Abstract
Background
Men who have sex with men (MSM) are more vulnerable to acquiring sexually transmitted infections (STIs). In 2019, for instance, 74% of European Neisseria gonorrhoeae (Ng) cases among males affected MSM. A recent report by the World Health Organization showed that most of the 2020’ interim targets to end STIs by 2030 had not been met. A broadened understanding of STI transmission networks could guide future elimination strategies and reduce the STI burden. Therefore, we used whole-genome sequencing (WGS) to determine Ng-clusters and assess sexual mixing.
Methods
WGS was performed on Ng-isolates collected at the Medical University of Vienna, Austria and was used for core genome multi-locus sequencing typing cluster analysis. Epidemiologic and infection-specific details were extracted from medical records.
Results
Genomic analysis and demographic data were available for 415 isolates, and 43.9% (182/415) were allocated to 31 Ng-clusters. Nine clusters comprised samples from heterosexual individuals only (women N = 4, human immunodeficiency virus (HIV)-negative men N = 49, HIV-positive man N = 1), nine clusters included MSM only (HIV-negative N = 22, HIV-positive N = 13) and 13 clusters included both heterosexuals and MSM (HIV-negative N = 75, HIV-positive N = 18). Current use of HIV pre-exposure prophylaxis (PrEP) was reported by 22.8% of MSM. In multivariate analysis, only ‘MSM’ predicted clustering with isolates from HIV-positive individuals (adjusted odds ratio 10.24 (95% CI 5.02–20.90)).
Conclusions
Sexual mixing of HIV-positive, HIV-negative MSM and non-MSM was frequently observed. Furthermore, HIV-serodiscordant clustering highlights the importance of PrEP rollout to avert HIV transmission. Our findings can inform future STI prevention strategies and continuous surveillance efforts are required to keep up with transmission dynamics.
Introduction
In 2016, the World Health Organization (WHO) set ambitious goals to reduce the global burden of epidemics by 2030 [Citation1]. Calculated projections included in an interim report published by the WHO in 2022 have shown that some progress has been made in the field of human immunodeficiency virus (HIV) and viral hepatitis. Still, the incidence of sexually transmitted infections (STIs) is stagnating at a high level, and a 90% reduction of gonorrhea and syphilis by 2030 seems currently out of reach [Citation2]. Men who have sex with men (MSM) are over-proportionally affected by HIV and STIs in high-income countries [Citation3]. A recent report from the European Centre for Disease Prevention and Control (ECDC) showed that in 2019, 54% of all 117,881 confirmed Neisseria gonorrhoeae (Ng) infections observed in 28 European countries affected MSM [Citation4]. Notably, the steady increase in Ng diagnoses over the last decade is primarily due to infections affecting MSM [Citation5]. Similar trends in transmission have been described by the Centre for Disease Prevention and Control (CDC) in the United States [Citation6]. Some increase in STI prevalence among MSM is due to the expansion of STI screening associated with the introduction of HIV pre-exposure prophylaxis (PrEP) [Citation7,Citation8]. Nevertheless, factors such as condomless anal intercourse and higher number of sexual partners [Citation7], commonly associated with international travel, facilitate frequent disease outbreaks among MSM. Recent examples include viral hepatitis [Citation9], Mpox [Citation10] as well as the still ongoing HIV epidemic [Citation11]. In addition, the burden of infectious diseases leads to repeated use of antibiotics and is linked to several reports of increased resistance/reduced susceptibility of some pathogens among MSM – examples include azithromycin for mycoplasma genitalium [Citation12] and Ng [Citation13], tetracycline for syphilis [Citation14] or multi-drug resistant Shigella [Citation15]. Previous data primarily describe the association between specific behavioral patterns and the incidence of STIs, which is used to identify ‘vulnerable populations’ [Citation16]. However, information on associated onward transmission and transmission networks is scarce. Yet, a better understanding of sexual mixing within the population (especially involving ‘MSM’ due to the high burden of STIs), including the interaction and potential onward transmission of STIs between ‘MSM’ and ‘non-MSM’, is crucial for targeted interventions. Therefore, to optimize future prevention strategies, we aimed to investigate patterns of sexual mixing based on genomic cluster analysis of Ng-isolates and to identify factors linked to possible transmission chains.
Methods
Study design and population
All positive cultures of Ng obtained from individuals attending the outpatient clinic at the Vienna General Hospital between January 2013 and March 2020 were collected and archived at −80 °C. The Vienna General Hospital works in conjunction with the Medical University of Vienna and is Austria’s largest tertiary care facility. Notably, it is also one of the few providers of HIV − and PrEP-services in Austria and open for STI-testing without a referral. All samples were obtained in the context of STI-testing in symptomatic patients (e.g. urethral or vaginal discharge) or following a positive nucleic acid amplification test (NAAT) performed as part of an STI-screening (e.g. individuals using PrEP). Urethral-, pharyngeal-, anal- and cervical samples were obtained.
In this retrospective cross-sectional study, we performed whole genome sequencing on all archived isolates to enable a genotypic cluster analysis. In addition, details on the clinical course and transmission types for each Ng case were extracted from the respective medical records. Subsequently, isolates/Ng cases were analyzed in terms of the genotypic clusters and transmission types to investigate sexual mixing within the study population. Moreover, factors associated with HIV-serodiscordant clustering were investigated.
In a previous study [Citation17], we reported on the phenotypic and genotypic antimicrobial resistance profiles of the isolates included in this work. Additionally, a subset of the samples was included in another study investigating genotypic resistance in Austria; accession numbers can be found here [Citation18]. The current study focuses exclusively on the analysis of sexual mixing as indicated by genotypic clustering and the corresponding transmission pattern.
Definitions
While an individual was defined as a single person, an ‘episode’ was defined as one Ng-isolate obtained from an individual. If more than one isolate was collected from a single individual at a given time (i.e. multiple sites were infected), this was considered a single episode (we did not find genotypically different Ng-isolates within an individual during a single episode). In case more than one isolate was collected from a single individual at different time points – with available negative tests of the affected location in between, it was considered multiple episodes. Therefore, the total number of episodes may comprise more than one isolate obtained from a single individual.
Details on sexual practices, transmission types and PrEP use are routinely requested and documented during a standard work-up in our STI-/HIV-outpatient clinic and thus, were readily available. Of note, ‘non-MSM’ comprises all men not identifying as MSM but also women. HIV and syphilis status were determined either by information from the medical history and/or by available serology. Information on the number of previous gonorrhoea episodes was determined based on previous Ng test results. In our clinic, an Ng-NAAT is primarily used for STI-screening instead of a culture. An Ng-culture is performed following a positive NAAT result and before treatment is administered. This procedural difference in sampling with NAAT versus culture explains the discrepancy between Ng-episodes with available genotypic/cluster information (primary objective – based on culture) and other previous Ng-episodes/multiple sites (covariate – based on NAAT).
Cluster identification
Details on DNA extraction and whole genome sequencing of the isolates as well as molecular work-up were performed as previously reported [Citation17]. Clusters were determined by Core-Genome-MLST (cgMLST) analysis using Seqshpere + version 8.5.1 (Ridom GmbH, Münster, Germany) based on PubMLST schemes as described by Harrison et al. using 1649 loci. Currently, no standardized method has been established to define sexual mixing based on genomic cluster analysis. However, it has been shown that Ng transmission networks can be determined reliably by cgMLST using only a low locus difference threshold (5 or fewer) [Citation19]. We thus deployed a cgMLST analysis to define clusters by a conservatively chosen allelic difference threshold of 12. In Austria, we do not have a mandatory reporting system or systematic surveillance structure and thus, we needed to acknowledge and account for missing links in the transmission chains. Notably, the genomic cluster analysis can only indicate a shared source of infection – which we consider as sexual mixing. N. gonorrhoeae whole genome sequencing assemblies were submitted to ENA-project PRJEB70864.
Data- and cluster analysis
GraphPad Prism 9.1.0 (GraphPad Software, San Diego, CA) and IBM SPSS Statistics 26 (SPSS Inc., Chicago, IL) were used to perform the statistical analysis. Individuals within clusters were stratified by HIV-status and transmission risk or sexual orientation group (women, men who have sex with women only, MSM). Continuous variables were reported as median (interquartile range) and we applied the Mann–Whitney U-test for group comparison. Nominal parameters were described as the proportion and number of individuals with or without a specific characteristic. We deployed the Chi-squared test and Fisher’s exact test to analyze differences in nominal parameters. Binary logistic regression models were calculated to further investigate the probability of clustering with isolates of HIV-positive individuals. p Values below or equal to .05 were considered statistically significant.
Ethics
The present study complies with the ethical standards of the 1964 Declaration of Helsinki and its subsequent amendments. This study was approved by the Ethics Committee of the Medical University of Vienna (EK number 1214/2019). As details on the clinical course and transmission patterns were collected retrospectively, informed consent was waived.
Results
Between January 2013 and March 2020, genomic data and clinical characteristics were available for 94.3% (415/440) of all collected isolates plus corresponding episodes, of which 43.9% (182/415) could be allocated to a cluster, whereas 56.1% (233/415) did not meet the criteria for clustering. The vast majority of all samples were collected from males (95.7% (397/415)), 11.3% (47/415) were taking PrEP and while 13.5% (56/415) had no information on HIV status, 21.7% (78/359) of those with available information were people living with HIV (PLWH). When comparing the infection characteristics of Ng-episodes from MSM (49.6% (206/415)) and non-MSM (50.4% (209/415)), anal (39.3% (81/206)) and multiple sites (34% (40/206)) infections were frequently observed in MSM, whereas non-MSM usually had symptomatic urethritis (93.3% (195/209)) (). This was also reflected in the reported sex practices: of those with available information, 99% (196/198) of MSM reported having anal sex, compared to 25% (20/80) of all non-MSM. Overall, concomitant infections with chlamydia were common (17.8% (64/359)), as were previous episodes of gonorrhea (28.7% (119/415)) or syphilis (23.1% (96/415)). However, previous infections with N. gonorrhoeae or T. pallidum were much more prevalent in MSM (41.8% (86/206) and 41.8% (86/206), respectively) than in non-MSM (15.8% (33/209) and 4.8% (10/209), respectively).
Table 1. Characteristics and comparison of all Ng episodes among non-MSM and MSM included in the dataset irrespective of genotypic clustering.
Cluster analysis
One hundred and eighty-two isolates were allocated to 31 different clusters. The cluster size ranged between 2 and 15 isolates (Table S3). All infection-specific characteristics were evenly distributed among those being within or outside a genotypic cluster (Table S1). Overall, there was a mixed picture between the clusters, including several combinations of men who have sex with women only (MSW) and women and MSM (). In addition, we identified 15 clusters that contained at least one sample from PLWH, while only one cluster that comprised 100% (6/6) isolates of PLWH – all MSM – was detected (). Seventy-six isolates clustered exclusively with samples from HIV-negative individuals. Non-MSM clusters were more common during the early observational period, whereas MSM-exclusive clusters and clusters comprising all transmission types occurred predominantly after 2016. Twelve individuals (nine MSM, three MSW) contributed two NG isolates, whereas one individual (one MSM) was infected three times with a clustering NG isolate. Only in two instances both isolates of a single individual were allocated to the same cluster, and in both cases, samples were obtained less than three months apart, suggesting reinfection at the same source the primary infection had occurred.
Figure 1. Genotypic clustering by transmission types and year of occurrence.
We identified 31 different clusters. In the upper part, each line depicts a single cluster starting and ending at the year the first and last isolates of the respective cluster had been detected. The gray scale indicates the mixing regarding HIV status, whereas the dotted lines group the clusters by transmission types (corresponding to ). The lower chart shows the isolates per year. Of note, 2020 included only the first quarter (i.e. 3 months). HIV: human immunodeficiency virus; MSM: men who have sex with men; MSW: men who have sex with women; PrEP: HIV pre-exposure prophylaxis.
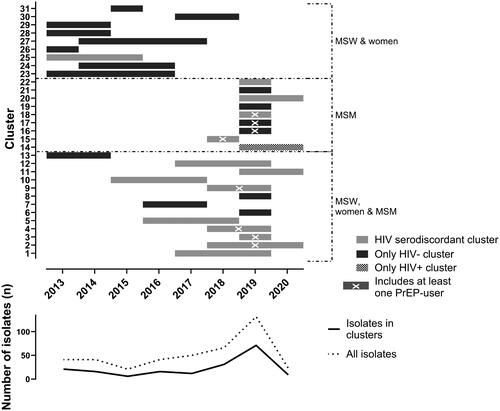
Table 2. Composition of clusters by transmission-groups.
The odds ratio (OR) for not being allocated to an all-HIV-negative cluster and thus for clustering with isolates from HIV-positive individuals were, in univariate analysis, 3.97 (95% CI 1.73–9.13) for age >24 years, 10.24 (95% CI 5.02–20.89) for MSM, 3.30 (95% CI 1.18–9.24) for the use of PrEP, 5.51 (95% CI 2.30–13.19) for being infected at multiple sites and 4.19 (95% CI 2.16–8.12) for ‘history of any STI’, respectively (). After adjusting for other co-variates, the only independent factor for clustering with isolates from HIV-positive individuals was ‘MSM’ with an OR of 10.24 (5.02–20.90). Since this analysis also included isolates from PLWH clustering with other isolates from PLWH, we wanted to rule out the possibility that the observed impact of ‘MSM’ on clustering with PLWH was solely due to ‘serosorting’ among MSM. Therefore, we performed a subgroup analysis (Table S2) including only isolates of HIV-negative individuals. The OR of clustering with a group that included at least one sample from PLWH was calculated, and again, the only independent statistically significant predictor for clustering with PLWH was ‘MSM’ with an OR of 7.02 (3.13–15.73).
Table 3. Risk to cluster with isolates from HIV-positive individuals.
Discussion
In this study, we detected 31 clusters among 415 NG isolates using core-genome MLST analysis following whole-genome sequencing. The composition of transmission types within the clusters was quite heterogeneous. It included combinations of MSW and women, MSW and women and MSM as well as HIV-positive and HIV-negative MSM, ultimately indicating sexual mixing independent of the conventional ‘risk group’ allocation. Importantly, ‘MSM’ was identified as an independent predictor of clustering with isolates of PLWH, suggesting transmission of Ng between HIV-serodiscordant MSM.
To our knowledge, only three studies have investigated sexual networks based on whole genome sequence analyses of Ng. The first study was published in 2017 by Peters et al. and included 100 Ng isolates from 83 patients in the UK; 14 clusters were identified. The vast majority of these patients were MSM, but several clusters included both HIV-positive and -negative individuals [Citation20]. Another UK study was published in 2020 by Town et al. and included results from 1277 Ng isolates corresponding to 213 clusters. Again, sexual mixing was observed in different ‘risk populations’ [Citation21]. The third study was conducted in Australia, published in 2019 by Williamson et al. and included 2186 Ng isolates. The authors pointed out that there was no clear separation between isolates from MSM and other transmission types and, in particular, MSM and women were distributed across several clusters, potentially linking the different populations [Citation22]. In all three studies and our study, the vast majority of Ng isolates were collected from men – with a substantial proportion being MSM. In addition, we observed a mixed picture of ‘MSM’ and ‘non-MSM’ clusters. We want to point out that Austria is a high-income country like Australia or the UK; nonetheless, the rollout of PrEP started later and PrEP is still not fully reimbursed [Citation8]. Furthermore, both Australia and the UK have recently reported decreasing numbers of hepatitis C among MSM [Citation23,Citation24], while an opposite trend has been observed in Central European countries like Germany [Citation25] or Austria [Citation26]. These substantial differences in STI care and epidemiologic trends in Austria highlight the importance of investigating the generalizability of the UK and Australian data on sexual mixing to other geographical regions. Our work bridges this gap and has important implications for future prevention strategies: our data suggest sexual mixing between MSM and non-MSM, it is reasonable to assume that interventions targeting MSM will also reduce the disease burden and, ultimately, the STI incidence among non-MSM. Men who have sex with men are a well-defined population with a strong peer network and thus, relatively easy to target with STI-specific harm reduction [Citation27]. Presumably, future interventions (e.g. information, screening) targeting MSM may not only benefit MSM but also indirectly other populations.
Another important finding of our study is the sexual mixing of HIV-serodiscordant MSM. We identified ‘MSM’ as a significant predictor of clustering with PLWH. This is of particular interest in the context of Ng because – as an STI – it is a marker for condomless intercourse and thus a risk factor for HIV transmission [Citation28]. We also know from previous studies that the presence of an STI promotes HIV transmission through mucosal damage and an increase in CD4+-cells in the inflamed tissue [Citation11,Citation29]. Nevertheless, 95% of all PLWH in Austria are on antiretroviral therapy (ART) [Citation30] so HIV transmission is very unlikely [Citation31]. This is also reflected in the recent decline in Austrian HIV incidence: in 2021, only 310 (∼3.46 per 100,000) new HIV infections were reported in Austria [Citation30]. Furthermore, HIV-negative individuals at increased risk for HIV infection can use PrEP for effective HIV prevention [Citation32,Citation33], and although PrEP has only been available in Austria since 2017, the number of PrEP users is slowly – but steadily – increasing [Citation8]. Eleven percent of the Ng isolates included in this study were collected from PrEP-users. Therefore, in the context of PrEP and the widespread availability of ART (‘treatment as prevention’), the mutual Ng clustering of HIV-serodiscordant individuals suggests that Ng transmission could occur without also transmitting HIV. Until a decade ago, frequent engagement in transmission-prone sex practices, which could also cause Ng infections, would frequently lead to HIV transmission, particularly among MSM [Citation34]. Today, modern HIV prevention measures (i.e. PrEP and ART) appear to be effective enough to ‘keep’ a substantial proportion of individuals in an HIV-negative status while Ng is still transmitted [Citation35]. Consequently, HIV status – historically considered an important ‘risk factor’ for STIs – is nowadays becoming less relevant as a predictor of STI incidence. PrEP use and a history of STIs should instead be taken into account when determining the population ‘at high risk’ for acquiring STIs. One drawback of consecutive PrEP initiation is the rising number of detected STIs among PrEP-users [Citation3]. While the term ‘risk compensation’ (i.e. disproportionate engagement in sex practices associated with STI/HIV transmission due to reduced risk for HIV acquisition under PrEP) was initially used to explain the residual HIV transmission in early PrEP trials [Citation32,Citation33], it is now widely considered as an explanation for the surge of STIs among PrEP-users [Citation36]. Notably, current guidelines recommend frequent STI screening among PrEP-users [Citation6,Citation37], and any expansion of testing will almost inevitably lead to an increase in positive test results. However, the increase in STI screening highlights another fact: many STIs – especially extragenital infections – are asymptomatic. In our study, 43% of Ng cases in MSM were asymptomatic, corresponding to the proportion of extragenital infections in this group. In a recent cross-sectional study from Austria, 40% of PrEP-users were found to have at least one STI, but only 10% of those were symptomatic [Citation8].
Numerous other studies have also found a high number of asymptomatic STIs among PrEP-users [Citation35,Citation38,Citation39]. Furthermore, spontaneous cure rates of STIs are relatively low: van Liere et al. described site-dependent clearance rates of 6.8–57.1% for chlamydia and 20.0–33.3% for gonorrhea between diagnosis and initiation of therapy [Citation40] while Chow et al. reported spontaneous cure of oropharyngeal gonorrhea or chlamydia after a median time of 4 and 2.5 weeks, respectively [Citation41]. Thus, asymptomatic extragenital infections appear to be key factors in maintaining the high STI burden among MSM, and as the data of our study show, sexual mixing occurs regardless of HIV status.
Our study provides substantial insights into sexual mixing in a high-income Central European country. Strengths of our work include that the cluster analysis was based on core genome MLST clustering of Ng isolates and that clinical information was included comprising not only the suspected mode of transmission but also the site of infection, symptoms and coinfections. In addition, regression models enabled us to identify independent predictors of clustering with PLWH. Nonetheless, we must also acknowledge several limitations, including the retrospective work-up of Ng cases, which may have led to selection bias, as well as collecting samples at only one geographical site. While it is relatively common in Austria, that STIs are treated in hospitals (gonorrhea and syphilis in particular since i.m. treatment is not readily available in office-based care in Austria), it is still very likely that we missed some patients who would avoid receiving treatment in a hospital. This might explain the low number of women in our analysis since women in Austria typically consult their office-based gynecologist first instead of an STI clinic. Furthermore, core-genome MLST analysis, which we used to determine clusters, cannot answer whether transmission occurred between two individuals directly or a third person was ‘linked in-between’. These potential ‘missing links’ are unidentified and might play an important role regarding transmission chains. Based on our data, we can only deduce that individuals within a cluster are connected; the extent of direct sexual mixing between two individuals harboring two clustering Ng isolates, remains unclear. This is further reflected in our dataset, as some clusters spanned over several years, and by the limited number of isolates that were connected to clusters in our study (44%). However, the proportion is on par with a comparable analysis from UK that included 1277 isolates of which 49% were allocated to clusters. Importantly, we did not see any significant differences in the population included in a cluster versus those not clustering. Another potential bias of our study is that isolates were analyzed upon cultivating Ng. During the observational period, 924 (urethral n = 300, cervical n = 8, anal n = 368, pharyngeal n = 248) Ng positive NAATs were obtained at our clinic, yet, only 415 isolates were included in this analysis. This discrepancy is mainly driven by the reduced sensitivity of Ng-culture for samples collected form the pharyngeal- or anal mucosa. Nonetheless, 23% of the 415 isolates were obtained from extra genital sites. Lastly, Austria lacks a surveillance network for Ng (and other STIs); thus, we are unable to put our data in context with national Ng transmission dynamics.
In conclusion, our analyses suggest that efforts to reduce the global burden of STIs, as called for by the WHO, should take into account sexual mixing between MSM and non-MSM. While ‘MSM’ remain the population with the highest burden of Ng in high-income countries, our data suggest that some infections also spread to non-MSM. Finally, in our analysis, ‘MSM’ was the only predictor for HIV-serodiscordant sexual mixing, underlining the importance of access to PrEP to avert HIV transmission.
Author contributions
A. Geusau, D. Chromy and S. Pleininger designed the study; D. Heissenberger performed the Neisseria gonorrhoeae cultures of the stored samples; K. Lippert and P. Hyden carried out the genetic analysis; D. Heissenberger, D. Chromy, F. Heger and K. Lippert collected the data; A. Geusau and D. Chromy verified the underlying data, A. Geusau, D. Chromy, D. Heissenberger, K. Lippert, S. Pleininger, B. Willinger and W.M. Bauer interpreted them; A. Geusau, D. Chromy, K. Lippert, S. Pleininger and B. Willinger drafted the manuscript. All authors revised the manuscript carefully for its content and approved the final version. All authors had full access to all the data in the study and agree to be accountable for all aspects of the work.
Supplemental Material
Download MS Word (18.4 KB)Acknowledgements
We would like to thank Elzbieta Motuk and Christopher Nava for their technical support. We would also like to thank Silke Stadlbauer, Petra Hasenberger and Anna Stöger for their logistical support throughout the study.
Disclosure statement
DC served as a speaker and/or advisory board member for Gilead, ViiV Healthcare and MSD, and received travel support from MSD, ViiV Healthcare and Gilead. DH has nothing to disclose. KL has nothing to disclose. FH has nothing to disclose. AI has nothing to disclose. PH has nothing to disclose. WMB served as a speaker and/or consultant and/or advisory board member for AbbVie, ViiV Healthcare and Takeda, and received travel support from AbbVie, MSD, ViiV Healthcare and Gilead. KGP served as a speaker and/or consultant and/or advisory board member for ViiV, Gilead, and received travel support from ViiV Healthcare, and Gilead. BW served as a speaker for Gilead and advisory board member for MSD. WW served as a speaker, consultant and/or advisory board member for LEO Pharma, Pfizer, Sanofi Genzyme, Eli Lilly, Novartis, Boehringer Ingelheim, AbbVie and Janssen. SP has nothing to disclose. AG participated in advisory boards and gave talks for Almirall and Sanofi.
Additional information
Funding
References
- World Health Organization. Report on global sexually transmitted infection surveillance. Geneva: WHO; 2018.
- World Health Organization. Global health sector strategies on, respectively, HIV, viral hepatitis and sexually transmitted infections for the period 2022–2030. Geneva: WHO; 2022.
- Ong JJ, Baggaley RC, Wi TE, et al. Global epidemiologic characteristics of sexually transmitted infections among individuals using preexposure prophylaxis for the prevention of HIV infection: a systematic review and meta-analysis. JAMA Netw Open. 2019;2(12):e1917134. doi: 10.1001/jamanetworkopen.2019.17134.
- European Centre for Disease Prevention and Control (ECDC). Annual epidemiological report for 2019. Stockholm; 2023.
- European Centre for Disease Prevention and Control (ECDC). Gonorrhoea. Annual epidemiological report for 2018. Stockholm; 2020.
- Workowski KA, Bachmann LH, Chan PA, et al. Sexually transmitted infections treatment guidelines, 2021. MMWR Recomm Rep. 2021;70(4):1–187. doi: 10.15585/mmwr.rr7004a1.
- Traeger MW, Cornelisse VJ, Asselin J, et al. Association of HIV preexposure prophylaxis with incidence of sexually transmitted infections among individuals at high risk of HIV infection. JAMA. 2019;321(14):1380–1390. doi: 10.1001/jama.2019.2947.
- Chromy D, Urban N, Grabmeier-Pfistershammer K, et al. High prevalence of asymptomatic sexually transmitted infections in Austrian pre-exposure prophylaxis users: a prospective observational study. AIDS Patient Care STDS. 2023;37(3):115–118. doi: 10.1089/apc.2022.0154.
- Chromy D, Bauer DJM, Simbrunner B, et al. The 'Viennese epidemic’ of acute HCV in the era of direct-acting antivirals. J Viral Hepat. 2022;29(5):385–394. doi: 10.1111/jvh.13665.
- Vaughan AM, Cenciarelli O, Colombe S, et al. A large multi-country outbreak of monkeypox across 41 countries in the WHO European region, 7 March to 23 August 2022. Euro Surveill. 2022;27(36):2200620. doi: 10.2807/1560-7917.Es.2022.27.36.2200620.
- Baggaley RF, White RG, Boily MC. HIV transmission risk through anal intercourse: systematic review, meta-analysis and implications for HIV prevention. Int J Epidemiol. 2010;39(4):1048–1063. doi: 10.1093/ije/dyq057.
- Ring A, Balakrishna S, Imkamp F, et al. High rates of asymptomatic Mycoplasma genitalium infections with high proportion of genotypic resistance to first-line macrolide treatment among men who have sex with men enrolled in the Zurich primary HIV infection study. Open Forum Infect Dis. 2022;9(6):ofac217. doi: 10.1093/ofid/ofac217.
- European Centre for Disease Prevention and Control (ECDC). Gonococcal antimicrobial susceptibility surveillance in the EU/EEA: summary of results for 2019. Stockholm: ECDC; 2021.
- Orbe-Orihuela YC, Sánchez-Alemán M, Hernández-Pliego A, et al. Syphilis as re-emerging disease, antibiotic resistance, and vulnerable population: global systematic review and meta-analysis. Pathogens. 2022;11(12):1546. doi: 10.3390/pathogens11121546.
- Moreno-Mingorance A, Mir-Cros A, Goterris L, et al. Increasing trend of antimicrobial resistance in Shigella associated with MSM transmission in Barcelona, 2020–21: outbreak of XRD Shigella sonnei and dissemination of ESBL-producing Shigella flexneri. J Antimicrob Chemother. 2023;78(4):975–982. doi: 10.1093/jac/dkad031.
- Skaletz-Rorowski A, Potthoff A, Nambiar S, et al. Sexual behaviour, STI knowledge and Chlamydia trachomatis (CT) and Neisseria gonorrhoeae (NG) prevalence in an asymptomatic cohort in Ruhr-area, Germany: PreYoungGo study. J Eur Acad Dermatol Venereol. 2021;35(1):241–246. doi: 10.1111/jdv.16913.
- Geusau A, Chromy D, Heissenberger D, et al. Resistance profiles of Neisseria gonorrhoeae isolates in Vienna, Austria: a phenotypic and genetic characterization from 2013 to 2020. Int J Antimicrob Agents. 2022;60(5–6):106656. doi: 10.1016/j.ijantimicag.2022.106656.
- Schaeffer J, Lippert K, Pleininger S, et al. Association of phylogenomic relatedness among Neisseria gonorrhoeae strains with antimicrobial resistance, Austria, 2016–2020. Emerg Infect Dis. 2022;28(8):1694–1698. doi: 10.3201/eid2808.220071.
- Harrison OB, Cehovin A, Skett J, et al. Neisseria gonorrhoeae population genomics: use of the gonococcal core genome to improve surveillance of antimicrobial resistance. J Infect Dis. 2020;222(11):1816–1825. doi: 10.1093/infdis/jiaa002.
- Peters J, Cresswell F, Amor L, et al. Whole genome sequencing of Neisseria gonorrhoeae reveals transmission clusters involving patients of mixed HIV serostatus. Sex Transm Infect. 2018;94(2):138–143. doi: 10.1136/sextrans-2017-053198.
- Town K, Field N, Harris SR, et al. Phylogenomic analysis of Neisseria gonorrhoeae transmission to assess sexual mixing and HIV transmission risk in England: a cross-sectional, observational, whole-genome sequencing study. Lancet Infect Dis. 2020;20(4):478–486. doi: 10.1016/s1473-3099(19)30610-3.
- Williamson DA, Chow EPF, Gorrie CL, et al. Bridging of Neisseria gonorrhoeae lineages across sexual networks in the HIV pre-exposure prophylaxis era. Nat Commun. 2019;10(1):3988. doi: 10.1038/s41467-019-12053-4.
- Hosseini-Hooshyar S, Martinello M, Yee J, et al. Low hepatitis C virus reinfection rate despite ongoing risk following universal access to direct-acting antiviral therapy among people living with HIV. AIDS. 2020;34(9):1347–1358. doi: 10.1097/qad.0000000000002562.
- Garvey LJ, Cooke GS, Smith C, et al. Decline in hepatitis C virus (HCV) incidence in men who have sex with men living with human immunodeficiency virus: progress to HCV microelimination in the United Kingdom? Clin Infect Dis. 2021;72(2):233–238. doi: 10.1093/cid/ciaa021.
- Marquez LK, Ingiliz P, Boesecke C, et al. Establishing a framework towards monitoring HCV microelimination among men who have sex with men living with HIV in Germany: a modeling analysis. PLOS One. 2022;17(5):e0267853. doi: 10.1371/journal.pone.0267853.
- Chromy D, Bauer D, Simbrunner B, et al. Progress of hepatitis C elimination in Viennese people living with HIV after two decades of increasing cure rates. Infect Dis. 2022;55(3):189–198. doi: 10.1080/23744235.2022.2153914.
- Jones J, Le Guillou A, Gift TL, et al. Effect of screening and treatment for gonorrhea and chlamydia on HIV incidence among men who have sex with men in the United States: a modeling analysis. Sex Transm Dis. 2022;49(10):669–676. doi: 10.1097/olq.0000000000001685.
- Mao L, Kippax SC, Holt M, et al. Rates of condom and non-condom-based anal intercourse practices among homosexually active men in Australia: deliberate HIV risk reduction? Sex Transm Infect. 2011;87(6):489–493. doi: 10.1136/sextrans-2011-050041.
- Ward H, Rönn M. Contribution of sexually transmitted infections to the sexual transmission of HIV. Curr Opin HIV AIDS. 2010;5(4):305–310. doi: 10.1097/COH.0b013e32833a8844.
- Leierer G, Rappold M, Strickner S, et al. 42th report of the Austrian HIV cohort study; 2022.
- Rodger AJ, Cambiano V, Bruun T, et al. Risk of HIV transmission through condomless sex in serodifferent gay couples with the HIV-positive partner taking suppressive antiretroviral therapy (PARTNER): final results of a multicentre, prospective, observational study. Lancet. 2019;393(10189):2428–2438. doi: 10.1016/s0140-6736(19)30418-0.
- Grant RM, Lama JR, Anderson PL, et al. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med. 2010;363(27):2587–2599. doi: 10.1056/NEJMoa1011205.
- Thigpen MC, Kebaabetswe PM, Paxton LA, et al. Antiretroviral preexposure prophylaxis for heterosexual HIV transmission in Botswana. N Engl J Med. 2012;367(5):423–434. doi: 10.1056/NEJMoa1110711.
- Jones J, Weiss K, Mermin J, et al. Proportion of incident human immunodeficiency virus cases among men who have sex with men attributable to gonorrhea and chlamydia: a modeling analysis. Sex Transm Dis. 2019;46(6):357–363. doi: 10.1097/olq.0000000000000980.
- Molina JM, Ghosn J, Assoumou L, et al. Daily and on-demand HIV pre-exposure prophylaxis with emtricitabine and tenofovir disoproxil (ANRS PREVENIR): a prospective observational cohort study. Lancet HIV. 2022;9(8):e554–e562. doi: 10.1016/s2352-3018(22)00133-3.
- Basten M, den Daas C, Heijne JCM, et al. The rhythm of risk: sexual behaviour, PrEP use and HIV risk perception between 1999 and 2018 among men who have sex with men in Amsterdam, The Netherlands. AIDS Behav. 2020;25(6):1800–1809. doi: 10.1007/s10461-020-03109-4.
- EACS guidelines version 11.0; 2021.
- Berçot B, Charreau I, Clotilde R, et al. High prevalence and high rate of antibiotic resistance of Mycoplasma genitalium infections in men who have sex with men. A sub-study of the ANRS Ipergay PrEP trial. Clin Infect Dis. 2020;73(7):e2127–e2133. doi: 10.1093/cid/ciaa1832.
- Johnson Jones ML, Chapin-Bardales J, Bizune D, et al. Extragenital chlamydia and gonorrhea among community venue-attending men who have sex with men – five cities, United States, 2017. MMWR Morb Mortal Wkly Rep. 2019;68(14):321–325. doi: 10.15585/mmwr.mm6814a1.
- van Liere G, Hoebe C, Dirks JA, et al. Spontaneous clearance of urogenital, anorectal and oropharyngeal Chlamydia trachomatis and Neisseria gonorrhoeae in women, MSM and heterosexual men visiting the STI clinic: a prospective cohort study. Sex Transm Infect. 2019;95(7):505–510. doi: 10.1136/sextrans-2018-053825.
- Chow EPF, Vodstrcil LA, Williamson DA, et al. Incidence and duration of incident oropharyngeal gonorrhoea and chlamydia infections among men who have sex with men: prospective cohort study. Sex Transm Infect. 2021;97(6):452–457. doi: 10.1136/sextrans-2020-054764.
