Abstract
Background
The coronavirus disease 2019 pandemic makes proper resource allocation and prioritisation important. Frailty increases the risk of adverse outcomes and can be quantified using the Clinical frailty scale. The aim of this study was to determine the role of the Clinical frailty scale, in patients ≥65 years of age with coronavirus disease 2019, as a risk factor either for critical coronavirus disease 2019 measured as intensive care unit admission or death or as a risk factor for death.
Methods
This was a retrospective observational study on patients ≥65 years hospitalised with coronavirus disease 2019 verified by polymerase chain reaction between 5 March 5 and 5 July 2020. The association between Clinical frailty scale and the composite primary outcome intensive care unit admission or death within 30 days post hospitalisation and the secondary outcome death within 30 days post hospitalisation was analysed using multivariable logistic regression models adjusting for gender, age, body mass index, hypertension, and diabetes. Clinical frailty scale was used as a categorical variable (fit score 1–4, frail score 5–6, and severely frail score 7–9).
Results
In total, 169 patients were included (47.3% women, mean age 79.2 ± 7.8 years). In the fully adjusted model, adjusted odds ratio for intensive care unit admission or death was 1.84 (95%-confidence interval 0.67–5.03, p = .234) for frail and 6.08 (1.70–21.81, p = .006) for severely frail compared to fit patients. For death, adjusted odds ratio was 2.81 (0.89–8.88, p = .079) for frail and 9.82 (2.53–38.10, p = .001) for severely frail compared to fit patients.
Conclusions
A high Clinical frailty scale score was an independent risk factor for the composite outcome intensive care unit admission or death and for the secondary outcome death.
Introduction
The first wave of the coronavirus disease 2019 (COVID-19) pandemic hit Sweden in the end of March 2020. The health care system faced a situation with impending lack of resources, especially regarding intensive care unit (ICU) beds, and the need for an adequate triage system became evident. There were no national guidelines in Sweden at the time-* regarding triaging of patients with COVID-19, as was the case in some other countries [Citation1,Citation2]. Age and comorbidities were identified as risk factors for mortality early in the pandemic [Citation3]. Guidelines in some countries recommended the use of the Clinical Frailty Scale (CFS) for triaging patients with COVID-19, for example the United Kingdom (NICE guidelines), France, Canada, and Belgium [Citation4–7].
Frailty is a multidimensional syndrome characterised by loss of reserves (in terms of energy, physical ability, cognition, and health) which results in vulnerability. For the purpose of triage the CFS is increasingly used to measure frailty. It is used as an evaluation tool to predict mortality and need for entry into an institutional facility [Citation8–10]. Studies on geriatric patients in intensive care [Citation11–14], patients hospitalised after trauma [Citation15], or following myocardial infarction [Citation16] show that a high CFS score is an independent risk factor for mortality. The CFS predicts outcomes more effectively than other commonly used ICU illness scores in elderly patients [Citation17]. While some studies have shown frailty to be a useful concept in younger patients [Citation18,Citation19], CFS has mainly been validated in patients ≥65 years of age [Citation8,Citation9]. During the first wave of the pandemic, the hospitals in Gävleborg County documented CFS scores as part of clinical routine.
In critically ill patients, the basis for decision making regarding ICU admission or treatment restrictions is often complex. Perceived frailty is a factor in this decision. Frailer individuals, while at increased risk for critical illness, might be less likely to be admitted to the ICU, especially in a setting of relative resource scarcity (such as the COVID-19 pandemic). The association between frailty and mortality thus may depend on factors relating to biological processes as well as resource allocation. Therefore, mortality as the sole measure of outcome may overestimate CFS score as a risk factor for critical illness. A composite outcome of need for ICU admission or death could be used to evaluate CFS score as a risk factor for critical illness.
Materials and methods
Aim
The aim of this study was to assess the CFS score as a risk factor for either critical illness, measured as ICU admission or death, or death in patients above 65 years of age with COVID-19.
Study design and setting
This was a retrospective observational cohort study conducted as a substudy of the Gävleborg COVID-19 cohort study. No interventions or examinations other than those routinely performed were done for the purpose of the study. Patients ≥65 years admitted to three hospitals (Gävle, Hudiksvall, and Bollnäs) in Gävleborg County (285,000 inhabitants) due to polymerase chain reaction (PCR) verified COVID-19, from 5 March 5 to 5 July 2020, were included. Patients who were screened positive for COVID-19, hospitalised due to other medical conditions were not eligible for inclusion. Patients were included only once. In cases with multiple ICU admissions, only the primary admission was included.
Patients were informed about the study when hospitalised, or contacted by phone after discharge. Information about the study and the consent form were translated from Swedish to English, Arabic, Somali and Tigrinya. If a patient did not speak one of those five languages, a translator or family member assisted with translation. Oral and written consent was received from all patients directly, or from relatives to those with severe cognitive impairment and to those who were deceased.
Data collection
Data on age, gender, smoking habits, living conditions (assisted/non assisted), treatment restrictions, comorbidities, symptoms, and status at admission were collected either directly at admission or retrospectively from electronic medical records. Premorbid frailty was assessed by the admitting or ward physician using the CFS.
The CFS is a 9-point assessment-based frailty tool that evaluates specific domains including comorbidity, function and cognition to generate a frailty score ranging from 1 (very fit) to 9 (terminally ill) [Citation9]. It has been validated in several languages including Swedish [Citation16]. The CFS is inter-rater reliable and can be scored retrospectively [Citation20,Citation21].
The information needed to calculate the score was obtained from the patient or family members. Physicians were trained in the use of the CFS at the beginning of the pandemic. If the CFS score was missing, it was evaluated by a physician or research assistant from the research group, and was based on information in the medical record. Data on ICU admission and decisions about treatment restrictions (i.e. do not resuscitate and decisions not to admit a patient to the ICU even if deteriorating) was gathered from the medical records.
In Sweden, medical records are linked to national registries, and information about mortality was obtained from the Swedish cause of death register for those who died within 30 days of hospital admission.
Outcome
The primary outcome was critical illness defined as the composite variable ICU admission or death within 30 days after hospital admission. The secondary outcome was death within 30 days after hospital admission.
Statistical analyses
Normally distributed continuous variables are expressed as mean ± standard deviation and skewed variables are expressed as median with range or interquartile range. Categorical variables are presented as frequencies and percentages. Differences between groups were analysed using chi-2 test for categorical variables and Student’s t-test or ANOVA-analysis for continuous variables. The ordinal scale CFS was used as a categorical, trichotomized variable: fit (score 1–4), frail (score 5–6), and severely frail (score 7–9) [Citation22]. The associations between CFS score and the primary and secondary outcomes were assessed using crude and multivariable logistic regression models. Odds ratios were adjusted (aOR) for age, gender, body mass index (BMI) and comorbidities (hypertension and diabetes) and are shown with 95% confidence intervals (CI). The choice of covariables was based on subject matter knowledge. The Kaplan-Meier survival estimate was used to visualise the primary and secondary outcomes in the trichotomized CFS categories. A p-value <.05 was considered to indicate statistical significance. Statistical analyses were conducted using the software packages Stata, version 16.0 (StataCorp LP; College Station, TX). A power calculation on the minimal sample size was not done, since the aim was to include as many patients as possible during the first wave of COVID-19. No values were imputed.
Ethical approval and consent to participate
The study protocol was approved by the Swedish Ethical Review Authority, Dnr 2020-01746. All participants who were alive and able gave written informed consent. For patients who had died or who had severe cognitive deficits, written informed consent was given by relatives.
Results
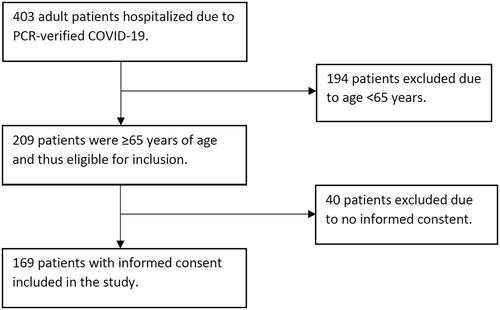
During the study period, 403 patients were hospitalised due to PCR-verified COVID-19. Of these, 209 were ≥65 years of age and thus eligible for inclusion and 169 gave informed consent (47.3% women, mean age 79.2 ± 7.8 years, ). Nineteen patients (11.4%) were nursing home residents and 152 (89.9%) were born in Sweden. The number of participants classified as fit, frail, and severely frail was 81 (48.5%), 57 (34.1%), and 29 (17.4%), respectively. CFS score was not possible to calculate from medical records in 2 patients, and they were thus not included in the regression analysis. Baseline characteristics are presented in and Citation2.
Table 1. Baseline characteristics.
Table 2. Co-morbidities and clinical features at admission.
Fifty patients died within 30 days after hospital admission due to COVID-19, 20 were admitted to the ICU and 63 either died or were admitted to the ICU within 30 days post admission. Those who were admitted to the ICU or died were of similar age (79.8 ± 7.4 vs. 78.9 ± 8.0 years, p = .490), and similar BMI (28.1 ± 5.4 vs 27.2 ± 5.5, p = .385), but more often had treatment restrictions (64.5% vs 45.7%, p = .019) than those who were not admitted to ICU and who survived ().
In the fully adjusted models, high CFS score was an independent risk factor for admission to the ICU or death (). For the composite outcome ICU admission or death, aOR was 1.84 (95% CI 0.67–5.03, p = .234) for frail and 6.08 (1.70–21.81, p = .006) for severely frail, when compared to fit patients.
Table 3. Logistic regression analyses of the associations between CFS categorised as fit (CFS 1–4), frail (CFS 5–6), and severely frail (CFS 7–9) and the composite outcome ICU admission or death or the secondary outcome death.
High CFS score was an independent risk factor for the secondary outcome, aOR for death within 30 days was 2.81 (0.89–8.88, p = .079) for frail and 9.82 (2.53–38.10, p = .001) for severely frail compared to fit patients.
Female sex, independent of other risk factors, significantly reduced the risk for ICU admission or death, (aOR 0.36, 0.15–0.84, p = .018) but not for death alone (aOR 0.60, 0.23–1.52, p = .282). Age in 10 years intervals was not an independent risk factor for the composite outcome, (aOR 0.87, 0.48–1.59, p = .658) nor for death (aOR 1.41, 0.71–2.77, p = .325).
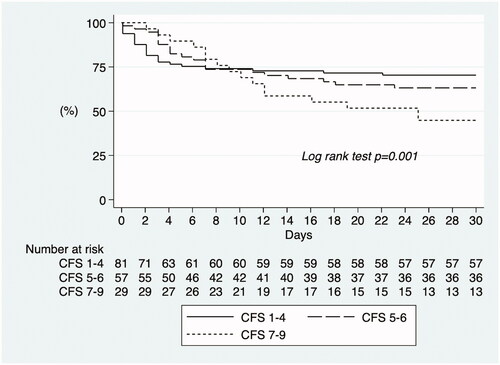
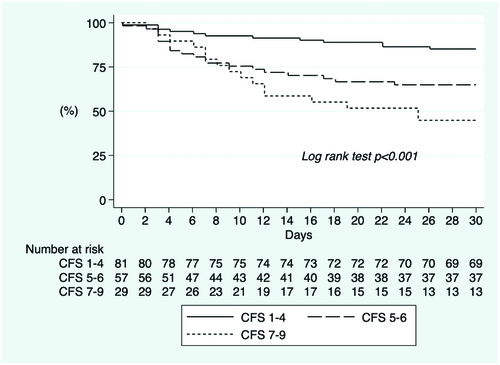
Kaplan-Meier survival estimate for the composite outcome ICU admission or death with frailty as a trichotomized variable showed that in the first days patients who were fit were more likely to reach the outcome. However, from day 9 until the end of the follow-up period of 30 days, those who were more frail were more likely to reach the composite outcome (Log rank test p < .001, ). The cumulative incidence of the composite outcome ICU admission or death was 29.6%, 36.8%, and 55.2% in patients who were fit, frail, and severely frail, respectively. Kaplan-Meier survival estimate for the secondary outcome death, with frailty as a trichotomized variable, showed that more frail patients were more likely to have died within 30 days (Log rank test p < .001, ). The cumulative incidence of death after 30 days was 14.8%, 35.1%, and 55.2% in patients who were fit, frail, and severely frail, respectively.
Discussion
The main finding of this study was that high CFS score was an independent risk factor for critical illness in patients above 65 years of age and hospitalised with COVID-19, as measured by the composite outcome ICU admission or death within 30 days. High CFS score was, in addition, an independent risk factor for the secondary outcome death within 30 days.
These findings are in line with prior studies that have shown frailty to be associated with mortality in patients with COVID-19. In a British study of 677 inpatients aged ≥65 years, CFS score 7–9 was associated with increased mortality compared with CFS score 1–3 [Citation23]. In a Swedish study of 250 geriatric patients, CFS score >5 was associated with increased in-hospital mortality [Citation24]. In the European multicentre COPE study (n = 1564), CFS score 7–9 was associated with increased 7-day mortality when compared to CFS score 1–2 [Citation25]. In the international multicentre COMET study (n = 2434), patients aged ≥65 years who had CFS scores 6–9 or 4–5 had higher in-hospital mortality than those with score 1–3 [Citation26]. In patients aged <65 years, mortality was higher in those who had CFS score 6–9 but not in those with CFS score 4-5 [Citation26]. While CFS measures frailty as a multidimensional syndrome of ageing in the elderly, high scores in younger patients likely reflect other mechanisms and thus do not necessarily reflect the risk for critical illness or the prognosis in the same way. Also, while patients <65 years of age were more likely to be admitted to the ICU with increasing frailty, frail patients aged ≥65 years (CFS score 6–9) were not more likely to be admitted, and those who were mildly frail (CFS score 4–5) were less likely to be admitted than those who were fit (CFS score 1–3) [Citation26].
The findings of the COMET study indicate that while frail patients with COVID-19 are at increased risk of death they are not more likely, and sometimes less likely, to be admitted to the ICU. High mortality in this patient population might thus be due to both biological processes relating to frailty itself and other factors such as perceived poor prognosis or scarcity of resources resulting in patients being less likely to be admitted to an ICU. A strength of our study is that the composite outcome ICU admission or death allows for estimation of the risk of critical illness which is not dependent on local clinical practice for ICU admission. However, local clinical practice might affect the patient population admitted to hospital. This might explain the low proportion of patients (11.4%) who were residents at a nursing home. The proportion of the most elderly and frail, who were cared for in nursing homes, as opposed to in a hospital, might vary between different countries, hospitals, and over time, which might affect the generalisability of our study. An additional limitation is the relatively low number of study participants, resulting in low statistical power and risk for type II errors.
Finally, our study was not designed to answer questions regarding the CFS as a predictor of outcomes in patients with COVID-19 admitted to an ICU. Frailty is a well-known risk factor for mortality in general in ICU patients [Citation14]. Future studies are needed to evaluate CFS as a risk factor for death or disability in COVID-19 patients admitted to an ICU.
Conclusions
A high CFS score was an independent risk factor for the composite outcome ICU admission or death within 30 days post hospitalisation, and the secondary outcome death within 30 days post hospitalisation, in patients above 65 years of age hospitalised due to COVID-19.
Author contributions
GM: first draft, writing, study design, data collection, statistical analysis, interpretation of data; MGL: writing, study design, data collection, interpretation of data; RR: writing, study design, data collection, interpretation of data; MF: writing, interpretation of data; FP: critical revision, interpretation of data; AB: critical revision, interpretation of data; CE: critical revision, interpretation of data, project management; AP: writing, study design, data collection, statistical analysis, interpretation of data, project management. All authors read and approved the final manuscript.
| Abbreviations | ||
| aOR | = | adjusted odds ratio |
| CI | = | confidence interval |
| COVID-19 | = | coronavirus disease 2019 |
| CFS | = | clinical frailty scale |
| ICU | = | intensive care unit |
| PCR | = | polymerase chain reaction |
Acknowledgements
We would like to thank research assistant Ingrid Olson and research nurse Lise-Lotte Sundgren for their help with data collection. We would also like to thank the staff and doctors at the COVID-19 wards in Gävleborg county for making the study possible.
Disclosure statement
GM has received consulting fees from Alnylam, MSD, and Internetmedicin. MGL, RR, MF, FP, AB, CE, and AP declare no competing interests.
Data availability statement
The datasets used and analysed during the current study might be available from the corresponding author on reasonable request.
Additional information
Funding
References
- Verweij M, van de Vathorst S, Schermer M, et al. Ethical advice for an intensive care triage protocol in the COVID-19 pandemic: lessons learned from The Netherlands. Public Health Ethics. 2020;13(2):157–165.
- Antommaria AHM, Gibb TS, McGuire AL, et al. Ventilator triage policies during the COVID-19 pandemic at U.S. Hospitals associated with members of the association of bioethics program directors. Ann Intern Med. 2020;173(3):188–194.
- Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. The Lancet. 2020;395(10229):1054–1062.
- Meyfroidt G, Vlieghe E, Biston P, et al. Ethical principles concerning proportionality of critical care during the 2020 COVID-19 pandemic in Belgium: advice by the Belgian Society of Intensive care medicine. [cited 2020 Mar 26]. Available from: https://www.siz.be/wp-content/uploads/COVID_19_ethical_E_rev3.pdf.
- Downar J. Clinical triage protocol for major surge in COVID pandemic. 2020. Available from: https://med.uottawa.ca/pathology/sites/med.uottawa.ca.pathology/files/clinical_triage_protocol_for_major_surge_in_covid_pandemic_-_march_28_20205.pdf.
- Azoulay É, Beloucif S, Guidet B, et al. Admission decisions to intensive care units in the context of the major COVID-19 outbreak: local guidance from the COVID-19 paris-region area. Crit Care. 2020;24(1):293.
- NICE. COVID-19 rapid guideline: critical care in adults NICE guideline [NG159] 2020. Available from: https://www.nice.org.uk/guidance/ng159.
- Rockwood K, Song X, MacKnight C, et al. A global clinical measure of fitness and frailty in elderly people. CMAJ. 2005;173(5):489–495.
- Pulok MH, Theou O, van der Valk AM, et al. The role of illness acuity on the association between frailty and mortality in emergency department patients referred to internal medicine. Age Ageing. 2020;49(6):1071–1079.
- Clegg A, Young J, Iliffe S, et al. Frailty in elderly people. Lancet. 2013;381(9868):752–762.
- Muessig JM, Nia AM, Masyuk M, et al. Clinical frailty scale (CFS) reliably stratifies octogenarians in german ICUs: a multicentre prospective cohort study. BMC Geriatr. 2018;18(1):162.
- Ferrante LE, Pisani MA, Murphy TE, et al. The association of frailty with Post-ICU disability, nursing home admission, and Mortality: A Longitudinal Study. Chest. 2018;153(6):1378–1386.
- Bagshaw SM, Stelfox HT, McDermid RC, et al. Association between frailty and short- and long-term outcomes among critically ill patients: a multicentre prospective cohort study. CMAJ. 2014;186(2):E95–102.
- De Geer L, Fredrikson M, Tibblin AO. Frailty predicts 30-day mortality in intensive care patients: a prospective prediction study. Eur J Anaesthesiol. 2020;37(11):1058–1065.
- Curtis E, Romanowski K, Sen S, et al. Frailty score on admission predicts mortality and discharge disposition in elderly trauma patients over the age of 65 y. J Surg Res. 2018;230:13–19.
- Ekerstad N, Pettersson S, Alexander K, et al. Frailty as an instrument for evaluation of elderly patients with non-ST-segment elevation myocardial infarction: a follow-up after more than 5 years. Eur J Prev Cardiol. 2018;25(17):1813–1821.
- Le Maguet P, Roquilly A, Lasocki S, et al. Prevalence and impact of frailty on mortality in elderly ICU patients: a prospective, multicenter, observational study. Intensive Care Med. 2014;40(5):674–682.
- Hewitt J, Carter B, McCarthy K, et al. Frailty predicts mortality in all emergency surgical admissions regardless of age. An observational study. Age Ageing. 2019;48(3):388–394.
- Smart R, Carter B, McGovern J, et al. Frailty exists in younger adults admitted as surgical emergency leading to adverse outcomes. J Frailty Aging. 2017;6(4):1– 23.
- Özsürekci C, Balcı C, Kızılarslanoğlu MC, et al. An important problem in an aging country: identifying the frailty via 9 Point Clinical Frailty Scale. Acta Clin Belg. 2020;75(3):200–204.
- Davies J, Whitlock J, Gutmanis I, et al. Inter-rater reliability of the retrospectively assigned clinical frailty scale score in a geriatric outreach population. Can Geriatr J. 2018;21(1):1–5.
- Church S, Rogers E, Rockwood K, et al. A scoping review of the Clinical Frailty Scale. BMC Geriatr. 2020;20(1):393.
- Aw D, Woodrow L, Ogliari G, et al. Association of frailty with mortality in older inpatients with Covid-19: a cohort study. Age Ageing. 2020;49(6):915–922.
- Hägg S, Jylhävä J, Wang Y, et al. Age, frailty, and comorbidity as prognostic factors for short-term outcomes in patients with coronavirus disease 2019 in geriatric care. J Am Med Dir Assoc. 2020;21(11):1555–1559.e2.
- Hewitt J, Carter B, Vilches-Moraga A, et al. The effect of frailty on survival in patients with COVID-19 (COPE): a multicentre, European, observational cohort study. Lancet Public Health. 2020;5(8):e444–e451.
- Sablerolles RSG, Lafeber M, van Kempen JAL, et al. Association between Clinical Frailty Scale score and hospital mortality in adult patients with COVID-19 (COMET): an international, multicentre, retrospective, observational cohort study. Lancet Healthy Longevity. 2021;2(3):e163–e70.