Abstract
Background
Male sex predicts case-fatality in SARS-CoV-2 (COVID-19) – a phenomenon linked to systemic inflammation. We compared sex-related associations of inflammation parameters and outcome in a population-based setting with low case-fatality prior to wide use of immunosuppressives.
Methods
A population-based quality registry with laboratory-confirmed COVID-19 cases of specialized hospitals of the Capital Province of Finland were analysed to compare inflammatory parameters by sex during the first COVID-19 wave February–June 2020.
Results
Altogether, 585 hospitalized patients (54% males) were included. Males required more often intensive care unit (ICU) treatment (26.9 vs. 17.5%) and had higher 90-d case-fatality (14.9 vs. 7.8%) compared with females. Highest association with case-fatality in males was seen for high neutrophil counts (median; interquartile range) (8.70; 7.10–9.10 vs. 5.60; 3.90–7.80) (E9/l), low monocyte (0.50; 0.20–1.50 vs. 0.70; 0.50–0.90) (E9/l) and lymphocyte (0.90; 0.70-1.40 vs. 1.50; 1.10-2.00) (E9/l) counts, and high levels of d-dimer (3.80; 1.80–5.30 vs. 1.10; 0.60–2.75) (mg/l) and C-reactive protein (CRP) (190; 85.5–290 vs. 77.0; 49.0–94.0) (mg/l). In females, low lymphocyte (0.95; interquartile range 0.60–1.28 vs. 1.50; 1.10–2.00) (E9/l) and thrombocyte counts (196; 132–285 vs. 325; 244–464) (E9/l) and high CRP values (95.0; 62.0–256 vs. 66.0; 42.5–89.0) (mg/l) were associated with case-fatality. In multivariable analysis for males, lymphocyte cut-off 0.85 (E9/l) (OR 0.02; 95% CI 0.002–0.260), d-dimer cut-off 1.15 (mg/l) (OR 7.29; 1.01–52.6) and CRP cut-off 110 (mg/l) (OR 15.4; 1.87–127) were independently associated with case-fatality. In female multivariable analysis, CRP cut-off 81 (mg/l) (OR 7.32; 1.44–37.2) was the only inflammatory parameter associated with case-fatality.
Conclusions
COVID-19 results in higher inflammation parameter levels in male vs. female patients irrespective of outcome. This study suggests that low lymphocyte, high d-dimer and high CRP cut-off values may serve as potential markers for risk stratification in male patients.
Introduction
The COVID-19 infection caused by coronavirus SARS-CoV-2 has rapidly become a world-wide pandemic [Citation1,Citation2]. Large scale meta-analyses, with up to 3.1 million patient cases, and ongoing global surveillance studies present similar numbers of COVID-19 diagnoses by sex, however, male patients have had a two-to-three-fold higher risk of intensive care unit (ICU) admission and death [Citation3,Citation4]. Reports suggest that sex-related illness severity and case-fatality is a multifactorial phenomenon with weaker male prognosis linked to poor health-seeking behaviour [Citation5], detrimental lifestyle habits, e.g. smoking and alcohol abuse [Citation6,Citation7], higher prevalence of age-adjusted co-morbidities [Citation8] and infrequent or delayed COVID-19 testing and hospital arrival [Citation9].
Furthermore, hyperinflammation due to sex-related immune response differences has received attention as an explanation for worse prognosis in men with COVID-19. Less robust t-cell response, higher C-reactive protein (CRP), ferritin and interleukin IL-6, IL-8 and CCL5 levels [Citation10–12] and higher antibody responses in convalescent plasma has been observed in male COVID-19 [Citation13,Citation14].
Although hyperinflammation may explain sex-related illness severity and prognosis of COVID-19, many reports are either hospital based, single centre or from areas with challenging COVID-19 epidemic situations. Moreover, many reports on sex-related hyper-inflammation include use of immunosuppressives, such as corticosteroids, e.g. dexamethasone or interleukin-6 receptor antagonist tocilizumab that may influence inflammatory parameters [Citation15–18] and very few reports have evaluated inflammatory parameter cut-off values as an instrument for risk stratification and prognosis [Citation19]. To the best of our knowledge, population-based studies on sex-related differences and prognostic value with usability of cut-off values for inflammatory parameters for hospitalized COVID-19 patients without wide use of immunosuppressives have not been performed.
The objective of the study was to quantify sex differences in inflammatory parameters and evaluate the association of cut-off values with case-fatality by using a population-based quality registry of all hospitalized patients treated in specialized healthcare in the Capital Province of Finland. By including only patients from the first wave of COVID-19 from February to June 2020, i.e. prior to large scale use, e.g. dexamethasone we could evaluate inflammatory parameters without potential disturbing impact from wide use of immunosuppressives.
Methods
This was a retrospective observational population-based quality registry study including all laboratory-confirmed cases of COVID-19 hospitalized in the specialized healthcare (including altogether 22 hospitals of specialized healthcare) of the Capital Province (southern Province of Helsinki and Uusimaa) of Finland during the first wave of COVID-19 from February to June 2020.
Study population
This study is a sequential second report from the COVID-19 quality registry including all specialized healthcare of the Capital Province of Finland (including Helsinki University Hospital). As described in our previous report [Citation20] the quality registry covers all specialized healthcare across the Province of Helsinki and Uusimaa, i.e. the capital region of Finland. The province is one of the largest healthcare organizations in Europe and the largest hospital district area in Finland. The HUS Helsinki University Hospital district of the Capital Province of Finland is a specialized healthcare organization with over 27,000 healthcare employees and approximately 2.7 million patient visits annually. The Finnish tax-funded universal healthcare system enables each health care district to provide specialist inpatient care for COVID-19 patients who have been previously independent in their daily life. Nursing home patients, i.e. assistance-dependent patients or permanently institutionalized patients may be treated by municipality run primary-care hospitals.
All patients in the specialized healthcare of the Capital Province of Finland during the first wave of the COVID-19 pandemic spring 2020 until 21 June 2020 with at least one positive test for COVID-19 RT-PCR (real-time reverse transcription-polymerase chain reaction) who received treatment at the hospital ward, ICU or spent a minimum of 6 h at the emergency room or deceased were included in the quality registry. Not included in the registry were patients managed at home, institution, or treated in primary-care hospitals as their data was not available.
Clinical patient data were gathered from both local and national electronic records as well as prescription database, referral letters and radiology and pathology information systems. As gender we used the data recorded in these data bases. Data regarding demographics, living and working arrangements, risk behaviour, underlying conditions, date and nature of initial COVID-19 symptoms, severity of illness at admission, laboratory results, radiological examinations, medicines used as well as dates for admission and hospital and ICU length-of-stay (LOS) and all-cause case-fatality were registered.
Definitions
We defined the first wave of the COVID-19 epidemic in Finland as the onset from the first diagnosed patient case of 27 February 2020 until 21 June 2020 when the epidemic clearly declined. The primary outcome measure was defined as all-cause case-fatality up to 90 d (calculated from hospital admission). Secondary outcome was admission to ICU.
Laboratory parameters
Laboratory values reflecting degree of inflammation and viewed as essential in COVID-19 were registered. We documented both the highest value for each parameter as well as the median for each parameter during the hospital treatment period. The following parameters were included: Blood count values; Leucocytes, neutrophils, monocytes, lymphocytes and thrombocytes; Kidney and liver function values; creatinine, urea, alanine aminotransferase, aspartate aminotransferase and thromboplastin time; Infectious and inflammation markers: CRP, ferritin and d-dimer.
Statistical analyses
Inflammatory parameters were reported as median (quartiles) (as they were not normally distributed). Univariate analyses were performed using the Mann–Whitney U-test. Receiver operating characteristic (ROC) curves were used to evaluate the association of laboratory values to 90-day case-fatality. The area under the curve (AUC) was calculated for each ROC curve. Youden function defining the difference between true positive rate and false-positive rate over all possible cut-point values, was used to identify the ROC-curve point maximizing both sensitivity and specificity values to locate the optimal cut-off point. These cut-off values were applied in multivariable logistic regression analysis for estimation of parameters associated with outcome. Univariate factors with p < .05 were included in multivariable logistic regression analysis for estimation of parameters associated with outcome. No missing data imputation analyses were performed and the multivariable analyses were performed with available data only (the data and N numbers available for each laboratory value are listed in the Tables). Tests were 2-tailed and p <.05 was considered significant. Analyses were done with SPSS version 25.0.0 IBM SPSS Statistics for Windows (IBM Corp, Armonk, NY).
Institutional review and patient consent
The quality registry was institutionally approved as a quality registry without requirement for patient consent (approvals HUS/1049/2020/§4 and HUS/157/2020/§94). Consent for registration was not sought, allowing all consecutive patients to be included.
Results
Altogether 585 patients (54% males) with positive RT-PCR testing received specialized healthcare hospital treatment and 132 (64% males) of them ICU treatment at the Capital Province of Finland during the first wave of COVID-19. All-cause case-fatality was 12% and ICU-related case-fatality 18%. Detailed results on demographics, underlying conditions, illness severity, complications and parameters associating to case-fatality have been described in our previous report [Citation20].
Underlying conditions
Underlying conditions with potential impact on inflammatory parameters for male vs. female patients have been compared previously. No significant sex differences in underlying chronic liver or kidney failure, malignancy, HIV infection or immunodeficiency disease was observed. Furthermore, only two patients (<0.5%) had systemic connective tissue disease [Citation20].
Immunosuppressive treatment
Altogether, 27 (4.6%) patients received corticosteroid treatment: Hydrocortisone (parenteral) 4 (<1%) patients, methylprednisolone (parenteral) 6 (1%) patients and oral prednisolone 19 (3%) of patients. Three of the patients who received oral prednisolone had the medication altered to oral dexamethasone which meant that only 0.5% of all patients were treated with dexamethasone. No difference in use of corticosteroids were observed between males (N = 13) and females (N = 14) (OR 0.78, p = .531). When repeating the ROC and multivariable analyses by excluding patients with corticosteroid treatment the main results did not change.
Laboratory parameters
Peak and median laboratory values were stratified by sex and grouped according to need for ICU and case-fatality and presented in and (Tables 1(a,b), Supplementary data).
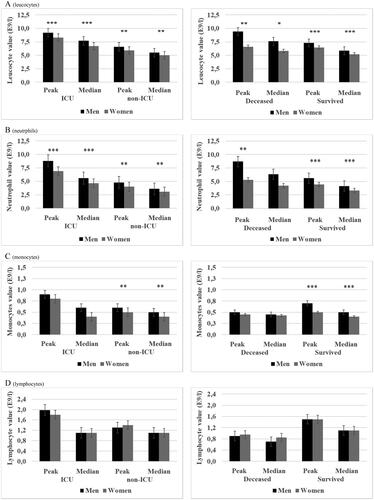
Figure 1. (A–E) Peak and median leucocyte, neutrophil, monocyte, lymphocyte and thrombocyte values for laboratory-confirmed COVID-19 patients (N = 562) in specialized healthcare hospitals stratified by mortality and intensive care unit (ICU) and sub-grouped by sex. ICU patients (N = 132) and deceased patients (N = 68). Data are median (quartiles). ***p Value < .001, **p Value < .01 and * p Value < .05.
Table 1. Multivariable analysis for parameters of 90-d case-fatality in patients with laboratory-confirmed COVID-19 treated in specialized healthcare hospitals and stratified by sex.
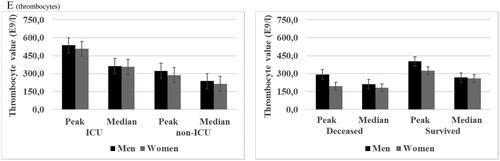
Significantly higher peak and median leucocyte, neutrophil and monocyte counts were observed in male patients who were managed without ICU and surviving males as compared with their female counterparts. Non-significant trends towards higher lymphocyte and thrombocyte counts were observed among male patients () and (Tables 1(a,b), Supplementary data). Furthermore, peak and median creatinine, alanine aminotransferase and ferritin were significantly higher among non-ICU and survived males than in females whereas for thromboplastin time the trend was the opposite with non-ICU and survived males presenting significantly lower peak and median levels compared with females () and (Tables 1(a,b), Supplementary data). No difference in peak or median d-dimer levels was observed for non-ICU male vs. female patients whereas CRP levels were significantly higher in non-ICU male compared with female patients. However, survived male patients had significantly higher peak and median d-dimer and significantly higher median CRP () and (Tables 1(a,b), Supplementary data) as compared with survived female patients.
When comparing laboratory values in ICU patients the sex differences were very similar to those seen in non-ICU patients. Male patients had significantly higher peak and median leucocyte and neutrophil counts and significantly higher creatinine and d-dimer levels compared with females. Male patients had also significantly higher median ferritin and median CRP levels but significantly lower thromboplastin time (peak and median) as compared with female patients (Tables 1(a,b), Supplementary data). Significantly higher peak and median leucocyte count and creatinine and significantly higher median CRP was observed in deceased males compared with deceased female patients (Tables 1(a,b), Supplementary data) ( and ).
Figure 2. (A–C) Peak and median creatinine and alanine aminotransferase (N = 562) and thromboplastin time (N = 341) for laboratory-confirmed COVID-19 patients in specialized healthcare hospitals stratified by mortality and intensive care unit (ICU) and sub-grouped by sex. ICU patients (N = 132) and deceased patients (N = 68). Data are median (quartiles). ***p Value < .001, **p Value < .01 and *p Value < .05.
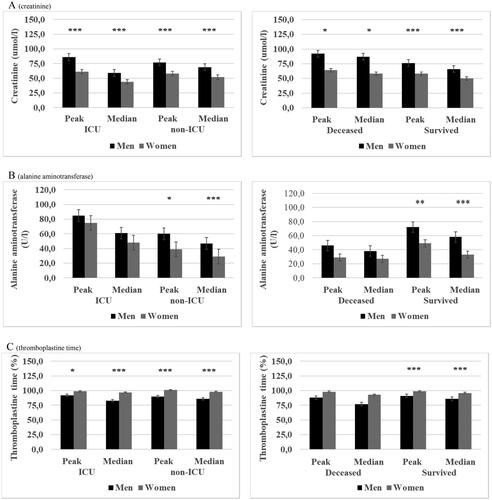
Figure 3. (A–C) Peak and median ferritin (N = 338), d-dimer (N = 356) and C-reactive protein (N = 562) in patients with laboratory-confirmed COVID-19 and treated in specialized healthcare hospitals stratified by mortality and intensive care unit (ICU) and sub-grouped by sex. ICU patients (N = 132) and deceased patients (N = 68). Data are median (quartiles). ***p Value < .001, **p Value < .01 and *p Value < .05.
Cut-off values of laboratory parameters for case fatality/mortality
In ROC analysis, higher peak and median values of leucocyte and neutrophil count, CRP, ferritin and d-dimer levels and lower peak and median monocyte, lymphocyte and thrombocyte counts were associated with case-fatality for male patients. The highest discriminative power for associating inflammatory parameters with 90-d case-fatality was seen for peak and median values of elevated neutrophil count, lower peak and median monocyte count and low median lymphocyte count and high peak and median CRP and d-dimer values ( and ).
Figure 4. (A,B) Receiver operating characteristic (ROC) curves for associating inflammatory parameters to case-fatality during 90 d follow-up in patients with laboratory-confirmed COVID-19 infection treated in specialized healthcare hospital. Among male (A) COVID-19 patients the ROC analysis was statistically significant for peak and median leucocyte, neutrophil, monocyte, lymphocyte and thrombocyte values: Sensitivity for peak and median values, respectively: leucocytes 62 and 71%, neutrophils 76 and 76%, monocytes 62 and 76%, lymphocytes 81 and 71% and thrombocytes 62 and 62%. Specificity for peak and median values, respectively: leucocytes 59 and 62%, neutrophils 72 and 73%, monocytes 66 and 73%, lymphocytes 70 and 77% and thrombocytes 60 and 63%. Cut-off values for peak and median: leucocytes 7.95 E9/l and 6.65 E9/l, neutrophils 7.05 E9/l and 4.95 E9/l, monocytes 0.55 E9/l and 0.35 E9/l, lymphocytes 1.15 E9/l and 0.85 E9/l and thrombocytes 360 E9/l and 230 E9/l. Among female (B) COVID-19 patients the ROC analysis was statistically significant for peak and median lymphocyte and thrombocyte values: Sensitivity for peak and median, respectively: lymphocytes 77 and 62% and thrombocytes 77 and 69%. Specificity for peak and median, respectively: lymphocyte 69 and 72% and thrombocytes 76 and 68%. Cut-off values for peak and median, respectively: lymphocytes 1.25 E9/l and 0.95 E9/l and thrombocytes 243 E9/l and 215 E9/l.
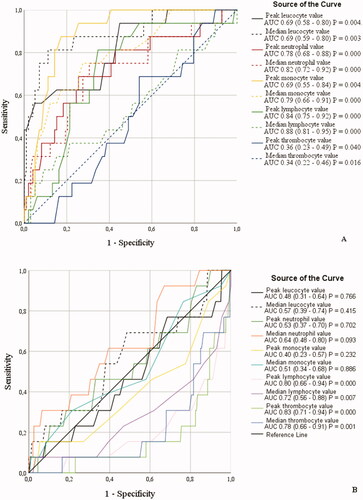
Figure 5. (A,B) Receiver operating characteristic (ROC) curves for associating inflammatory parameters to case-fatality during 90 d follow-up in patients with laboratory-confirmed COVID-19 infection treated in specialized healthcare hospital. Among male (A) COVID-19 patients the ROC analysis was statistically significant for peak and median C-reactive protein, ferritin and d-dimer values and peak creatinine value. Sensitivity, peak and median: C-reactive protein 75 and 88%, ferritin 69 and 69%, d-dimer 88 and 75% and peak creatinine 69%. Specificity, peak and median: C-reactive protein 62 and 76%, ferritin 75 and 72%, d-dimer 83 and 72% and peak creatinine 67%. Cut-off values, peak and median: C-reactive protein 89 and 110 mg/l, ferritin 1347 and 1310 ug/l, d-dimer 2.15 mg/l and 1.15 ug/l and peak creatinine 81 umol/l. Among female (B) COVID-19 patients the ROC analysis was statistically significant for peak and median C-reactive protein and peak creatinine value. Sensitivity, peak and median: C-reactive protein 73 and 82%. Specificity, peak and median: C-reactive protein 70 and 64%. Cut-off values, peak and median: C-reactive protein 87 and 81 mg/l.
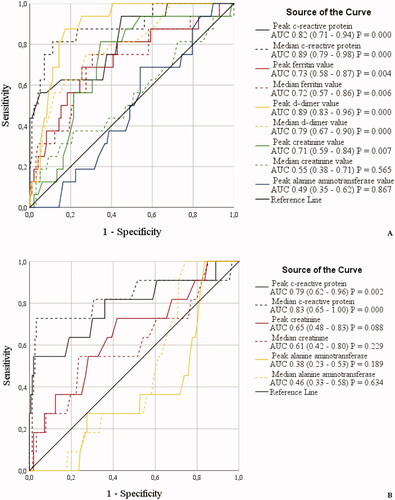
In female patients, a significant association of inflammatory parameters with 90-d case-fatality in ROC analysis were observed for low peak and median lymphocyte and thrombocyte count and high peak and median CRP ( and ). The highest discriminative power for associating inflammatory parameters with case-fatality was observed for low peak lymphocyte and thrombocyte count and high median CRP ( and ). However, the low overall case-fatality among female patients and the low N number for retrieved ferritin and d-dimer laboratory values among females did not allow for reliable ROC analyses and these were excluded from .
Multivariable analysis
The cut-off values derived from the ROC analyses were applied in multivariable analysis to evaluate parameters that independently associate with 90-d case-fatality for male and female patients separately (). In male patients, age over 65 years (OR 12.1, p = .018), median d-dimer above 1.15 mg/l (OR 7.29, p = .049) and median CRP over 110 mg/l (OR 15.4, p = .011) connected to poor prognosis whereas lymphocyte count above 0.85 E9/l (OR 0.02, p = .002) associated to improved prognosis (). For female patients, only age above 65 years (OR 10.6, p = .005), previous dementia diagnosis (OR 25.5, p = .003) and median CRP over 81 mg/l (OR 7.32, p = .016) were independently associated with 90-d case-fatality ().
Discussion
The main observation of this study was a clear difference in the inflammatory response of male and female patients due to COVID-19 infection, assessed by commonly used clinical laboratory parameters. The sex difference in levels of inflammatory parameters was observed irrespective of disease severity or outcome. Survived and non-ICU male patients presented significantly higher peak and median values for leucocyte, neutrophil and monocyte counts and d-dimer, ferritin and median CRP as compared with female patients. In ROC analysis, a stronger connection and higher cut-off values of inflammatory parameters to case-fatality were observed in male vs. female patients. Taking all prognostic parameters into account, an independent association of high median d-dimer, CRP and low lymphocyte count with 90-d case-fatality was observed in male patients whereas a corresponding association was observed for high CRP only in female patients. This population-based study from a low epidemic area with low overall case-fatality and minimal prior use of immunosuppressives suggests that a more disadvantageous systemic inflammation response with higher inflammatory parameters could be at least one explanation behind higher case-fatality of males in COVID-19.
The role of inflammatory parameters, reflecting hyperinflammation and predicting prognosis, has received much attention during the COVID-19 epidemic. However, the exact underlying mechanism for elevated inflammatory parameters is only partly understood. COVID-19 induced cytokine storms with release of reactive oxygen radicals and sequential cell DNA damage has been suggested as one mechanism for leukocytosis, neutrophilia and elevated neutrophil/lymphocyte ratio as well as high ferritin and elevated CRP [Citation21–24]. Traditionally, lymphocytosis may be encountered in viral infections, but lymphopenia in COVID-19 has been explained by hyper-inflammation-induced lymphocyte apoptosis, defective haematopoiesis or lymphocyte migration to lung tissue due to angiotensin-converting enzyme 2 receptor expression on lymphocytes that the COVID-19 virus targets [Citation25–27]. Hyperinflammation has been postulated as a driver for hypercoagulation resulting in elevated d-dimer and diminished partial thromboplastin time [Citation28–30].
Reports associate severely ill and ICU patients as well as deceased patients to higher inflammatory parameters, such as blood white cell count values, ferritin, CRP, lactate dehydrogenase and d-dimer [Citation30–38]. However, to the best of our knowledge, few studies have evaluated sex difference in inflammatory parameters and compared the prognostic value and cut-of values of inflammatory parameters between male and female patients. Scully et al. [Citation16], Vahidy et al. [Citation17] and Lau et al. [Citation18], compared inflammatory parameters in 781 − 4785 patients (51–58% males) of which 31–32% required ICU and 9–15% died with male patients more often presenting a severe disease, more ICU need or higher case-fatality. Scully et al. observed higher peak and median neutrophil/lymphocyte ratio, ferritin and CRP within 48 h of hospital admission among severely ill or deceased male patients as compared with females. However, they observed comparable levels of d-dimer for male and female patients at hospital presentation and when subgrouping by illness severity and case-fatality [Citation16]. Vahidy et al. presented higher lymphocyte count, thrombocyte count, CRP and creatinine in male patients as compared with female patients but no sex differences in d-dimer levels was observed [Citation17] whereas Lau et al. observed higher CRP and ferritin values at initial admission and overall higher peak levels in male patients but no sex differences in d-dimer levels [Citation18]. This study observed a significant difference in inflammatory parameter response of male vs. female patients due to COVID-19, irrespective of outcome, an observation made without disturbance from immunosuppressives. In line with above previous studies, we observed that changes in leucocyte and lymphocyte counts, CRP, ferritin and creatinine were more marked in male patients but a novel finding was that also blood coagulation parameters d-dimer and thromboplastin time as well as alanine aminotransferase were more affected in male patients. Together all these reports suggest that a more forceful inflammatory activation contributes to a more severe and more often a fatal COVID-19 disease in males as compared with female patients [Citation15–18]. Hence, morbidity and case-fatality related to COVID-19 seems to be mediated through hyperactivated viral stimulation of inflammation expressed as cytokine elevation with sequential increase in inflammatory biomarker levels, a phenomenon dominant in male patients.
Comparison of sex-related inflammatory parameter results or overall prognostic impact of cut-off values from previous studies is challenging due to differences in laboratory collection time-points, patient categorization, i.e. most studies analyse male and female patients en bloc, variable disease severity, use of immunosuppressive medication and reported follow-up time and endpoint status. In this study, altogether 23% received ICU treatment and the overall case-fatality was 12% and ICU case-fatality 18% during 90-d follow-up which is lower as compared with 27 − 46% critical illness or ICU treatment and 9 − 19% overall case-fatality and 32 − 46% ICU related (or severe disease) case-fatality presented in many previous reports on sex-differences or overall prognostic impact of inflammatory parameters in patients with COVID-19 [Citation10,Citation15–19].
Many previous studies report higher occurrence of malignancies, chronic kidney disease and HIV-infection but less often rheumatic diseases and autoimmune diseases in male vs. female patients, i.e. inequality in occurrence of underlying conditions with potential impact on inflammatory parameters [Citation16–18]. Furthermore, use of corticosteroids in 5 − 49% and/or tocilizumab in 1 − 16% [Citation16–18,Citation21,Citation31] or overall use of immunosuppressants before COVID-19 diagnosis in 6 − 8% of patient cases [Citation18,Citation31] with male patients receiving significantly more often tocilizumab [Citation16,Citation17] or corticosteroids [Citation17] during COVID-19 treatment has been presented in previous reports. We observed no differences in underlying conditions, such as liver or kidney failure, malignancy, HIV infection or immunodeficiency disease or use of corticosteroids between male and female patients in this study. Moreover, only 4.6% received corticosteroids and no one received tocilizumab. Hence, the detailed patient data of this study enabled thorough evaluation of factors that may impact inflammatory parameters and we could compare sex differences in inflammatory parameters without disturbance from conditions or medications with potential impact on systemic inflammation. This is an evident advantage as compared with previous studies.
The ROC analyses of this study observed highest discriminative power for predicting case-fatality (p < .001) in males within neutrophil, monocyte and lymphocyte count and d-dimer and CRP levels. Corresponding results were seen only within lymphocyte and thrombocyte count and CRP in female patients. Most previous studies on cut-off values regarding inflammatory parameters have been performed with male and female patients analysed en bloc and comparison of results from previous studies is challenging due to various ROC analysis end points with varying definitions [Citation21,Citation22,Citation32,Citation33], case-fatality [Citation19,Citation30,Citation33,Citation36] or a combination of severe disease and case-fatality [Citation10].
Previous reports have presented ROC analysis derived cut-off values for leucocyte count (6.01 − 7.70 E9/l), neutrophil count (4.11 − 5.93 E9/l) and lymphocyte count (0.98 − 1.35 E9/l) [Citation21,Citation22,Citation32,Citation33] for predicting a severe disease and leucocyte count (7.46 E9/l) and neutrophil count (4.53 E9/l) [Citation33] for predicting mortality. Corresponding cut-off results for d-dimer (0.335 − 0.565 mg/l) [Citation21,Citation33], CRP (20.3 − 87 mg/l) [Citation21,Citation22,Citation32] and ferritin (163.5 ug/l) [Citation21] predict a severe disease whereas d-dimer (1.11 − 2.52 mg/l) [Citation19,Citation30,Citation35] and CRP (63.3 − 100 mg/l) [Citation31,Citation33] predict mortality. One report connected cut-off values for CRP (100 mg/l), ferritin (400 ug/l) and lymphocyte count (0.8 E9/l) to severe disease or case-fatality analysed en bloc [Citation10] whereas one study associated CRP and ferritin to case-fatality in ROC analysis but without numerical cut-off values [Citation36]. When comparing results of inflammatory parameter cut-off values of the present and previous reports a certain trend may be observed. Many of the cut-off values for male patients in this study exceed or are in line with those of previous reports. However, for female patients an opposite trend may be observed, i.e. the cut-off values of this study are mainly lower or in line with those of previous reports.
We observed that multivariable analysis associated d-dimer, CRP and lymphocyte count independently to case-fatality in male patients whereas in female patients CRP was the only inflammatory parameter associated independently with prognosis. Furthermore, high age was an independent parameter for case-fatality both in male and female patients whereas dementia diagnosis was a strong prognostic parameter in female patients. Many reports have applied multivariable analysis to evaluate the impact of individual inflammatory parameters on severe disease or case-fatality. High age [Citation19,Citation22,Citation37,Citation38], elevated leucocyte or neutrophil count [Citation32,Citation37] or increased neutrophil/lymphocyte ratio [Citation21,Citation33] and high ferritin [Citation21,Citation36], d-dimer [Citation19,Citation21,Citation31,Citation33,Citation38], and CRP [Citation31–33] have been independently associated with severe disease and/or case-fatality in hospitalized patients with COVID-19. However, to the best of our knowledge, dementia diagnosis as a prognostic parameter in female COVID-19 patients has not been presented in the references cited in this study. Lau et al. observed that in male patients peak CRP was a stronger marker for ICU admission and case-fatality (OR 9.19, p < .001) than in females (OR 2.81, p = .009) [Citation18]. Higher neutrophil/lymphocyte ratio, CRP or lactate dehydrogenase has been observed in deceased male patients as compared with female patients [Citation15]. Qin et al. demonstrated higher levels of CRP, ferritin, interleukin-10 and lower levels of lymphocyte count even after statistical correction for age and comorbidities and a poorer outcome in hospitalized male patients as compared with female patients [Citation10]. These reports are in line with this study, but we show that the association of inflammatory parameters to case-fatality were accentuated in male patients.
The influence of biological sex on pathophysiology is not yet fully understood in COVID-19. Sex-biased collateral cardiovascular damage and endocrinological aspects have been presented as parameters that most likely affect disease progression and prognosis more than previously known [Citation39–42]. Cardiometabolic disorders are overrepresented among males and patients with cardiometabolic disorders comorbidities are at risk of suffering from a more severe infection in COVID-19 [Citation39–41]. Moreover, sex hormones oestrogen and testosterone are suggested to have anti-inflammatory effect which may be one explanation for why younger females and male patients (high oestrogen and testosterone levels) experience a better prognosis whereas aged males experience a poor prognosis in COVID-19 [Citation42]. Furthermore, we have previously shown that diabetes mellitus is more common among male vs. female COVID-19 patients [Citation20] and diabetes mellitus is known to be a parameter for a more severe COVID-19 disease [Citation43]. To the best of our knowledge, so far it is not clear whether biological sex should be viewed as an independent risk or whether the prognostic impact of sex is mediated through pre-existing factors (e.g. male sex-related detrimental lifestyle habits such as smoking and alcohol abuse) [Citation6,Citation7,Citation39,Citation40].
However, within the scope of this study, the N number does not allow for robust statistical analyses that would evaluate how cardiometabolic disorders or diabetes mellitus affect sex-related inflammatory parameters and endocrinological data, e.g. hormone levels have not been recorded.
There are limitations, as well as strengths, in this study that are to be accounted for when interpreting the results. Strengths; first, the universal thorough electronic patient and laboratory records in combination with the unique national personal identification code given to all Finnish residents, enabled us to identify and record all laboratory-confirmed COVID-19 hospitalized patients across different sections of the specialized healthcare hospitals of the Capital Province. These patient cases were recorded into the quality registry and applied for this study. This ensured that no laboratory positive hospitalized COVID-19 patients have been lost. Second, the potential case-fatality of patients transferred to municipality run primary-care hospitals could be followed through the National causes of death registry. This enabled us to follow-up all-cause case-fatality for all deceased patients. Third, this study evaluated patients from the first COVID-19 wave February–June 2020, i.e. prior to wide use of corticosteroids. Limitations; first, this study included patients from the first COVID-19 wave, i.e. at a time period when knowledge on COVID-19 was scarce and all inflammatory parameters were not yet routinely and repeatedly collected from all patients, which was the case at a later stage of the epidemic. This explains the low N number for some of the inflammatory parameters. The low N number decreases the statistical power of the analyses, however, does not affect the main results. Second, this study was a retrospective registry report with a retrospective observational setting. Hence, the multivariable analyses must be considered hypothesis generating although the observations are reliable. Third, the primary prevention of COVID-19 was quite successful during the first wave of COVID-19 in Finland which explains the overall small number of patients hospitalized in specialized healthcare. Fourth, the results presented in this study comprise the COVID-19 situation in spring 2020, i.e. the Wuhan COVID-19 virus variant. Throughout the pandemic new virus variants have emerged and the disease severity and need for hospital treatment have varied as new virus variants have developed. Hence, it is very likely that the impact of COVID-19 on inflammatory parameters and the capability of inflammatory parameter cut-off values to serve as potential markers for risk stratification in male vs. female patients may vary. New research is needed to evaluate whether the results observed in this study are valid for new COVID-19 virus variants as well.
In conclusion, we observed higher peak and median inflammatory parameter levels in male as compared with female patients irrespective of outcome. A strong connection of several inflammatory parameters, including higher cut-off values, to case-fatality, was observed especially in male and to a lesser degree in female patients. These observations were made in a study setup with minimal disturbance from underlying conditions or immunosuppressive treatment. This study suggests that inflammatory parameter cut-off values may serve as a potential tool for risk stratification in male patients. Additional research is warranted to further identify the usability and robustness of inflammatory parameter cut-off values as an instrument for risk stratification and intervention strategies among hospitalized male patients with COVID-19.
Supplemental Material
Download MS Word (34.4 KB)Disclosure statement
AJ reports speaker honoraria from Astellas, GlaxoSmithKline, Gilead, Sanofi, ThermoFisher and consultation fees from Gilead, GlaxoSmithKline, Sanofi, Sobi and Roche outside the scope of this publication. AM and SS none.
Additional information
Funding
References
- Zhu A, Zhang DZ, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382(8):727–733.
- Guan W, Ni Z, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708–1720.
- Peckham H, de Gruijter NM, Raine C, et al. Male sex identified by global COVID-19 Meta-analysis as a risk factor for death and ITU admission. Nat Commun. 2020;11(1):6317.
- The Sex, Gender and COVID-19 Project. 5050 Global Health. https://globalhealth5050.org/covid19/.
- Thompson AE, Anisimowicz Y, Miedema B, et al. The influence of gender and other patient characteristics on health care-seeking behaviour: a QUALICOPC study. BMC Fam Pract. 2016;17:38.
- Reitsma MB, Kendrick PJ, Ababneh E, et al. Spatial, temporal, and demographic patterns in prevalence of smoking tobacco use and attributable disease burden in 204 countries and territories, 1990–2019: a systematic analysis from the global burden of disease study 2019. Lancet. 2021;397(10292):2337–2360.
- GBD 2016 Alcohol Collaborators. Alcohol use and burden for 195 countries and territories, 1990-2013; a systematic analysis for the global burden of disease study 2016. Lancet. 2018;392(10152):1015–1035.
- Bots SH, Peters SAE, Woodward M. Sex differences in coronary heart disease and stroke mortality: a global assessment of the effect of ageing between 1980 and 2010. BMJ Glob Health. 2017;2(2):e000298.
- Jarva H, Lappalainen M, Luomala O, et al. Laboratory-based surveillance of COVID-19 in the greater helsinki area, Finland, February–June 2020. Int J Infect Dis. 2021;104:111–116.
- Qin L, Li X, Shi J, et al. Gendered effects on inflammation reaction and outcome of COVID-19 patients in Wuhan. J Med Virol. 2020;92(11):2684–2692.
- Del Valle DM, Kim-Schulze S, Huang HH, et al. An inflammatory cytokine signature predicts COVID-19 severity and survival. Nat Med. 2020;26(10):1636–1643.
- Takahashi T, Ellingson MK, Wong P, et al. Yale IMPACT research team. Sex differences in immune responses that underlie COVID-19 disease outcomes. Nature. 2020;588(7837):315–320.
- Klein SL, Pekosz A, Park HS, et al. Sex, age, and hospitalization drive antibody responses in a COVID-19 convalescent plasma donor population. J Clin Invest. 2020;130(11):6141–6150.
- Robbiani DF, Gaebler C, Muecksch F, et al. Convergent antibody responses to SARS-CoV-2 in convalescent individuals. Nature. 2020;584(7821):437–442.
- Meng Y, Wu P, Lu W, et al. Sex-specific clinical characteristics and prognosis of coronavirus disease-19 infection in Wuhan, China: a retrospective study of 168 severe patients. PLoS Pathog. 2020;16(4):e1008520.
- Scully EP, Schumock G, Fu M, et al. Sex and gender differences in testing, hospital admission, clinical presentation, and drivers of severe outcomes from COVID-19. Open Forum Infect Dis. 2021;31;8(9):ofab448.
- Vahidy FS, Pan AP, Ahnstedt H, et al. Sex differences in susceptibility, severity, and outcomes of coronavirus disease 2019: cross-sectional analysis from a diverse US metropolitan area. PLoS One. 2021;16(1):e0245556.
- Lau ES, McNeill JN, Paniagua SM, et al. Sex differences in inflammatory markers in patients hospitalized with COVID-19 infection: insights from the MGH COVID-19 patient registry. PLoS One. 2021;16(4):e0250774.
- Poudel A, Poudel Y, Adhikari A, et al. D-dimer as a biomarker for assessment of COVID-19 prognosis: D-dimer levels on admission and its role in predicting disease outcome in hospitalized patients with COVID-19. PLoS One. 2021;16(8):e0256744.
- Forsblom E, Silén S, Kortela E, et al. Male predominance in disease severity and mortality in a low Covid-19 epidemic and low case-fatality area – a population-based registry study. Infect Dis (Lond). 2021;53(10):789–799.
- Bastug A, Bodur H, Erdogan S, et al. Clinical and laboratory features of COVID-19: predictors of severe prognosis. Int Immunopharmacol. 2020;88:106950.
- Yang AP, Liu JP, Tao WQ, et al. The diagnostic and predictive role of NLR, d-NLR and PLR in COVID-19 patients. Int Immunopharmacol. 2020;84:106504.
- Wang J, Jiang M, Xin Chen X, et al. Cytokine storm and leukocyte changes in mild versus severe SARS-CoV-2 infection: review of 3939 COVID-19 patients in China and emerging pathogenesis and therapy concepts. J Leukoc Biol. 2020;108(1):17–41.
- Hu B, Huang S, Yin L. The cytokine storm and COVID-19. J Med Virol. 2021;93(1):250–256.
- Xu H, Zhong L, Deng J, et al. High expression of ACE2 receptor of 2019-nCoV on the epithelial cells of oral mucosa. Int J Oral Sci. 2020;12(1):8–5.
- Sun S, Cai X, Wang H, et al. Abnormalities of peripheral blood system in patients with COVID-19 in Wenzhou China. Clin Chim Acta. 2020;507:174–180.
- Wang F, Hou H, Luo Y, et al. The laboratory tests and host immunity of COVID-19 patients with different severity of illness. JCI Insight. 2020;5(10):e137799.
- Di Micco P, Russo V, Carannante N, et al. Clotting factors in COVID-19: epidemiological association and prognostic values in different clinical presentations in an Italian cohort. J Clin Med. 2020;9(5):1371.
- Tang N, Bai H, Chen X, et al. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost. 2020;18(5):1094–1099.
- Luo HC, You CY, Lu SW, et al. Characteristics of coagulation alteration in patients with COVID-19. Ann Hematol. 2021;100(1):45–52.
- Peiró ÓM, Carrasquer A, Sánchez-Gimenez R, et al. Biomarkers and short-term prognosis in COVID-19. Biomarkers. 2021;26(2):119–126.
- Shi S, Nie B, Chen X, et al. Clinical and laboratory characteristics of severe and non-severe patients with COVID-19: a retrospective cohort study in China. Clin Lab Anal. 2021;35(1):e23692.
- Wang Q, Cheng J, Shang J, et al. Clinical value of laboratory indicators for predicting disease progression and death in patients with COVID-19: a retrospective cohort study. BMJ Open. 2021;1;11(10):e043790.
- Lv Z, Wang W, Qiao B, et al. The prognostic value of general laboratory testing in patients with COVID-19. J Clin Lab Anal. 2021;35(2):e23668.
- Zhang L, Yan X, Fan Q, et al. D-dimer levels on admission to predict in-hospital case-fatality in patients with COVID-19. J Thromb Haemost. 2020;18(6):1324–1329.
- Deng F, Zhang L, Lyu L, et al. Increased levels of ferritin on admission predicts intensive care unit mortality in patients with COVID-19. Med Clin. 2021;156(7):324–331.
- Zeng Z, Yu H, Chen H, et al. Longitudinal changes of inflammatory parameters and their correlation with disease severity and outcomes in patients with COVID-19 from Wuhan, China. Crit Care. 2020;24(1):525.
- Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020; 395(10229):1054–1062.
- Kararigas G. Sex-biased mechanisms of cardiovascular complications in COVID-19. Physiol Rev. 2022;102(1):333–337.
- Ritter O, Kararigas G. Sex-biased vulnerability of the heart to COVID-19. Mayo Clin Proc. 2020;95(11):2332–2335.
- Ji HL, Zhao R, Matalon S, et al. Elevated plasmin(ogen) as a common risk factor for COVID-19 susceptibility. Physiol Rev. 2020;100(3):1065–1075.
- Al-Lami RA, Urban RJ, Volpi E, et al. Sex hormones and novel corona virus infectious disease (COVID-19). Mayo Clin Proc. 2020;95(8):1710–1714.
- Apicella M, Campopiano MC, Mantuano M, et al. COVID-19 in people with diabetes: understanding the reasons for worse outcomes. Lancet Diabetes Endocrinol. 2020;8(9):782–792.