ABSTRACT
Under growth condition, the Golgi reassembly-stacking protein of 55 kDa (GRASP55)/GORASP2 serves as the “glue” to hold adjacent Golgi cisternae into stacks by forming trans-oligomers. Upon glucose starvation, GRASP55 is de-O-GlcNAcylated and functions as a membrane tether to facilitate autophagosome-lysosome fusion through the interactions with LC3-II and LAMP2.
The basic structure of the Golgi apparatus in mammalian cells is a stack of multilayer flattened cisternae; formation of the stacks is required for proper functioning of the Golgi in protein trafficking, glycosylation, and sorting.Citation1 Two Golgi stacking proteins, Golgi reassembly stacking protein 1 (GORASP1, best known as the Golgi reassembly-stacking protein of 65 kDa, GRASP65) and GORASP2/GRASP55, have been identified in Golgi stack formation. GRASP55/65 are peripheral membrane proteins that form homo-dimers, and dimers from adjacent cisternae oligomerize in trans to hold the cisternae together into stacks. GRASP55 and GRASP65 localize to the medial-trans and cis-Golgi, respectively, and play complementary roles in maintaining the Golgi structure.Citation2 Recent studies reported that GRASP55 is also required for unconventional protein secretion of cytosolic proteins, which is independent of the Golgi,Citation3 but the mechanism remains unknown. It is generally believed that unconventional secretion requires autophagy, but whether GRASP55 functions in autophagy has not been determined.
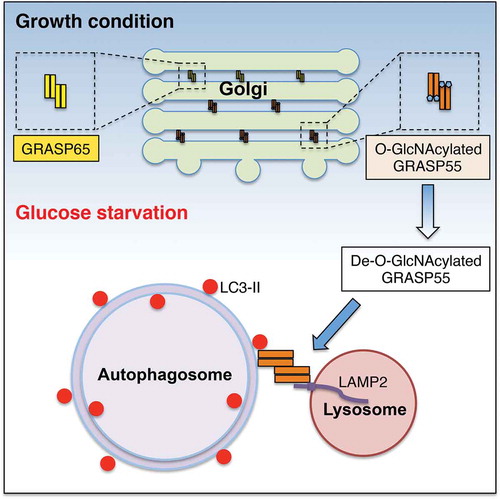
Autophagy, most often referred to as macroautophagy, is an evolutionarily conserved intracellular degradation process required for the clearance of protein aggregates and damaged organelles, in particular, under nutrient and energy deprivation.Citation4 Autophagy consists of several sequential steps, including the induction and initiation of the isolation membranes, expansion and closure of isolation membranes into autophagosomes, fusion of autophagosomes and lysosomes into autolysosomes, and degradation of the sequestrated materials.Citation4 Distinct protein complexes are responsible for different steps. In a recent study, we uncovered a novel role of GRASP55 in autophagosome maturation (). Upon glucose starvation, GRASP55 is targeted to the autophagosome-lysosome interface, where it functions as a membrane tether to facilitate autophagosome-lysosome fusion.Citation5
Figure 1. Dual function of GRASP55 in the organization of intracellular membranes. Under growth condition, GRASP55 is O-GlcNAcylated and localized in the Golgi, where it serves as the “glue” to hold adjacent Golgi cisternae into stacks by forming trans-oligomers. Upon glucose starvation, GRASP55 is de-O-GlcNAcylated and targeted to the autophagosome-lysosome interface, where it interacts with LC3-II and LAMP2 and functions as a membrane tether to facilitate autophagosome-lysosome fusion.
In an effort to study how the Golgi adjusts its structure and function in response to energy deprivation, we surveyed a number of Golgi structural proteins for O-GlcNAcylation, a cytosolic glycosylation that functions in energy sensing.Citation6 We found that only GRASP55 is O-GlcNAcylated under growth conditions by the O-GlcNAc transferase (OGT) and de-O-GlcNAcylated upon glucose deprivation. Given that glucose starvation induces autophagy, we determined the role of GRASP55 in autophagy. Indeed, depletion of GRASP55 in cells increased the number of autophagosomes but decreased the autophagy flux as indicated by the increased Sequestosome 1 (SQSTM1, best known as p62) protein level,Citation5 suggesting a defect in autophagy-mediated protein degradation.
The next question concerns how such a Golgi structural protein functions in autophagy. Subcellular localization by light and immuno-electron microscopy (Immuno-EM) demonstrated that GRASP55 is partially localized to autophagosomes in addition to the Golgi upon glucose starvation. Using wild type (WT) GRASP55 or an O-GlcNAcylation deficient mutant called “5A”, we showed that targeting of GRASP55 to autophagosomes requires de-O-GlcNAcylation of the protein. Expression of the 5A mutant of GRASP55 increased GRASP55 targeting to autophagosomes as well as autophagy flux. In addition to autophagosomes, GRASP55 also colocalizes with lysosomal associated membrane protein 2 (LAMP2), a maker for late endosome/lysosome.Citation5 These results suggest a role of GRASP55 in autophagosome-lysosome fusion.
GRASP55 has been shown to function as a “glue” to hold adjacent Golgi cisternae into stacks by forming trans-oligomers. The colocalization of GRASP55 with autophagosomes and lysosomes under glucose starvation suggests that GRASP55 oligomers may also tether autophagosomes and lysosomes to facilitate fusion. Subsequent biochemical assays demonstrated that GRASP55 preferably interacts with the lipidated form of microtubule associated protein 1 light chain 3 (LC3) after glucose deprivation, and a direct GRASP55-LC3 interaction is required for GRASP55 targeting to autophagosomes. GRASP55 also binds LAMP2, a major membrane component of lysosomes. More importantly, we found that GRASP55 bridges LC3 and LAMP2 for complex formation, which requires de-O-GlcNAcylation of GRASP55. GRASP55 deletion reduced the colocalization between LC3 and LAMP2 after glucose starvation, which was rescued by the addition of de-O-GlcNAcylated but not O-GlcNAcylated GRASP55. These results demonstrated that de-O-GlcNAcylation of GRASP55 facilitates autophagy flux by tethering autophagosomes and lysosomes through the interactions with LC3 and LAMP2 (). Therefore, we uncovered an unexpected role of the Golgi stacking protein GRASP55 in autophagosome-lysosome fusion upon energy deprivation.
Several important questions remain. One concerns how GRASP55 is targeted to autophagosomes upon glucose starvation. We propose that a small cytosolic pool of GRASP55 dynamically exchanges with Golgi-anchored GRASP55. When cytosolic GRASP55 is de-O-GlcNAcylated, it is recruited to autophagosomes by the increased interaction with LC3. It is worth mentioning that GRASP55 de-O-GlcNAcylation occurs not only after glucose deprivation, but also upon inhibition of the mechanistic target of rapamycin kinase (MTOR, also called the mammalian target of rapamycin) by Torin 1 or amino acid starvation, suggesting a common mechanism of GRASP55 de-O-GlcNAcylation in the regulation of autophagy. Another question concerns the relationship between GRASP55 and other known proteins in autophagosome maturation. In autophagy, RAB7, the homotypic fusion and protein sorting (HOPS) tethering complex, and the Syntaxin 17 (STX17)-Synaptosome Associated Protein 29 (SNAP29)-Vesicle associated membrane protein 7/8 (VAMP7/8) complex are well documented for their roles in autophagosome-lysosome tethering and fusion.Citation7–Citation10 The relative relationship between the RAB7/HOPS machinery and LC3/GRASP55/LAMP2 is so far unknown, but depletion of GRASP55 significantly reduced autophagosome-lysosome colocalization, suggesting that GRASP55 plays an indispensable role in autophagosome-lysosome tethering. It is possible that GRASP55 and Rab7/HOPS function as a cascade or coordinate with each other. This will be a future direction of this study.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Additional information
Funding
References
- Xiang Y, Zhang X, Nix DB, Katoh T, Aoki K, Tiemeyer M, Wang Y. Regulation of protein glycosylation and sorting by the Golgi matrix proteins GRASP55/65. Nat Commun. 2013;4:1659. doi:10.1038/ncomms2669.
- Xiang Y, Wang Y. GRASP55 and GRASP65 play complementary and essential roles in Golgi cisternal stacking. J Cell Biol. 2010;188(2):237–251. doi:10.1083/jcb.200907132.
- Jiang S, Dupont N, Castillo EF, Deretic V. Secretory versus degradative autophagy: unconventional secretion of inflammatory mediators. J Innate Immun. 2013;5(5):471–479. doi:10.1159/000346707.
- Feng Y, He D, Yao Z, Klionsky DJ. The machinery of macroautophagy. Cell Res. 2014;24(1):24–41. doi:10.1038/cr.2013.168.
- Zhang X, Wang L, Lak B, Li J, Jokitalo E, Wang Y. GRASP55 senses glucose deprivation through O-GlcNAcylation to promote autophagosome-lysosome fusion. Dev Cell. 2018;45(2):245–261. e6. doi:10.1016/j.devcel.2018.03.023.
- Yang X, Protein O-GlcNAcylation: QK. emerging mechanisms and functions. Nat Rev Mol Cell Biol. 2017;18(7):452–465. doi:10.1038/nrm.2017.22.
- Gutierrez MG, Munafo DB, Beron W, Colombo MI. Rab7 is required for the normal progression of the autophagic pathway in mammalian cells. J Cell Sci. 2004;117(Pt 13):2687–2697. doi:10.1242/jcs.01114.
- Jager S, Bucci C, Tanida I, Ueno T, Kominami E, Saftig P, Eskelinen EL. Role for Rab7 in maturation of late autophagic vacuoles. J Cell Sci. 2004;117(Pt 20):4837–4848. doi:10.1242/jcs.01370.
- Itakura E, Kishi-Itakura C, Mizushima N. The hairpin-type tail-anchored SNARE syntaxin 17 targets to autophagosomes for fusion with endosomes/lysosomes. Cell. 2012;151(6):1256–1269. doi:10.1016/j.cell.2012.11.001.
- Jiang P, Nishimura T, Sakamaki Y, Itakura E, Hatta T, Natsume T, The MN. HOPS complex mediates autophagosome-lysosome fusion through interaction with syntaxin 17. Mol Biol Cell. 2014;25(8):1327–1337. doi:10.1091/mbc.E13-08-0447.
