ABSTRACT
Lactate-based metabolic symbiosis between glycolytic and oxidative cancer cells is known to facilitate tumor growth. We have recently demonstrated that 7ACC2 blocks extracellular lactate uptake via the inhibition of mitochondrial pyruvate carrier. 7ACC2 also prevents compensatory glucose oxidation, induces tumor reoxygenation and potentiates radiotherapy, making it a promising anticancer drug.
In the field of cancer biology, lactate is not perceived any more as a simple waste product of the glycolytic pathway. In the last decade, we and others have shown that lactate constitutes an alternative metabolic fuel for cancer cells. Indeed, oxidative tumor cells can use lactate instead of (or in addition to) glucose to generate pyruvate and fuel the tricarboxylic acid (TCA) cycle, thereby increasing the glucose availability for hypoxic tumor cells, located at far distance from tumor blood vessels. This tumor lactate-based metabolic symbiosis has been reported in various cancer types and shown to also involve cancer-associated fibroblasts and angiogenic endothelial cells.Citation1 More recently, in vivo 13C-lactate tracing experiments revealed extensive labeling of TCA cycle intermediates in human patients and genetically engineered mouse models with non-small-cell lung cancers.Citation2,Citation3 These studies have documented that circulating (and not only tumor-derived) lactate may be captured and oxidized in cancer cells and importantly that the contribution of lactate to TCA cycle intermediates could be greater than that of glucose.Citation2,Citation3 Other investigators also reported that lactate-based metabolic symbiosis directly participated to an adaptive resistance mechanism to anti-angiogenic treatments.Citation4,Citation5 Altogether, these studies point towards the regulation of lactate flux as a particularly druggable process to impact on tumor progression.
Monocarboxylate transporter 1 (MCT1) is an obvious target for such pharmacological intervention. Indeed, MCT1 shows a greater affinity for lactate (Km≈3–6 mmol/L) than MCT4 (Km≈25–30 mmol/L), and represents the main entry path for lactate in oxidative cancer cells and tumor-associated endothelial cells.Citation1 Moreover, the preferential expression of MCT1 at the vicinity of blood vessels (vs MCT4 in distant hypoxic regions) makes this transporter an easily reachable pharmacological target. For several decades, the only MCT inhibitors described like α-cyano-4-hydroxycinnamate (CHC), organomercurials, and stilbene disulfonates exhibited a poor selectivity. More recently, a new class of high-affinity MCT1/MCT2 inhibitors such as AR-C155858 was developed by Astra-Zeneca. From our own drug discovery program, we also identified the 7-aminocarboxycoumarin 2 (7ACC2) as a potent inhibitor of lactate influx, but not efflux.Citation6 The above compounds have thus the capacity to interfere with the lactate-based metabolic symbiosis in tumors. Nevertheless, whether and (if so) how glucose may affect their action was unclear.
In a recent article published in Nature Communications,Citation7 we used several pre-clinical models including Xenopus oocytes, 3D tumor spheroids and human tumor xenografts in nude mice, combined with state-of-the-art metabolomics strategies (Seahorse respirometer analysis, in vitro 13C tracing experiments, and in vivo hyperpolarized 13C-pyruvate monitoring) to evaluate and compare the anti-tumor effects of two compounds previously reported to interfere with lactate uptake, namely AR-C155858 and 7ACC2. Interestingly, while both compounds prevented the uptake and the use of the extracellular lactate by cancer cells, their respective mechanism of action was different. Indeed, 7ACC2 was identified as a blocker of mitochondrial pyruvate transport through the inhibition of the mitochondrial pyruvate carrier (MPC) in a MCT1-independent manner (). Blockade of pyruvate import into mitochondria actually prevented lactate uptake as efficiently as the MCT1 inhibitor AR-C155858.Citation7 However, 7ACC2 but not AR-C155858 induced cytosolic accumulation of pyruvate, which in turn rapidly prevented the intracellular conversion of lactate into pyruvate. Furthermore, although both MCT1 and MPC blockers inhibited cancer cell growth and mitochondrial respiration when lactate was the only nutrient available, the presence of glucose overrode the MCT1 inhibition but not the 7ACC2-mediated MPC blockade.Citation7 These results indicate that pharmacological MPC targeting may offer further advantages over MCT1 inhibition and thus represents a more attractive strategy to block tumor cell oxidative metabolism whatever the energetic fuel that is used (i.e. glucose, lactate or pyruvate).
Figure 1. 7ACC2-mediated mitochondrial pyruvate carrier inhibition prevents lactate uptake by cancer cells and radiosensitize tumors. Inhibition of the mitochondrial pyruvate carrier (MPC) activity by 7ACC2 induces cytosolic accumulation of pyruvate, which in turn prevents the uptake and the use of extracellular lactate. 7ACC2 blocks both lactate- and glucose-fueled mitochondrial respiration, leading to a local tumor reoxygenation that considerably improves the anticancer efficacy of radiation therapy. αKG: α-ketoglutarate; GLUT: glucose transporter; MCT: monocarboxylate transporter; OAA: oxaloacetate; OXPHOS: oxidative phosphorylation; TCA: tricarboxylic acid.
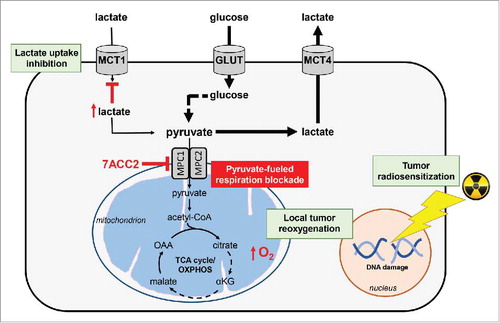
Accordingly, while both inhibitors blocked the growth of 3D tumor spheroids, cytostatic effects were observed with the MCT1 inhibitor AR-C155858 while 7ACC2 showed significant cytotoxic effects. This potent growth inhibitory action was associated with an exacerbated glycolysis as revealed in vitro by increased glucose uptake and lactate efflux and a subsequent lactate accumulation in the extracellular compartment (). In vivo nuclear magnetic resonance (NMR) data with hyperpolarized 13C-pyruvate also documented a 7ACC2-driven increase in pyruvate to lactate conversion.Citation7 Importantly, we also observed that these metabolic alterations (i.e. blockade of lactate- and glucose-fueled TCA cycle) led to a reduction in hypoxia as proven in spheroids via pimonidazole and carbonic anhydrase IX (CAIX) staining and in vivo through electron paramagnetic resonance (EPR) measurements. Interestingly, we showed that this induced tumor reoxygenation could benefit radiotherapy (). Indeed, pre-challenge of tumor-bearing mice with 7ACC2 (2 hours pre-treatment) considerably improved the anticancer efficacy of either single high dose or fractionated low dose radiation therapy.Citation7
Like for any potential treatments targeting tumor metabolism, possible limitations to the use of MPC inhibitors could exist. First, as MPC is also expressed in healthy tissues, the potential toxicity of such inhibitors could represent a concern for cancer patients. However, while we observed exercise intolerance in 7ACC2-treated mice (vs. sham-treated mice), this was documented by measuring elapse time until exhaustion, an extreme situation not encountered in cancer patients maintained at rest during their treatment. Second, some cancers such as colorectal cancers do not express MPCCitation8 and should thus not respond to a MPC blocker. In other tumors like prostate and ovarian cancers, the effects of MPC inhibition on the fate of cancer stem cells is also difficult to anticipate.Citation8 More work is thus warranted to determine which cancer patients will benefit the most from the inhibition of MPC activity but the recently identified link between mitochondrial activity and tumor malignancyCitation9,Citation10 certainly positions 7ACC2 as a very promising anticancer drug.
In conclusion, our study highlights the inhibition of mitochondrial pyruvate transport as a much more attractive anticancer strategy than MCT1 blockade to inhibit lactate-based metabolic symbiosis since it also prevents the (compensatory) use of glucose. Importantly, inhibition of both lactate- and glucose-fueled mitochondrial respiration by 7ACC2 also supports a potent reoxygenation phenomenon that may be exploited to sensitize tumors to radiation therapy.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
This work was supported by grants from the Fonds National de la Recherche Scientifique (FRS-FNRS). C.V.L. is the recipient of a PhD fellowship from FNRS-Télévie and C.C. is a senior FRS-FNRS postdoctoral fellow.
References
- Draoui N, Feron, O. Lactate shuttles at a glance: from physiological paradigms to anti-cancer treatments. Dis Model Mech. 2011;4:727–32. doi:10.1242/dmm.007724. PMID:22065843.
- Faubert B, et al. Lactate metabolism in human lung tumors. Cell. 2017;171:358–371 e359. doi:10.1016/j.cell.2017.09.019.
- Hui S, et al. Glucose feeds the TCA cycle via circulating lactate. Nature. 2017;551:115–8. doi:10.1038/nature24057. PMID:29045397.
- Allen E. et al. Metabolic symbiosis enables adaptive resistance to anti-angiogenic therapy that is dependent on mTOR signaling. Cell Rep. 2016;15:1144–60. doi:10.1016/j.celrep.2016.04.029. PMID:27134166.
- Pisarsky L, et al. Targeting metabolic symbiosis to overcome resistance to anti-angiogenic therapy. Cell Rep. 2016;15:1161–74. doi:10.1016/j.celrep.2016.04.028. PMID:27134168.
- Draoui N, et al. Antitumor activity of 7-aminocarboxycoumarin derivatives, a new class of potent inhibitors of lactate influx but not efflux. Mol Cancer Ther. 2014;13:1410–8. doi:10.1158/1535-7163.MCT-13-0653. PMID:24672058.
- Corbet C, et al. Interruption of lactate uptake by inhibiting mitochondrial pyruvate transport unravels direct antitumor and radiosensitizing effects. Nat Commun. 2018;9:1208. doi:10.1038/s41467-018-03525-0. PMID:29572438.
- Corbet C. Stem cell metabolism in cancer and healthy tissues: pyruvate in the limelight. Front Pharmacol. 2017;8:958. doi:10.3389/fphar.2017.00958. PMID:29403375.
- Corbet C, Feron O. Cancer cell metabolism and mitochondria: Nutrient plasticity for TCA cycle fueling. Biochim Biophys Acta. 2017;1868:7–15. PMID:28110019.
- Corbet, C, Feron O. Emerging roles of lipid metabolism in cancer progression. Curr Opin Clin Nutr Metab Care. 2017;20:254–60. doi:10.1097/MCO.0000000000000381. PMID:28403011.
