ABSTRACT
Caspase-3 is known to play a critical function in the process of apoptosis. Recently, we have discovered a non-apoptotic role of Caspase-3 as a key regulator of cell proliferation and organ size. Caspase-3 cleaves α-Catenin, which sequesters Yes-associated protein 1 (Yap1) in the cytoplasm, thus facilitating the activation and nuclear translocation of Yap1. These findings reveal that the apoptotic machinery can be refocused to regulate cell proliferation and organ size.
Keywords:
Apoptosis is a central mechanism responsible for the elimination of undesired and potentially dangerous cells. Apoptosis culminates in the activation of a family of cysteine proteases, known as caspases, which are expressed as inactive zymogens in virtually all cells.Citation1 In response to either intrinsic or extrinsic apoptotic stimuli, caspase zymogens are cleaved and converted to an active state. One of the most important members of the caspase family is Caspase-3, which plays an instrumental role in cleaving a variety of vital substrates to implement the cell death program.Citation2 For many years, activation of Caspase-3 has been synonymous with the implementation of apoptosis and cleaved Caspase-3 has been widely accepted as a marker of an apoptotic cell. However, emerging findings now challenge the simplistic view of Caspase-3 as an obligated executioner and reveal that Caspase-3 has critical non-lethal functions.Citation3
Recently, we uncovered an important non-apoptotic function of Caspase-3 in the mouse epidermis.Citation4 The epidermis is comprised of several compartments: the hair follicle (HF), sweat gland, interfollicular epidermis (IFE) and sebaceous gland (SG). The HF cycles between phases of anagen (growth), catagen (destruction) and telogen (rest), a process fueled by distinct subpopulations of HF stem cells (HFSCs).Citation3 During catagen, apoptosis eliminates the lower transient portion of the HF in a cohort manner, while the permanent upper part and SG remain intact.Citation2,Citation5 In contrast to the HF, the SG is under a constant state of renewal. Briefly, proliferating cells situated along the SG proliferative zone (SGPZ) differentiate into lipid-filled sebocytes in the inner SG compartment.Citation4 As these differentiated sebocytes mature they accumulate lipids, grow in size, erupt and release their sebum. Importantly, while the HF and IFE have been the focus of numerous investigations, extremely little is known regarding homeostasis of the SG and whether it is regulated by apoptotic machinery proteins.
Initially, we tested the function of key apoptotic proteins in the SG and performed staining with the cleaved Caspase-3 (CP3) antibody. Surprisingly, in contrast to CP3+ HFSCs and HF catagenic cells that underwent apoptosis, we found that a large portion of the SGPZ cells were CP3+ but did not exhibit the morphological characteristics of apoptosis. In fact, the vast majority of these CP3+ SGPZ cells were undergoing proliferation.Citation4
Since Caspase-3 did not instruct apoptosis in the SG, we utilized Caspase-3−/- mice to examine its function. Caspase-3−/- mice displayed a significant decrease in sebocyte number, as well as a dramatic reduction in SG size.Citation4 Since organ size can result from alterations in either cell size or number we examined both scenarios. While deletion of Caspase-3 did not have any effect on cell size, we observed a reduction in the number of cells in each individual SG. This decline could be attributed to a dramatic decrease in the number of proliferating SGPZ cells. Furthermore, when Caspase-3 inhibitors or activators were administered in vivo, they altered cell proliferation and SG size, verifying our genetic results.Citation4 These findings suggest that utilizing Caspase-3 inhibitors or activators for therapeutic purposes may yield significantly broader consequences than merely hindering or driving apoptosis and advocate that, under specific conditions, they could be utilized for manipulating cell proliferation.
An essential regulator of cell proliferation and organ size is the Yes-associated protein 1 (Yap1, best known as Yap) co-activator.Citation6 Given that manipulation of Caspase-3 affected both proliferation and organ size we examined whether it could be mediated via Yap. Results from various experimental approaches, including wholemount confocal analysis, nuclear/cytoplasmic fractionation, western blotting and real time PCR analysis indicated that Caspase-3 regulates the activation and nuclear translocation of Yap. Importantly, employing two in vivo models (grafting and wounding), which result in de novo formation of SGs and HFs, indicated that Caspase-3 regulates cell proliferation and SG size in a Yap-dependent manner.Citation4
Next, we sought to understand the specific mechanism enabling Caspase-3 to activate Yap. Given that Caspase-3 functions as a cysteine protease, we hypothesized that it might liberate Yap by cleaving an upstream target. It is key to note that although in many scenarios Yap is regulated by the Hippo pathway, in the epidermis Yap regulation is Hippo-independent.Citation7 In this setting, α-Catenin, a key component of adherent junctions, retains inactivated Yap via interaction with the 14–3-3 protein.Citation7 Interestingly, we found that α-Catenin encompasses two exposed Caspase-3 sites that are highly conserved across different species. By performing cleavage assays coupled with mass spectrometry, as well as binding assays, we found that Caspase-3-mediated cleavage facilitates the liberation of Yap from the α-Catenin complex.Citation4
Once Yap is liberated it translocates to the nucleus and drives expression of various target genes.Citation6 In Drosophila, Yorkie (Yap homolog) directly regulates the transcription of Death-associated inhibitor of apoptosis 1 (Diap1), which is the functional equivalent of X-linked inhibitor of apoptosis (Xiap), a potent mammalian caspase inhibitor.Citation2 Close examination indicated that the Caspase-3/Yap module regulates Xiap expression. Intriguingly, deletion of Xiap had no effect on apoptotic levels in the SG, but resulted in a significant increase in the number of CP3+ SGPZ cells, proliferating cells and SG size. These findings suggest that Xiap expression generates a negative feedback loop to prevent SG overgrowth.
Taken together, this study uncovers an important mechanism for the regulation of cell proliferation and organ size. In this model, the activation of Caspase-3 does not lead to cellular elimination. Rather, Caspase-3 governs the liberation of Yap by cleaving α-Catenin, thus allowing it to translocate to the nucleus and drive the expression of Xiap. In turn, Xiap functions as a feedback antagonist for the Caspase-3/Yap signaling circuit to determine proliferation and proper organ size,Citation4 ().
Figure 1. Non-apoptotic role of Caspase-3 in Yap signaling.
α-Catenin sequesters phosphorylated inactive Yes-associated protein 1 (pYap) in the cytoplasm via interaction with 14–3-3. Upon Caspase-3 activation, α-Catenin is cleaved leading to the dissociation of 14–3-3 and pYap from the complex. pYap is then dephosphorylated, which enables it to translocate to the nucleus where it regulates transcription of its target genes, including X-linked inhibitor of apoptosis (Xiap). In turn, Xiap generates a feedback loop by blocking Caspase-3 activity. This molecular signaling circuit dictates cell proliferation, organ size and potentially plays a role in tumorigenesis.
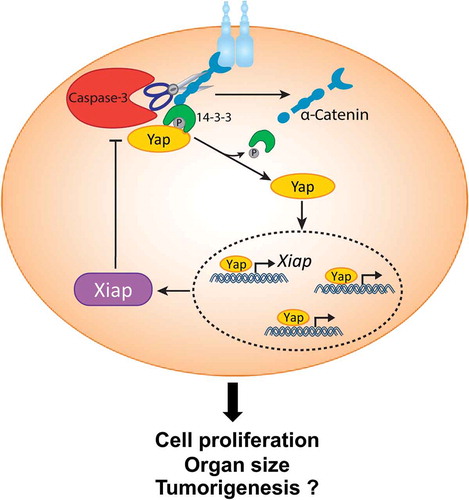
We speculate that this mechanism could also play an important role in tumor development. It has been shown that deletion of α-Catenin (Ctnna1) can result in hyperproliferation and lead to squamous cell carcinoma (SCC).Citation8 This suggests that α-Catenin levels must be tightly regulated to prevent Yap overstimulation. Notably, deletion of Yap attenuates the formation of papillomas and SCCs, while Yap gain-of-function phenotypes resemble those of α-Catenin ablation.Citation8 Intriguingly, Caspase-3−/- mice develop fewer skin carcinomasCitation9 and in human tumors high levels of cleaved Caspase-3 are correlated with poor prognosis.Citation10 Thus, future work should address whether Caspase-3 regulates Yap activation in the tumor setting and whether targeting the Caspase-3/Yap module could be beneficial for tumor therapy.
Declaration of interests
The authors declare no financial competing interests.
Additional information
Funding
References
- Fuchs Y, Steller H. Programmed cell death in animal development and disease. Cell. 2011;147:742–758. doi:10.1016/j.cell.2011.10.033.
- Fuchs Y, Steller H. Live to die another way: modes of programmed cell death and the signals emanating from dying cells. Nat Rev Mol Cell Biol. 2015;16:329–344. doi:10.1038/nrm3999.
- Soteriou D, Fuchs Y. A matter of life and death: stem cell survival in tissue regeneration and tumour formation. Nat Rev Cancer. 2018;18:187–201. doi:10.1038/nrc.2017.122.
- Yosefzon Y, Soteriou D, Feldman A, Kostic L, Koren E, Brown S, Ankawa R, Sedov E, Glaser F, Fuchs Y. Caspase-3 regulates YAP-dependent cell proliferation and organ size. Mol Cell. 2018;70:573–587e4. doi:10.1016/j.molcel.2018.04.019.
- Perez-Garijo A, Fuchs Y, Steller H. Apoptotic cells can induce non-autonomous apoptosis through the TNF pathway. Elife. 2013;2:e01004. doi:10.7554/eLife.01004.
- Piccolo S, Dupont S, Cordenonsi M. The biology of YAP/TAZ: hippo signaling and beyond. Physiol Rev. 2014;94:1287–1312. doi:10.1152/physrev.00005.2014.
- Schlegelmilch K, Mohseni M, Kirak O, Pruszak J, Rodriguez JR, Zhou D, Kreger BT, Vasioukhin V, Avruch J, Brummelkamp TR, et al. Yap1 acts downstream of alpha-catenin to control epidermal proliferation. Cell. 2011;144:782–795. doi:10.1016/j.cell.2011.02.031.
- Silvis MR, Kreger BT, Lien W-H, Klezovitch O, Rudakova GM, Camargo FD, Lantz DM, Seykora JT, Vasioukhin V. alpha-catenin is a tumor suppressor that controls cell accumulation by regulating the localization and activity of the transcriptional coactivator Yap1. Sci Signal. 2011;4:ra33. doi:10.1126/scisignal.2001823.
- Liu X, He Y, Li F, Huang Q, Kato TA, Hall RP, Li C-Y. Caspase-3 promotes genetic instability and carcinogenesis. Mol Cell. 2015;58:284–296. doi:10.1016/j.molcel.2015.03.003.
- Huang Q, Li F, Liu X, Li W, Shi W, Liu -F-F, O’Sullivan B, He Z, Peng Y, Tan A-C, et al. Caspase 3-mediated stimulation of tumor cell repopulation during cancer radiotherapy. Nat Med. 2011;17:860–866. doi:10.1038/nm.2385.
