ABSTRACT
Our recent work demonstrates that inactivating mutations in phosphatase and tensin homolog (PTEN) are sufficient to drive macropinocytosis in the context of AMP-activated protein kinase (AMPK) activation. Given that blocking macropinocytosis limits PTEN-deficient prostate tumor growth, AMPK or phosphatidylinositol-4,5-bisphosphate 3-kinase (PI3K) inhibitors could have therapeutic value in castration-resistant prostate cancer patients, particularly when used in combination with standard of care therapies.
Abbreviations: ATG5: autophagy related 5; NHE: Na(+)/H(+) exchanger; PAK1: p21-activated kinase 1; PI3K: phosphatidylinositol-4,5-bisphosphate 3-kinase; PIP3: phosphatidylinositol (3,4,5)-trisphosphate; PIP2: phosphatidylinositol (4,5)-bisphosphate; RAC1: Rac family small GTPase 1
Inadequate perfusion makes it difficult for tumor cells to acquire sufficient nutrients to sustain their non-homeostatic, oncogene-driven proliferation.Citation1 Macropinocytosis, an actin-dependent form of bulk endocytosis, allows Kirsten rat sarcoma viral oncogene homolog (KRAS) driven pancreatic ductal adenocarcinomas (PDAC) to proliferate despite amino acid depletion.Citation2,Citation3 PhosphatidylinositolCitation3-Citation5-trisphosphate (PCitation3-Citation5P3; best known as PIP3) generated by class 1 phosphatidylinositol-4,5-bisphosphate 3-kinase (PI3K) is necessary for macropinosome formation.Citation4 Tumor suppressor phosphatase and tensin homolog (PTEN), which is deleted or inactivated in many cancer classes, opposes PI3K by converting PIP3 to phosphatidylinositol 4,5-bisphosphate (PCitation4,Citation5P2; best known as PIP2). Our finding that macropinocytosis also supports the growth of PTEN-deficient prostate cancer cells in vitro and in vivo demonstrates that the potential impact of therapeutic macropinocytosis inhibitors could be much greater than previously appreciated.Citation5
PTEN loss and AMPK activation promote macropinosome formation
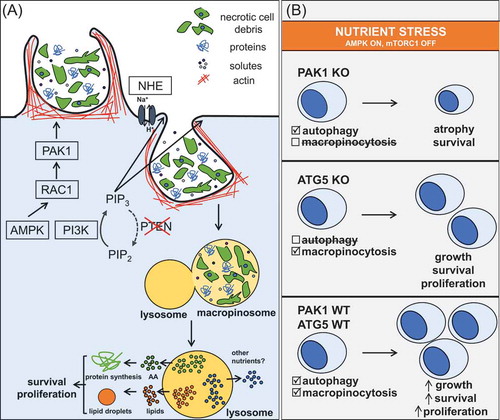
PTEN null or deficient prostate cancer cell lines, patient-derived organoids, and autochthonous, patient-derived xenograft, and isograft prostate tumors exhibit macropinocytosis that is blocked by class I PI3K inhibitors or PTEN reconstitution.Citation5 An unexpected role for AMP-activated protein kinase (AMPK) in promoting macropinocytosis was also uncovered. AMPK promotes macropinosome formation by activating Rac family small GTPase 1 (RAC1) that coordinates the actin remodeling necessary for membrane ruffling. Although AMPK activated RAC1 in PTEN-replete cells, macropinosomes only formed if PTEN was inactivated in keeping with previous studies showing that PIP3 is necessary for macropinosome closure (). These mechanistic studies add to a growing body of literature suggesting that AMPK inhibition makes more therapeutic sense than activation in established prostate tumors.Citation6 AMPK inhibitors would undermine not only macropinocytosis, but also autophagy. It will be important to conduct genetic experiments where AMPK is inhibited in established tumors to evaluate this hypothesis.
Figure 1. Macropinocytosis allows PTEN-deficient prostate cancer cells to proliferate in nutrient-limiting conditions. (A) Phosphatase and tensin homology (PTEN) loss and AMP-activated protein kinase (AMPK) activation is sufficient to induce macropinosome formation. Macropinocytosis allows non-selective uptake of necrotic cell debris, proteins, and extracellular fluid. Lysosomal catabolism of the macromolecules present in macropinosomes provides anabolic substrates. Boxed proteins are potential targets for pleiotropic macropinocytosis inhibitors. (B) Recycling intracellular components via cell autonomous autophagy fuels survival, but not growth, in nutrient-limiting conditions. Because macropinocytosis provides access to extracellular macromolecules, it can support both survival and proliferation.
Targeting macropinocytosis for cancer therapy
To date, no specific macropinocytosis inhibitors have been identified. The most commonly used macropinocytosis inhibitor, EIPA, targets Na+/H+ exchangers (NHE) resulting in pleiotropic antitumor effects that could make EIPA an excellent therapeutic.Citation7 As systemic administration of EIPA blocks macropinocytosis and significantly reduces prostate tumor growth, even producing some regressions,Citation5 the conventional wisdom that EIPA’s pharmacologic properties preclude its use in the clinic merits reevaluation. More potent and selective NHE inhibitors could also be effective. While RAC1 and p21-activated kinase 1 (PAK1) inhibitors block macropinocytosis and are valuable as tool compounds, viable clinical candidates are lacking. In PTEN null MEFs, inhibition of both α and β isoforms of PI3K was necessary to block macropinocytosis. Combining α and β isoform-selective class 1 PI3K inhibitors that are in late-stage clinical trials may be a better approach than deploying pan-PI3K inhibitors which have been plagued by on-target toxicities. Given our finding that macropinocytosis, but not autophagy, drives growth in nutrient-limiting conditions, the beneficial effects of lysosomal inhibitors like chloroquine may be derived more from the inhibition of macropinocytosis than from blocking autophagy ().
Necrocytosis: a new tumor-microenvironment interaction
Scavenging extracellular matrix proteins and albumin can provide amino acids, but poor perfusion is also likely to compromise tumor cell access to lipids, sugars and nucleotides. Our demonstration that macropinocytic prostate cancer and PDAC cell lines can use necrotic cell corpses to fuel proliferation in amino acid- and glucose-deficient medium implies that this scavenging pathway has more to offer than just amino acids. Macropinocytosis of necrotic cell debris, but not albumin, increased lipid droplet content in starved prostate cancer cells suggesting that lipids are also harvested from cellular corpses. Macropinocytic consumption of necrotic corpses (necrocytosis) also affords unique opportunities for isotopic labeling studies, allowing us to demonstrate that even in nutrient-replete medium 15–25% of the amino acids in newly synthesized proteins were derived from macropinocytosis. Advanced, drug resistant solid tumors often contain necrotic regions,Citation8 and cytotoxic standard of care therapies may generate cellular corpses that paradoxically fuel the growth of the surviving cells. Thus, macropinocytosis inhibitors may be particularly effective as part of combination therapies. Combining α and β isoform-selective class 1 PI3K inhibitors with the androgen receptor inhibitor enzalutamide produced regressions in pre-clinical, PTEN-deficient prostate cancer models;Citation9 macropinocytosis inhibition could contribute to the enhanced activity of this combination. While the pan-PI3K inhibitor BKM120 has been tested in combination with enzalutamide in a clinical trial,Citation10 enzalutamide substantially increased the metabolism and clearance of BKM120, and it was not clear whether PI3K was inhibited in patients receiving the combination. Patients were also not selected for mutations that would drive macropinocytosis (e.g. PTEN inactivation or loss). Thus, whether inhibiting macropinocytosis will enhance androgen deprivation therapy or overcome resistance in castration-resistant prostate cancer patients remains an open question.
In conclusion, our study suggests that the contribution of macropinocytosis to tumor anabolism has been significantly underestimated. Several drugs in the clinical pipeline could be used to block macropinocytosis in patient tumors and will likely be most effective when used in combination with existing therapies. Because these agents all have pleiotropic actions, more selective approaches will be required to dissect the contribution macropinocytosis makes to tumor growth and metastasis.
Disclosure of Potential Conflicts of Interest
No potential conflict of interest was disclosed.
Additional information
Funding
References
- Forster, J., Harriss-Phillips, W., Douglass, M., Bezak, E. A review of the development of tumor vasculature and its effects on the tumor microenvironment. Hypoxia. 2017;5:21–32. PMID: 28443291. doi:10.2147/HP.S133231.
- Commisso C, Davidson SM, Soydaner-Azeloglu RG, Parker SJ, Kamphorst JJ, Hackett S, Grabocka E, Nofal M, Drebin JA, Thompson CB, et al. Macropinocytosis of protein is an amino acid supply route in Ras-transformed cells. Nature. 2013;497(7451):633–637. PMID: 23665962. doi:10.1038/nature12138.
- Davidson SM, Jonas O, Keibler MA, Hou HW, Luengo A, Mayers JR, Vander Heiden MG, Hagenkort A, Kutzner J, Page BDG, et al. Direct evidence for cancer-cell-autonomous extracellular protein catabolism in pancreatic tumors. Nat Med. 2017;23(2):235–241. PMID: 2802408. doi:10.1038/nm.4256.
- Egami, Y., Taguchi, T., Maekawa, M., Arai, H., & Araki, N, et al. Small GTPases and phosphoinositides in the regulatory mechanisms of macropinosome formation and maturation: gtpases and phosphoinositides in macropinocytosis. Front Physiol. 2014;11–31. PMID: 25324782. doi:10.3389/fphys.2014.00374.
- Kim SM, Nguyen TT, Ravi A, Kubiniok P, Finicle BT, Jayashankar V, Malacrida AL, Hou J, Robertson J, Gao D, et al. PTEN deficiency and AMPK activation promote nutrient scavenging and anabolism in prostate cancer cells. Cancer Discov. 2018;8:.866–883. doi:10.1158/2159-8290.CD-17-1215.
- Khan AS, Frigo DE. A spatiotemporal hypothesis for the regulation, role, and targeting of AMPK in prostate cancer. Nat Rev Urol. 2017;14(3):164–180. PMID: 28169991. doi:10.1038/nrurol.2016.272.
- Matthews H, Ranson M, Kelso MJ. Anti-tumour/metastasis effects of the potassium-sparing diuretic amiloride: an orally active anti-cancer drug waiting for its call-of-duty? Int J Cancer. 2011;129(9):2051–2061. PMID: 21544803. doi:10.1002/ijc.26156.
- Caruso RA, Branca G, Fedele F, Irato E, Finocchiaro G, Parisi A, Ieni A. Mechanisms of coagulative necrosis in malignant epithelial tumors. Oncol Lett. 2014;8(4):1397–1402. PMID: 25202341. doi:10.3892/ol.2014.2345.
- Schwartz S, Wongvipat J, Trigwell CB, Hancox U, Carver BS, Rodrik-Outmezguine V, Will M, Yellen P, de Stanchina E, Baselga J, et al. Feedback suppression of PI3Kα signaling in PTEN-mutated tumors is relieved by selective inhibition of PI3Kβ. Cancer Cell. 2015;27(1):109–122. PMID: 25544636. doi:10.1016/j.ccell.2014.11.008.
- Armstrong AJ, Halabi S, Healy P, Alumkal JJ, Winters C, Kephart J, … George DJ. Phase II trial of the PI3 kinase inhibitor buparlisib (BKM-120) with or without enzalutamide in men with metastatic castration resistant prostate cancer. Eur J Cancer. 2017;81:228–236. PMID: 28502694. doi:10.1016/j.ejca.2017.02.030.
