosphorylated, a mechanism that is driven by c-Jun N-terminal kinase (JNK) and spleen tyrosine kinase (Syk).Citation6 In our study, we demonstrated that JNK activity is elevated in Ptpn2-deficient macrophages, resulting in elevated ASC phosphorylation, subsequently promoting exacerbated inflammasome activity and IL-1β/IL-18 secretion. Inhibition of IL-1β demonstrated that exacerbated IL-1β production drives elevated intestinal inflammation in mice lacking Ptpn2 in the myeloid compartment. Of interest, however, increased IL-1β levels at the same time conferred protection from tumor development.
This is of great interest, since in colitis-associated tumors, it is generally accepted that inflammation is a main driver of tumor development. Nevertheless, inflammation also results in the recruitment/expansion of anti-tumor immune cells. IL-1β is importantly involved in recruiting immune cells into tissues, and promotes the differentiation of pro-inflammatory (M1) macrophages, as opposed to anti-inflammatory (M2) macrophages that have been associated with suppression of anti-tumor immune responses.Citation7
It is noteworthy that in our study, besides highly elevated levels of the inflammasome product IL-1β, we also observed increased levels of IFN-γ in the inflamed intestine and in tumor tissue of Ptpn2-LysMCre mice, potentially induced via elevated production of IL-18 from PTPN2-deficient macrophages. IL-18 induces IFN-γ production from NK and T cells, and it has been shown that IFN-γ-signaling is important to confer susceptibility to immune cell mediated cancer cell depletion.Citation8 Hence, elevated IFN-γ production might confer an additional mechanism how loss of Ptpn2 results in less tumor development (). In a recent study to identify targets that might enhance immune checkpoint inhibitor treatment, Manguso et al showed that deletion of PTPN2 in cancer cells promotes the response to checkpoint inhibitors.Citation8 Of note, this effect was dependent on intact IFN-γ signaling within the cancer cells. PTPN2 is an important intracellular regulator of IFN-γ-induced signaling, and its depletion therefore increases the response of cancer cells to IFN-γ. On the other hand, our work demonstrates that loss of PTPN2 in non-cancer cells promoted IFN-γ production, hence PTPN2 might further act as a tumor suppressor in an indirect manner ().
Summarized, our work, together with other recent publications, demonstrates the important immune-regulatory role of PTPN2 on one hand, but also its important role in suppressing anti-cancer immune responses.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- Spalinger MR, McCole DF, Rogler G, Scharl M. Role of protein tyrosine phosphatases in regulating the immune system: implications for chronic intestinal inflammation. Inflamm Bowel Dis. 2015;21:645–55. doi:10.1097/MIB.0000000000000297. PMID:25581833.
- You-Ten KE, Muise ES, Itie A, Michaliszyn E, Wagner J, Jothy S, Lapp WS, Tremblay ML. Impaired bone marrow microenvironment and immune function in T cell protein tyrosine phosphatase-deficient mice. J Exp Med. 1997;186:683–93. doi:10.1084/jem.186.5.683. PMID:9271584.
- Spalinger MR, Manzini R, Hering L, Riggs JB, Gottier C, Lang S, Atrott K, Fettelschoss A, Olomski F, Kündig TM, et al. PTPN2 Regulates Inflammasome Activation and Controls Onset of Intestinal Inflammation and Colon Cancer. Cell Rep. 2018;22:1835–48. doi:10.1016/j.celrep.2018.01.052. PMID:29444435.
- Martinon F, Mayor A, Tschopp J. The inflammasomes: guardians of the body. Annu Rev Immunol. 2009;27:229–65. doi:10.1146/annurev.immunol.021908.132715. PMID:19302040.
- Takeda K, Tsutsui H, Yoshimoto T, Adachi O, Yoshida N, Kishimoto T, Okamura H, Nakanishi K, Akira S. Defective NK cell activity and Th1 response in IL-18-deficient mice. Immunity. 1998;8:383–90. doi:10.1016/S1074-7613(00)80543-9. PMID:9529155.
- Hara H, Tsuchiya K, Kawamura I, Fang R, Hernandez-Cuellar E, Shen Y, Mizuguchi J, Schweighoffer E, Tybulewicz V, Mitsuyama M. Phosphorylation of the adaptor ASC acts as a molecular switch that controls the formation of speck-like aggregates and inflammasome activity. Nat Immunol. 2013;14:1247–55. doi:10.1038/ni.2749. PMID:24185614.
- Genard G, Lucas S, Michiels C. Reprogramming of Tumor-Associated Macrophages with Anticancer Therapies: Radiotherapy versus Chemo- and Immunotherapies. Front Immunol. 2017;8:828. doi:10.3389/fimmu.2017.00828. PMID:28769933.
- Manguso RT, Pope HW, Zimmer MD, Brown FD, Yates KB, Miller BC, Collins NB, Bi K, LaFleur MW, Juneja VR, et al. In vivo CRISPR screening identifies Ptpn2 as a cancer immunotherapy target. Nature. 2017;547:413–8. doi:10.1038/nature23270. PMID:28723893.
