ABSTRACT
The canine transmissible venereal tumor (CTVT) is one of the few clonally transmissible cancers in nature and the only one that fully regresses following treatment with vincristine. The molecular signature of CTVT regression has been described in a recent paper published in Cancer Cell, revealing some fundamental insights into cancer regression.
The canine transmissible venereal tumor (CTVT) is a contagious cancer already described in the XIX century as a fungous excrescence with ulcerations growing from the genital mucosae or skin. CTVT is naturally transmitted between dogs by coitus, biting or licking affected areas and was the first tumor to be experimentally transplanted, well before the introduction of inbred mice.Citation1 Although the etiology of this cancer was proposed to be a viral or parasitic infection, CTVTs collected from different dogs in different continents share a similar karyotype and a Long Interspersed Nuclear Element (LINE) insertion into the MYC gene, which raised the possibility that CTVT might have originated from a common ancestor.Citation1 In 2006 we proved the clonal origin of CTVT, demonstrating that a mammalian cancer cell can transmit as a “parasite”.Citation2 Our results received further support from the analysis of CTVT mitochondrial DNA and the sequencing of its genome, which indicated that this cancer originated around 11,000 years ago.Citation3 Remarkably, CTVT is not unique. Two transmissible cancers have been found in Tasmanian devils, collectively known as Tasmanian devil facial tumor disease (DFTD), and two in clams.Citation4 Hence transmissible cancers may emerge in the right ecological conditions.
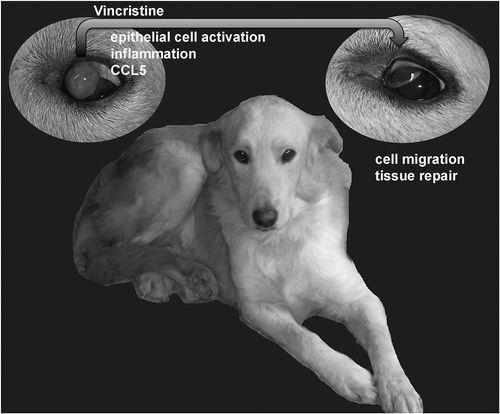
Figure 1. Mechanisms underlying the regression of ocular canine transmissible venereal tumor (CTVT) upon chemotherapy in mixed breed dog.
Upper left, ocular CTVT treated with vincristine. Treatment induces activation and differentiation of host epithelial cells surrounding (or within) the tumour, an acute inflammatory response and a strong upregulation of Chemokine (C-C motif) ligand 5 (CCL5), which recruits and retains immune cells into the tumor. This early response is followed by infiltration of CD8 and CD4 T lymphocytes, natural killer (NK) cells, B lymphocytes and upregulation of C-C chemokine receptor type 5 (CCR5). Lastly, at regression (upper right), there is upregulation of genes for cell migration and tissue repair.
CTVT is a very interesting model for cancer immunology.Citation5 Firstly, this tumor has evolved to escape immune detection, despite extensive dog leukocyte antigen (DLA) polymorphisms. Secondly, naturally transmissible CTVT often fully regresses in dogs treated with vincristine, sometimes even a single dose being sufficient to elicit a full response. Histological data on CTVTs pointed to an immune-mediated mechanism of regression.Citation1 Thirdly, many dog cancers share close similarities with human cancers, providing important parallels to study their etiology and behavior.
We therefore investigated the mechanisms leading to the regression of naturally transmitted CTVTs to address some fundamental questions: how is tolerance to this cancer broken? What is the role of chemotherapy? How is the cancer rejected? And what is its cellular origin?
To address these questions, we obtained sequential biopsies before and after vincristine administration from four CTVTs that regressed and four CTVTs that did not regress following treatment. We analyzed by RNAseq gene expression changes in these biopsies; we contrasted regressing and non-regressing CTVTs and, using a stringent statistical approach, we identified a core of 127 genes strictly associated with regression. This analysis revealed that regression proceeds in steps: first, we detected a strong induction of the innate immune response characterized by acute expression of interferon-stimulated genes and Chemokine (C-C motif) ligand 5 (CCL5) and Chemokine (C-C motif) ligand 28 (CCL28). Simultaneously, we found a clear signature of epithelial cell activation and differentiation. Second, we found a substantial loss of expression of genes related to the cell-cycle and a striking increase in abundance of gene markers for CD8 and CD4 T lymphocytes, natural killer (NK) cells and B lymphocytes, and inflammatory mediators such as Interleukin-17 and C-C chemokine receptor type 5 (CCR5). Lastly, we detected upregulation of genes related to cell movement and differentiation, similar to wound healing.Citation6
The picture that emerged from these results () indicates that vincristine initially induces a strong inflammatory response, even before it arrests the cell cycle by affecting microtubule depolarization. A key component of this response is CCL5, which attracts to, and retains into the tumor site T-lymphocytes, NK cells and myeloid cells that are crucial to reject the cancer.Citation6 This agrees with previous observations that higher CCL5 levels correlate with slower disease progression and better response to therapy in lung adenocarcinoma.Citation7 Furthermore, it supports recent work showing that certain chemotherapeutic agents generate an inflammatory response that sensitizes tumors to immune check-point therapy.Citation8 However, whilst it is generally thought that the tumor cells contribute to the inflammation, we found that, in CTVT, this acute response is dictated mainly by the host cells surrounding or within the tumor itself. Indeed, because CTVT has a clonal origin and is different from the host, we were able to differentiate host from tumor genes in our expression analysis.Citation6 Most likely, this early inflammation to which epithelial cells seem to contribute, is caused by the release of damage associated molecular patterns (DAMPs) from dying cancer cells after vincristine administration.
We also analyzed changes in DNA methylation by methylation-dependent DNA immunoprecipitation (MeDIP) in the CTVT biopsies and found that up-regulated genes were de-methylated at the first and 3ʹ most exon (rather than at promoter CpG islands) in regressing CTVTs. Strikingly, however, we found that non-regressing CTVTs, whose DNA was also initially demethylated at the same sites, actually re-methylated their DNA later on. This is in agreement with the notion that de-methylating agents may trigger re-expression of genes for inflammation and reverse tumor immune evasion,Citation8 but also suggests that de-methylation may not be stable.
To understand the cell origin of CTVT, we examined genes profoundly down regulated in the last biopsy of the regressing tumors, when the tumor mass disappeared. Gene pathway analysis indicated that CTVT is similar to melanoma at the transcriptional level. This is plausible because melanoma can form on genital mucosae or skin, which is suitably positioned for venereal transmission.Citation6
Our study has provided fundamental insights into the pivotal role of innate immunity in breaking tolerance to this cancer and its link with epigenetic modifications. It also lends support to the exciting concept that chemotherapy may act in novel ways, beyond its classical cytostatic effect, that can be harnessed to break tumor tolerance.Citation9 The dramatic improvement in survival of non-small cell lung cancer patients obtained by combining chemotherapy with anti-programmed cell death protein-1 (PD1) antibodies lends strong support to this idea.Citation10
Additional information
Funding
References
- Cohen D. The canine transmissible venereal tumor: a unique result of tumor progression. Adv Cancer Res. 1985;43:75–112.
- Murgia C, Pritchard JK, Kim SY, Fassati A, Weiss RA. Clonal origin and evolution of a transmissible cancer. Cell. 2006;126(3):477–487.
- Murchison EP, Wedge DC, Alexandrov LB, Fu B, Martincorena I, Ning Z, Tubio JM, Werner EI, Allen J, De Nardi AB, et al. Transmissible [corrected] dog cancer genome reveals the origin and history of an ancient cell lineage. Science. 2014;343(6169):437–440. doi:10.1126/science.1247167.
- Metzger MJ, Goff SP. A sixth modality of infectious disease: contagious cancer from devils to clams and beyond. PLoS Pathog. 2016;12(10):e1005904. doi:10.1371/journal.ppat.1005904.
- Fassati A, Mitchison NA. Testing the theory of immune selection in cancers that break the rules of transplantation. Cancer Immunol Immunother. 2010;59(5):643–651. doi:10.1007/s00262-009-0809-1.
- Frampton D, Schwenzer H, Marino G, Butcher LM, Pollara G, Kriston-Vizi J, Venturini C, Austin R, De Castro KF, Ketteler R, et al. Molecular signatures of regression of the canine transmissible venereal tumor. Cancer Cell. 2018;33(4):620–633 e626. doi:10.1016/j.ccell.2018.03.003.
- Moran CJ, Arenberg DA, Huang CC, Giordano TJ, Thomas DG, Misek DE, Chen G, Iannettoni MD, Orringer MB, Hanash S, et al. RANTES expression is a predictor of survival in stage I lung adenocarcinoma. Clin Cancer Res. 2002;8(12):3803–3812.
- Zappasodi R, Merghoub T, Wolchok JD. Emerging concepts for immune checkpoint blockade-based combination therapies. Cancer Cell. 2018;33(4):581–598. doi:10.1016/j.ccell.2018.03.005.
- Galluzzi L, Buque A, Kepp O, Zitvogel L, Kroemer G. Immunological effects of conventional chemotherapy and targeted anticancer agents. Cancer Cell. 2015;28(6):690–714. doi:10.1016/j.ccell.2015.10.012.
- Gandhi L, Rodriguez-Abreu D, Gadgeel S, Esteban E, Felip E, De Angelis F, Domine M, Clingan P, Hochmair MJ, Powell SF, et al. Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med. 2018;378:2078–2092. doi:10.1056/NEJMoa1801005.
