ABSTRACT
MAD2L1 (Mitotic Arrest Deficient 2 Like 1), a member of the mitotic checkpoint, maintains the genomic stability by insuring the proper segregation of the sister chromatids. Deregulation of MAD2L1 protein expression is a recurrent feature in cancer cells. In our recent publication, we uncovered a role for its yeast homolog, Mad2p, in protein synthesis during S-phase.
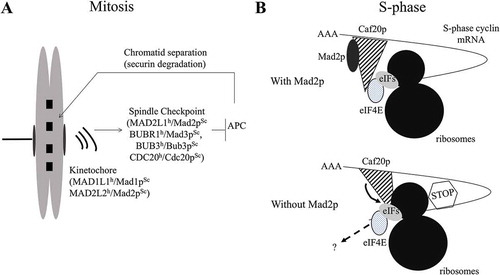
Genomic instability, a hallmark of cancer cells, is often associated with tumor progression and bad survival prognosis. Different checkpoint pathways ensure the correct progression through the different phases of the cell cycle in order to maintain genomic integrity. The intra-S checkpoint controls the accuracy of the DNA replication during S-phaseCitation1 by stabilizing replication forks following replication stress, by maintaining the levels of the dNTP pools, and by controlling the firing of replication origins. The intra-S checkpoint is mediated by a phosphorylation chain reaction dependent on the ATR (Ataxia Telangiectasia and Rad3-related protein) and CHEK1 (Checkpoint Kinase 1) kinases in mammals, Rad3p (Radiation sensitive 3) and Cds1p (Checkpoint DNA synthesis 1) in Schizosaccharomyces pombe (S. pombe), and Mec1p (Mitosis entry checkpoint 1) and Rad53p (Radiation sensitive 53) in Saccharomyces cerevisiae (S. cerevisiae). The mitotic checkpoint, also called the Spindle Assembly Checkpoint (SAC), ensures the correct segregation of the sister chromatids during mitosis through the MAD1L1 (Mitotic Arrest Deficient 1 Like 1) and MAD2L2 (Mitotic Arrest Deficient 2 Like 1) proteins that are localized at the kinetochores of mitotic chromosomesCitation2 (). In the absence of a bipolar attachment of microtubules to chromosomes, MAD2L1 conformation is modified, promoting its association with the proteins BUBR1 (BUB1-Related (BUB1: budding uninhibited by benzymidazol 1)), BUB3 (Budding Uninhibited by Benzymidazol 3) and with CDC20 (Cell Cycle Deficient 20), an activator of the Anaphase Promoting Complex (APC). This complex inhibits APC activity, responsible for the securin and mitotic cyclin degradation and, subsequently, for the metaphase/anaphase transition. This pathway is highly conserved from yeast to mammals. In agreement with their protective role of the genomic stability, the genes encoding the mitotic spindle checkpoint proteins have been originally classified as tumor suppressors. However, careful analyses of the relative mRNA expression in tumor cells have pointed out that many tumor cells show over-expression of the mitotic checkpoint componentsCitation3 leading to the hypothesis that these proteins could have additional functions outside of mitosis. Additional functions in protein transport, protein secretion and cell migration have already been attributed to MAD1L1 homologs.Citation4,Citation5 In our recent study,Citation6 we unmasked intriguing genetic interactions between MAD2, the yeast homolog of MAD2L1, and the intra-S checkpoint genes. Deletion of MAD2 in cells deficient for the intra-S checkpoint causes an increased sensitivity to replication stress, but not to DNA damaging agents. We found that this synthetic genetic interaction is due to a general decrease in the efficiency of origin firing, caused by a strong reduction in the levels of the S-phase cyclins. Our results indicate that, while the intra-S checkpoint inhibits S phase-cyclin degradation during S-phase when cells experience replication stress, Mad2p stimulates the translation of the S-phase cyclin mRNAs, both in the presence and in the absence of replication stress. Mad2p co-sediments with polysomes, strongly suggesting that Mad2p could be directly implicated in translation. Moreover, we found that Mad2p modulates the interaction of the translation inhibitor Caf20p (Cap associated factor 20) with the translation machinery: the presence of Mad2p inhibits the interaction of Caf20p with the translation machinery, with the exception of the translation initiator factor eIF4E (eukaryotic Initiation Factor 4E) (). We were able to suppress the defects of MAD2 deletion on cyclin mRNA translation by ablating CAF20, suggesting that the effect of Mad2p on translation should be at least partly mediated by the translational inhibitor Caf20p. Notably, Mad2p, out of the other proteins of the mitotic checkpoint pathway, is the only one contributing to the translational control of S-phase cyclins. The results of this study, carried out in S. cerevisiae, need to be further confirmed in other organisms. However, several observations suggest that the contribution of Mad2p in translation may be conserved through the evolution. First, the genetic interaction between MAD2 and the intra-S checkpoint is also conserved in S. pombe.Citation7 In addition, physical interactions between the translation machinery and the plant and human homologs of Mad2p have already been described.Citation8,Citation9 Finally, this new function may very well account for the selective up-regulation of MAD2L1 protein expression in cancer cells since tumor cells are considered “translation addicted”.Citation10 In the future, more work will be needed to understand if this novel function of Mad2p is conserved in human cells and whether this function might have therapeutic relevance.
Figure 1. Role of MAD2L1h/Mad2pSc in the S and M phases of the cell cycle. (A) During mitosis, MAD2L1h/Mad2pSc acts as a component of the spindle assembly checkpoint. (B) During S-phase, Mad2p promotes S-phase cyclin mRNA translation by modulating the association of the translational inhibitor Caf20p with the replication machinery. Abbreviations: APC: Anaphase promoting complex; h: human; Sc: Saccharomyces cerevisiae; eIF: eukaryotic initiation factor.
Disclosure of Potential Conflicts of Interest
The author has no conflicts of interest.
Additional information
Funding
References
- Branzei D, Foiani M. Maintaining genome stability at the replication fork. Nat Rev Mol Cell Biol. 2010;11:208–219. PMID:20177396. doi:10.1038/nrm2852.
- Kapanidou M, Curtis NL, Bolanos-Garcia VM. Cdc20: at the crossroads between chromosome segregation and mitotic exit. Trends Biochem Sci. 2017;42:193–205. PMID:28202332. doi:10.1016/j.tibs.2016.12.001.
- Suijkerbuijk SJ, Kops GJ. Preventing aneuploidy: the contribution of mitotic checkpoint proteins. Biochim Biophys Acta. 2008;1786:24–31. PMID:18472014. doi:10.1016/j.bbcan.2008.04.001.
- Cairo LV, Ptak C, Wozniak RW. Mitosis-specific regulation of nuclear transport by the spindle assembly checkpoint protein Mad1p. Mol Cell. 2013;49:109–120. PMID:23177738. doi:10.1016/j.molcel.2012.10.017.
- Wan J, Zhu F, Zasadil LM, Yu J, Wang L, Johnson A, Berthier E, Beebe DJ, Audhya A, Weaver BA. A Golgi-localized pool of the mitotic checkpoint component Mad1 controls integrin secretion and cell migration. Curr Biol. 2014;24:2687–2692. PMID:25447996. doi:10.1016/j.cub.2014.09.052.
- Gay S, Piccini D, Bruhn C, Ricciardi S, Soffientini P, Carotenuto W, Biffo S, Foiani M. A Mad2-mediated translational regulatory mechanism promoting S-phase cyclin synthesis controls origin firing and survival to replication stress. Mol Cell. 2018;70:628–638e625. PMID: 29775579. doi:10.1016/j.molcel.2018.04.020.
- Sugimoto I, Murakami H, Tonami Y, Moriyama A, Nakanishi M. DNA replication checkpoint control mediated by the spindle checkpoint protein Mad2p in fission yeast. J Biol Chem. 2004;279:47372–47378. PMID:15347659. doi:10.1074/jbc.M403231200.
- Hein MY, Hubner NC, Poser I, Cox J, Nagaraj N, Toyoda Y, Gak IA, Weisswange I, Mansfeld J, Buchholz F, et al. A human interactome in three quantitative dimensions organized by stoichiometries and abundances. Cell. 2015;163:712–723. PMID:26496610. doi:10.1016/j.cell.2015.09.053.
- Van Leene J, Hollunder J, Eeckhout D, Persiau G, Van De Slijke E, Stals H, Van Isterdael G, Verkest A, Neirynck S, Buffel Y, et al. Targeted interactomics reveals a complex core cell cycle machinery in Arabidopsis thaliana. Mol Syst Biol. 2010;6:397. PMID:20706207. doi:10.1038/msb.2010.53.
- De La Parra C, Walters BA, Geter P, Schneider RJ. Translation initiation factors and their relevance in cancer. Curr Opin Genet Dev. 2018;48:82–88. PMID:29153484. doi:10.1016/j.gde.2017.11.001.
