ABSTRACT
This study aimed to investigate the leaf anatomy of Jacquinia armillaris plants in two different Venezuelan xeric shrublands to evaluate possible variations caused by the environments, which differ in rainfall and soil salinity. Leaf samples were collected in two sites: La Tortuga Island, a dryer and salty environment than the other collecting site, Turpialito, a coastal location in the mainland. The epidermis and the mesophylls were observed with a light microscope and measured with an ocular micrometer inserted in one of the eyepieces. The results show that J. armillaris has many characteristic anatomical traits of xerophytes, among them: thick cuticles and thick epidermis in both leaf surfaces, stomata only in the abaxial side and epidermal depressions lodging a glandular trichome. Leaves are bifacial and present multistratified palisade parenchyma facing the adaxial epidermis and abaxial spongy parenchyma with abundant intercellular spaces. Furthermore, the hypodermis is composed of one or two cell layers below the upper epidermis. Leaf lamina dimension, petiole, cuticles, epidermis and hypodermis cells, as well as the stomatal occlusive cells and number of trichomes, had differences in size and number when the plants of both sites were compared, being higher in plants from La Tortuga. On the other hand, palisade parenchyma and stomatal density were higher in Turpialito plants. Plants collected in La Tortuga Island showed characteristics that suggest adaptation to the island’s saline and more arid conditions in comparison to plants from Turpialito, which presented more leaf traits related to adaptations to water deficit. These results demonstrate the phenotypic plasticity of J. armillaris plants that grow in two different xeric shrublands.
Este estudio tuvo como objetivo investigar a anatomía foliar de plantas de Jacquinia armillaris en dos matorrales xerófilos venezolanos diferentes para evaluar posibles variaciones causadas por los ambientes, que difieren en la pluviosidad y en la salinidad del suelo. Se recolectaron muestras foliares en dos sitios: isla La Tortuga, un ambiente más seco y salino que el otro sitio de recolección, Turpialito, un lugar costero en el continente. La epidermis y los mesófilos se observaron con un microscopio óptico y se midieron con un micrómetro ocular insertado en uno de los oculares. Los resultados muestran que J. armillaris presenta muchos rasgos anatómicos característicos de xerófitas, entre ellos: cutículas y epidermis gruesas en ambas superficies foliares, estomas solo en el lado abaxial y depresiones epidérmicas que alojan un tricoma glandular. Las hojas son bifaciales y presentan parénquima en empalizada multiestratificado próximo a la epidermis adaxial y parénquima esponjoso abaxial con abundantes espacios intercelulares. Además, la hipodermis está compuesta por una o dos capas de células situada debajo de la epidermis adaxial. La dimensión de la lámina foliar, pecíolo, cutículas, células epidermis e hipodermis, así como las células oclusivas estomáticas y número de tricomas, tuvieron diferencias en tamaño y número cuando comparadas las plantas de ambos sitios, siendo mayores en plantas de La Tortuga. Por otro lado, el parénquima en empalizada y la densidad estomática fueron mayores en las plantas de Turpialito. Las plantas colectadas en la isla La Tortuga mostraron características que sugieren una adaptación a las condiciones salinas y más áridas de la isla en comparación con las plantas de Turpialito, que presentaron más rasgos foliares relacionados con adaptaciones al déficit hídrico. Estos resultados demuestran la plasticidad fenotípica de las plantas de J. armillaris que crecen en dos matorrales xerófilos diferentes.
PALABRAS-CLAVES:
Introduction
It is known that leaves play a key role in plant function and long-term adaptation to the environment. Moreover, leaves show differences between plant species due to phylogenetic relationships and to their adaptation to specific environments [Citation1,Citation2]. Plants adjust their morphological, anatomical and/or physiological traits in response to the deficit of resources. These adjustments are known as “plastic responses” and can facilitate plant acclimation to new environmental conditions [Citation3]. Climate diversity is one of the causes of morphotypic differentiation observed in plants; geographic parameters such as altitude, latitude, temperature, and to a lesser extent, soil type, among others, are responsible for inducing leaf‐trait variation [Citation4].
Arid and semi-arid vegetation types comprise 41,023 km2 of Venezuela [Citation5]. These regions form an almost continuous narrow belt along the Caribbean coast whose altitude does not exceed 500 m above sea level and the mean annual rainfall is lower than 800 mm [Citation6]. La Tortuga Island is located in the South of the Caribbean Sea and its environment is characterized by strong winds, high solar radiation, elevated temperature, low water availability (less than 250 mm annual rainfall) and by the prevalence of xerophilous vegetation [Citation7]. On the other hand, Turpialito is located in the Southern coast of Gulf of Cariaco and it is characterized by a warm semi-arid environment. It presents mean temperature 27 °C, altitude 400 m above sea level, mean annual rainfall 440 mm [Citation8], and prevalence of spiny shrubland vegetation [Citation9].
Studies about the leaf anatomy of arid-region plants composing the Venezuelan flora have shown significant environmental influence on the development of adaptive anatomical traits, fact that indicated the phenotypic plasticity of the investigated species [Citation10–12]. Despite the importance of island areas, mainly La Tortuga Island, whose ecosystems host a wide variety of animal and plant species yet to be identified and studied, anatomical studies about the coastal xerophytic flora in Venezuela remain scarce.
Jacquinia armillaris Jacq. (Theophrastoideae – Primulaceae) stands out among natural tree species to the arid and semiarid regions in Venezuela. This plant species is widely distributed in the Caribbean [Citation13], both in island and continental areas, besides growing in regions from Southern Florida to Northern South America and in Eastern Brazil [Citation14]. J. armillaris is a shrub or small tree (4 m tall) that presents straight, cylindrical and light-gray trunk, as well as simple and pseudoverticillate leaves with curved margin at the edges towards the underside thereof [Citation15]. Aboriginal people living in Venezuela use the extract of this plant for fishing, since it is capable of suffocating fish which, once dead, float on the water surface and are easily captured [Citation16].
Leaf epidermal anatomy of J. armillaris grown in La Tortuga Island has been described [Citation17]; nevertheless, the possible morphoanatomical variations induced by this highly saline and dry environment have not been clarified, due to an absence of studies on this species in nearby areas and with more or less similar characteristics. Kuster et al. [Citation18] report that, the fact of living in rocky outcrops or post-beach formation can trigger the anatomical and morphological plasticity in J. armillaris. It is known that climate and leaf nutrients are the main factors that regulate leaf morphological and anatomical traits [Citation2], and even that soil is more important than climate in shaping latitudinal variation in leaf traits, therefore a good approach to interpretation of leaf traits must take into account the interaction of soil factors and annual precipitation [Citation19]. In this way, it is expected that a species that possesses such phenotypic plasticity as J. armillaris (still growing in similar latitudes) might vary its anatomy in response to differences in the characteristics of the island’s soil (more saline and with less humidity) and due to the rainfall amount (slightly higher in Turpialito). Previous work [Citation17,Citation18] suggests that specific structures such as stomatal and trichomes could play an important role in reducing the impact of salinity; Even, recently trichomes have been confirmed as salt excretory glands [Citation20,Citation21]. So, it was hypothesized that leaf anatomical and structural traits associated with drought and salinity tolerance would correspond with a habitat. Accordingly, the aim of the present study was to investigate the anatomical traits of J. armillaris leaves in two xeric shrublands: La Tortuga Island and Turpialito, in order to determine the anatomical differences and the relation to the typical habitats.
Materials and methods
Study sites
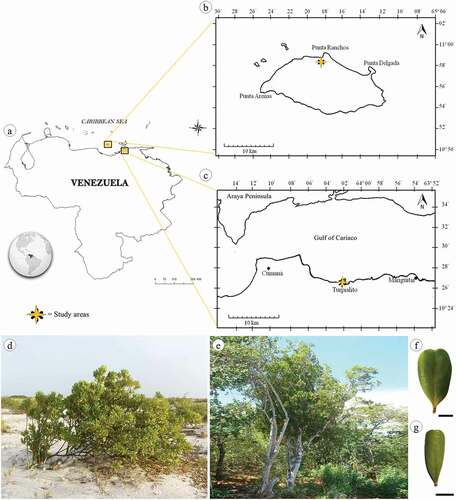
Jacquinia armillaris Jacq. specimens were collected in two xeric shrublands in Venezuela ()); according to the Köppen-Geiger classification, the climate in the region is hot semi-arid (BSh). Punta Ranchos (10º58ʹ90” N, 65º18ʹ44” W), in La Tortuga (TO) Island, was the first collection site. It is a Federal Dependency of Venezuela located in the Caribbean Sea between the Central-eastern Venezuelan coasts and the Western of the Margarita Island (). The second collection area was Turpialito (TU) (10º26ʹ37” N, 64º01ʹ54” W), which is located on the southern coast of the Gulf of Cariaco, in Sucre State, in the mainland of the Venezuelan coast (). Mean temperatures and annual rainfalls in the two study sites were 27.9 °C/102.51 mm, and 28.0 °C/246.72 mm in La Tortuga and Turpialito, respectively (Meteorological Station 8044200, SVCU at Antonio José de Sucre International Airport, Cumaná). La Tortuga Island has rocky soil mostly formed by huge plates or loose pieces of limestone rocks of coral origin, or by large sandy stretches in areas near the sea [Citation7]. Whereas the soil in Turpialito comprises sedimentary rocks and interstratifications of limestones, sandstones, shales and clays [Citation8]. For each site, soil samples were collected. Ten sampling points were established randomly in each habitat at regular intervals of 10 m apart, at a depth of 0–20 cm. All 10 samples (500 g each) were subsequently mixed and transformed into one composite sample. In the laboratory, for textural determination, the Bouyoucos technique was used; sodium and potassium contents were determined by photoelectric flame photometer, calcium and magnesium through volumetry with EDTA, chloride by Mohr method, pH and electrical conductivity (EC) in suspension by potentiometric and conductimetric methods, respectively [Citation22].
Plant material
Leaf samples were collected from ten individuals in each study site (). Fully-expanded and healthy leaves () were fixed in formalin – glacial acetic acid – 96% ethanol (FAA) [Citation23] and transported to the Plant Physiology and Anatomy Laboratory of Universidad de Oriente, where they were analyzed.
Leaf morphoanatomical traits
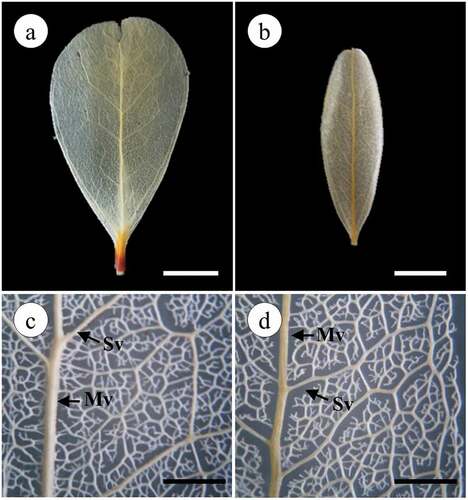
Leaf length and width, as well as petiole length, width, and thickness, were measured with a Vernier caliper (Impact Tools) at two-decimal precision. The diaphanization technique was realized by method proposed by Dilcher [Citation24]. Fresh leaves were boiled in a 5% sodium hydroxide solution for 10 minutes. Subsequently, leaves were washed in cold water and the venous skeleton was obtained, placed in a diluted 2–5% commercial chlorine solution to bleach it and dried between two sheets of filter paper. Posteriorly, venation patterns were observed in an Optima ST-604 stereoscopic microscope and classified according to the “Dicotyledonous Leaf Architecture Classification System” proposed by Hickey [Citation25].
The epidermis was detached from the leaf based on the technique described by Ram and Nayyar [Citation26]. Fragments (approximately 1 cm2) of the leaf blade edge were cut and heated in 5% aqueous cupric sulfate solution for 5 minutes. Subsequently, 5 mL of concentrated hydrochloric acid was added to the solution and left to boil for 4 minutes; the epidermis was washed with distilled water, stained with 1% aqueous Safranin and mounted on histological slides with aqueous glycerin (1:1). Mesophyll free-hand cross-sections were made in order to obtain fragments (1 cm2) from the middle region of the leaves, including the primary vein. The fragments were stained with 1% Safranin and 0.5% Astra blue (1:9 v/v), and, finally, they were mounted in aqueous glycerin (1:1).
The fixed samples were observed in a light microscope (Globe Germany n-200), which was previously calibrated for 10X and 40X objective lenses, and had an ocular micrometer incorporated into it. Length, width and thickness of epidermal as well as thickness of mesophyll cells were measured, and the number of trichomes and stomata were counted. Each individual was randomly measured 10 times (n = 100 for each variable). Photomicrographs were taken in an HP Photosmart 7 MP camera attached to the microscope.
Statistical analyses
Data were tested for normality (Shapiro–Wilk test) and homogeneity of variances (Levene’s test) and for the variable nº of trichomes that did not comply with the supposed normality, a transformation of the data was performed using log10. t-test (at p ≤ 0.05) was applied to determine significant differences between plant traits in each site. To assess the degree of association between the anatomical features and to determine how increases in the values of some of them are accompanied by increases or decreases in other features, a Pearson correlation analysis was applied once all data were compiled. The software used was Statgraphics Centurion XVIII.
Results
Characteristics of soil
The soil analysis conducted in both sites showed that the soil in La Tortuga (TO) is conformed to a higher degree of sand (73.66%) while soil in Turpialito (TU) holds 56.17% sand and 36.32% clay (). The soil of TU was approximately 5.2 times more humid than the soil of TO (15.39% versus 2.48%, respectively). In TU the soil showed a more acidic pH (7.72) when compared to that of TO (8.11). In general, salt contents were higher in the TO soil, which had 2.3, 2.3, 12.6 and 107 times more content of calcium, sodium, chloride and potassium, respectively, than soil from TU. By other hand, in TU site, the soil had 40.7% more magnesium than soil collected in TO site. Soil from TO had higher electrical conductivity (2.56 dS m−1) than the soil from TU (1.86 dS m−1).
Table 1. Physiochemical characteristics of the soils from the collection sites, La Tortuga (TO) and Turpialito (TU), Venezuela
Leaf morphological traits
Jaquinia armillaris leaves from both sites showed similar qualitative traits, although there were significant differences in the size and frequency of some variables. Leaves were simple, whole, and all of them presented revolute margin, as well as similar blade shape and base. Leaf size was significantly different between plants from both sites: leaves collected in TU were smaller in length (t = 23.59, p ˂ 0.001) and width (t = 28.40, p < 0.001), and presented shorter (t = 10.04, p < 0.001) and narrower (t = 19.46, p < 0.001) petioles than the ones collected at TO site (). Length and width of the leaves showed a strong positive correlation with each other, as well as with the length and width of the petiole ().
Table 2. Morphometric characteristics of the leaf blade and petiole of Jacquinia armillaris from La Tortuga (TO) and Turpialito (TU), Venezuela. Different letters indicate differences between localities (t-test at p ≤ 0.05)
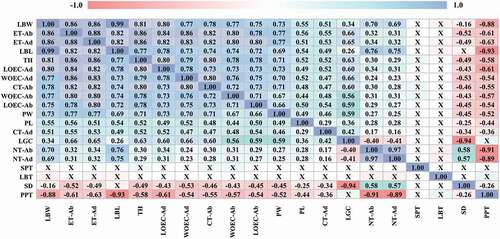
Figure 2. Pearson’s correlations coefficients between morphological and anatomical variables of Jacquinia armillaris of La Tortuga and Turpialito, Venezuela. Abbreviations: LBL: leaf blade length; LBW: leaf blade width; LBT: leaf blade thickness; PL: petiole length; PW: petiole width; CT: cuticle thickness; ET: epidermis thickness; LOEC-ad: length of ordinary epidermal cell; WOEC-ad: width of ordinary epidermal cell; NT: number of trichomes; -ad or -ab indicate adaxial or abaxial surface; LGC: length of guard cell; SD: stomatal density; TH: thickness of hypodermis; PPT: palisade parenchyma thickness; SPT: spongy parenchyma thickness. * X indicates correlations with no significance at p ≤ 0.05
The leaves of the plants from both locations have the same leaf venation pattern. Both leaves are pinnate, that is, they present a middle vein from which secondary veins originate. Was observed a camptodromous–brochidodromous secondary venation pattern (veins do not end at the margin) (). Secondary ribs are joined together forming prominent arches, the inter-secondary veins are composite, the pattern of the tertiary veins is reticulated-random-orthogonal, the margin is lobed, the areoles are imperfectly developed, randomly arranged and polygonal in shape, and the veins are branched ().
Epidermis
The cross-section of J. armillaris leaves collected in both sites showed a uniseriate adaxial epidermis composed of tabular cells and wrinkled cuticle (). Based on the superficial view, the ordinary cells are polyhedral, with straight thickened anticlinal cell walls and no intercellular spaces (). There were differences between the variables in the adaxial epidermis. Greater cuticle thickness (t = 10.02, p < 0.001) and epidermal thickness (t = 47.69, p < 0.001) were recorded for specimens collected at the TO site. Also, there were differences in the length (t = 25.81, p < 0.001) and width (t = 26.61, p < 0.001) of ordinary epidermis cells, with the largest cell sizes corresponding to the TO samples. The abaxial surface of leaves collected at both sites showed uniseriate epidermis with tabular or cuboidal cells and wrinkled cuticle in the cross-section view (). The frontal view allowed seeing polyhedral-shaped cells, as well as straight and thick anticlinal cell walls with numerous epidermal stomata and depressions (). Differences were found in cuticular (t = 23.65, p < 0.001) and epidermal (t = 43.11, p < 0.001) thicknesses, the highest values were recorded for plants from TO site (). Cell sizes were positively correlated with cuticle thickness, as well as length and width of leaf ().
Table 3. Morphometric characteristics of leaf epidermis in Jacquinia armillaris from La Tortuga (TO) and Turpialito (TU), Venezuela. Different letters indicate differences between localities (t-test at p ≤ 0.05). – absence of the characteristic
Figure 4. Epidermis adaxial (a–d) and abaxial (e–h) of Jacquinia armillaris. (a, b, e, f, i, j, k, l): in cross-section. (c, d, g, h): surface view. (a, c, e, g, i, k): of TO samples. (b, d, f, h, j, l): of TU samples. a-b: general aspect of the cuticle and epidermis cells with straight outline of the anticlinal cell walls. (c,d): Epidermis cells, note the depressions. (e,f): Epidermis cells covering by cuticle and stomata guard cells. (g–h): general aspect of cuticle and epidermis straight thickened anticlinal cell walls, note the depressions and stomata. (i–l): Glandular trichomes in depressions in cross-section of epidermis adaxial (i, j) and abaxial (k, l). Abbreviations: cu: cuticle; st: stomata. Scale bars: 50 μm
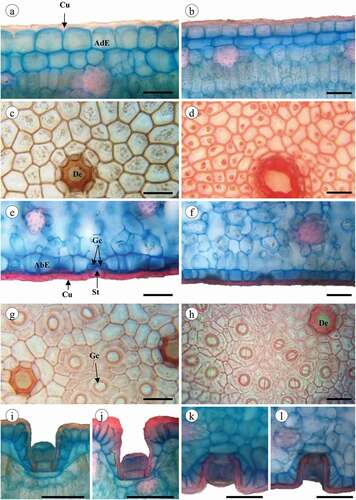
The stomata of plants from both sites were anomocytic (), located only on abaxial surfaces and at the level of ordinary epidermal cells (), besides being smaller (t = 13.90, p < 0.001) and numerous (t = −9.10, p < 0.001) in plants grown at the TU site (). The length of the guard cell showed a negative correlation with stomatal density, which in turn showed an inverse relationship with almost all the variables, except for those related to the density of trichomes (). The adaxial and abaxial epidermis of all specimens showed deep epidermal depressions (, 4(g,h)), which cavities covered by cuticle, and harboring a glandular trichome (). The glandular trichomes inserted in the epidermal depressions of both leaf surfaces of plants from TO and TU were formed by a unicellular base and a globular pluricellular head. Such structures did not show significant differences in total length (t = 1.16, p = 0.247) and head diameter (t = −1.05, p = 0.295) at adaxial level, but there were statistically significant differences between the trichome number per mm2 (t = 4.72, p < 0.001). At adaxial level, the trichome number per mm2 also was statistically different between sites (t = 4.86, p < 0.001). The glandular trichome was more abundant in both surfaces of the TO site plants ().
Mesophyll
J. armillaris leaves were bifacial, presented palisade parenchyma on the upper surface and spongy parenchyma on the lower surface, as well as hypodermis (comprised by one-to-two cell layers) below the adaxial epidermis (). A midrib and vascular bundles surrounded by fibers are embedded in the mesophyll (). Under the hypodermis are located cortical fibers (). The shape of the hypodermis cells ranged from round to irregularly elongated and their major axis was parallel to the leaf surface, some cases presented a remarkable number of druses and prismatic crystals. The thickest hypodermic tissue (t = 29.11, p < 0.001) was found in samples collected in TO (). The thickness of the hypodermis was positively correlated with the other cell sizes, with the exception of palisade parenchyma, with which it showed a negative correlation ().
Table 4. Morphometric characteristics of mesophyll in Jacquinia armillaris from La Tortuga (TO) and Turpialito (TU), Venezuela. Different letters indicate differences between localities (t-test at p ≤ 0.05)
Figure 5. Jacquinia armillaris leaves in cross-section. (a, c, g, i): of TO samples. (b, d, e, f, h, j): of TU samples. (a,b): Leaf section of the primary vein. (c,d): Leaf detail showing palisade and spongy parenchyma. (e,f): primary vein and vascular bundle, xylem and phloem recovery with fibers. (g,h): adaxial region showing the hypodermis and palisade parenchyma cells. (i,-j): Leaf abaxial region, note the spongy parenchyma cells and intercellular spaces (*). Abbreviations: Cf: cortical fibers; Md: midrib; Pp: palisade parenchyma; Sp: spongy parenchyma; VB: vascular bundle; Xy: xylem; Ph: Phloem; Pf: perivascular fibers; Hy: hypodermis; Dr: druses. Scale bars: 100 μm
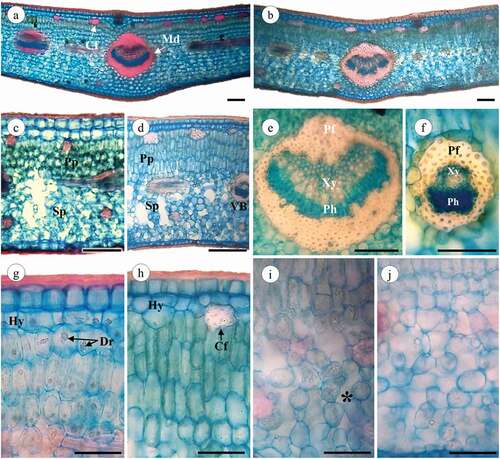
The palisade parenchyma of plants from both sites was multilayered: TO individuals presented two to four cell layers (mostly three), whereas TU individuals also presented two to four layers (often four) and thicker palisade parenchyma (t = −11.55, p < 0.001) (). The spongy parenchyma in leaves collected at both sites was composed of lobed cells, with abundant chloroplasts and numerous intercellular spaces (); there were no differences in spongy parenchyma thickness (t = −0.20, p = 0.842) between sites (). In contrast to the spongy parenchyma, the palisade parenchyma was negatively correlated with almost all the variables studied, especially those referring to the size of the leaf lamina.
Discussion
It is known that plant species can inhabit arid and semiarid regions since they develop resistance or tolerance mechanisms. Jacquinia armillaris individuals showed many traits characteristic of xerophytic species, as well as traits typical of halophytes, a fact that enables their growth in different arid and/or saline environments.
J. armillaris leaves showed revolute-leaf margins, which are typical for semi-arid habitats [Citation27]. The reduced leaf area of TU plants is a common trait of plant species grown in arid and heavily-insolated areas. This trait helps to decrease water loss caused by transpiration because a small surface area allows leaves to avoid overheating by remaining close to ambient air temperature [Citation4]. Differences in leaf size have been described in plants under contrasting light conditions, such as sun and shade environments or even in leaves occupying different regions in the canopy [Citation28–30]. Thus, given the high luminosity condition in both environments, it was expected to find leaves with similar size or even bigger size in plants from TU (once they coexist with trees around). However, the leaf blade of TU samples was smaller than those of TO (). In reference to this finding, Gong and Gao [Citation19] emphasize that, in addition to the climate factor, soil factors play in the irreplaceable role in determining the specific leaf area. In this regard, the soil analyzes showed higher K+ ion contents in soils from TO. Potassium was found to provide abiotic stress tolerance. Under drought stress conditions, K+ regulates stomatal opening, improves water uptake and water conservation, helps to increase the utilization of carbohydrates and all this increases leaf area [Citation31,Citation32]. Taking this into account, the presence of higher amounts of this element in the TO soils could have favored the size of the leaves.
Thick and wrinkled cuticles, which are common traits of xerophytes, were observed in J. armillaris. Accordingly, Lee et al. [Citation33] conducted a study with ginseng leaves and suggested that the epicuticular wrinkle in this plant could help to prevent excessive sunlight absorption. Thick cuticles limit leaf transpiration, mainly when the stomata are closed, besides working as a drought-tolerance mechanism [Citation34,Citation35]. In addition, thick cuticles provide more resistance to tearing and can increase the mechanical resistance of leaves [Citation36]. Another aspect that must be considered is the composition of the cuticle, since it has been shown that cuticle thickness has little impact on its function as a water barrier when compared to the wax composition [Citation37]. This makes it necessary to develop new studies involving the chemical composition of this layer.
Plants that grow in xeric shrublands or in arid climates are expected to present slightly-thick epidermis, generally, in desert plants the leaf epidermis rarely exceed 30 μm [Citation38]. This adaptation reduces the mean cell size, decreases cellular osmotic potential, and increases the ability of plants to maintain turgor; thus, they can be more tolerant to low water availability [Citation39]. The epidermal thickness of TU plants was lower than 30 μm, corresponding to this premise. TO plants presented both upper and lower surfaces that exceeded this size. Valerio et al. [Citation17] recorded epidermal thickness around 29.65 μm (upper) and 31.99 μm (lower) in this species growing in a rocky area of La Tortuga Island.
The reduced size of the ordinary epidermal cells in both leaf surfaces of plants grown at the TU site could be interpreted as an anatomical adaptation to arid conditions, whereas the size of the cells in TO plants may be the resulted from high saline concentrations in the soil of the island. Previous studies [Citation40] show that high NaCl concentrations in salt-tolerant species, such as Medicago arborea, increase the thickness of the epidermis cells, this one being a salinity-resistance mechanism triggered to osmoregulate the turgor potential. This mechanism (or a similar one) could also be present in J. armillaris.
J. armillaris presented stomata characteristic of Subfamily Theophrastoideae [Citation41]. Stomata in small size and high density, like the ones from the TU site (), are related to drought-resistant plants. Small stomata allow them to control turgor pressure and respond to environmental changes by opening and closing in rapid succession [Citation42]. This factor, in association with high stomatal density, enables rapid increase in stomatal conductance. These observations have also been reported in other plants native from xerophilous scrublands, as in Oyedaea verbesinoides [Citation43] and Capparis flexuosa [Citation12], which inhabit environments with low water availability on the Venezuelan Coast. On the other hand, TO plants presented stomata with a bigger size and lower-density. Previous studies in other xerophyte plants have revealed that this response can be triggered by high sodium or chloride concentrations (quite abundant in the soil of this site) [Citation40,Citation44,Citation45]. These traits could be correlated with a water-saving process via intensive accumulation of Na+ in leaves which consequently resulted in a marked decrease in water loss rate through down-regulation of stomatal aperture size and decrease in density [Citation44]. Glandular trichomes observed in J. armillaris are adapted to salt secretion, characterizing as salt glands that participate in sodium and others ion excretion [Citation20,Citation21]. Na+ and Cl− ion exclusion is one of the most vital phenomena to enable high salt-tolerance in plants [Citation46]. These structures found on both leaf surfaces in TO and TU plants can be seen as an anatomical adaptation to the saline environment where the species is distributed, since both sites presented sodic soils. Kuster et al. [Citation18] related a higher density of trichomes to the amount of salty spray in the studied environments. Considering the characteristics of both soils in the locations studied in the present work, it is possible to reinforce this hypothesis. Plants from TO showed a higher number of trichomes/mm2 as well as higher sodium content in soil.
Multistratified hypodermis, as reported in J. armillaris, increases leaf stiffness and controls the passage of light to the palisade [Citation47]. The fact that TO plants presented thickened hypodermis often composed of two layers can be interpreted as an adaptation mechanism used to maintain leaf turgor and/or to protect the palisade parenchyma from damages caused by the strong incidence of light. Another possible J. armillaris adaptation to the salt in the soil can be seen in the hypodermic tissue, which presented druses and prismatic crystals (mostly calcium oxalate crystals). Plants growing in environments where calcium is relatively abundant in the soil continuously absorb this ion through transpiration, Ca+ accumulated in these plants can disturb their physiological function and damage them [Citation48]. So, Ca+ is stored in the form of crystals. Jaramillo-Pérez et al. [Citation49] observed abundant crystals in the leaves of Alvaradoa amorphoides, a xerophytic plant growing in Mexico, and correlated them to edaphological conditions and high evapotranspiration in the environment.
Some epidermal traits of J. armillaris plants comply with the ones described by Valerio et al. [Citation17] in four species grown in La Tortuga, such as, thick and wrinkled cuticle in one, or both, leaf surfaces, along with thickened cell walls and abundant crystals. All these traits strongly correlated, in combination with the thicker hypodermis observed, could prevent damages to the leaves by strong and never-ceasing winds. According to Jáuregui et al. [Citation50], A. oestophora coming from Venezuelan coastal zones, which are characterized by elevated temperatures, high solar radiation, and sandy soils with high salt content, presented high salt-secreting trichome density, high-volume and hypodermis-abundant cells. Thus, these traits could be favored by similar climatic conditions in the Venezuelan semi-arid areas.
TO and TU plants presented more than two palisade parenchyma layers, which is widely reported in evergreen sclerophyllous shrubs [Citation51]. An abundant palisade parenchyma has been pointed out as one of the most outstanding characteristics of plants that grow in arid environments, possibly because the development of this tissue is stimulated by light, which tends to be intense in these areas [Citation29,Citation52]. It is worth highlighting that the increased development of palisade tissue was correlated with the smaller size of the leaves in TU plants, characterizing them as sun leaves. Some authors suggest that only an abundant palisade parenchyma develops if the plant receives not only high light radiation but also has sufficient water in the soil [Citation10]. This could help interpret why TO leaves did not show such a developed parenchyma.
The intercellular spaces in the spongy mesophyll of Jacquinia could increase the internal leaf surface, and the air in these spaces can also help mitigating temperature fluctuations [Citation53], fact that could be beneficial to plants exposed to mean temperatures of approximately 28 °C. The similar temperatures at both collection sites, and the presence of other characteristics that minimize water loss and leaf heating could be interpreted as reasons why this variable did not differ between the two places.
The vascular bundles in plants grown at both sites were abundant and surrounded by perivascular fibers. The association between mechanical and vascular tissues provided protection to, and prevented the collapse of, leaf tissues undergoing excessive water loss [Citation54]; thus, sclerenchyma cells can work as water storage tissue and increase water conduction in the mesophyll [Citation47,Citation55]. Similar traits, such as fibers in the vascular bundles, thick cuticle and hypoestomatic leaf, were described by Lemos et al. [Citation30] as being very plastic in Eugenia luschnathiana grown in Brazilian restinga areas. These traits enabled plant acclimatization to the environment, which presents some similarities with the one in La Tortuga and Turpialito, among them: intense sunlight and water deficit.
The plasticity of the anatomical variables herein studied could indicate that the climatic conditions (in TO and TU) enabled J. armillaris to change their leaf traits in order to better adjust to the environmental conditions and to assure its proper functioning. Overall, most anatomical traits found in plants grown at both collection sites, such as thick cuticle and epidermis, salt-excreting trichomes, hypodermis, and cortical and perivascular fibers, are anatomical traits typical of xerophytes. Moreover, it was possible concluding that TO plants presented more obvious salinity-tolerance adaptations such as lower stomata density and turgid cells, and higher trichome density. On the other hand, TU plants presented traits, such as smaller leaf size and cells, higher stomatal density, and smaller stomata capable of minimizing evaporative water loss. This outcome suggested that their leaves were well-adapted to dry and sunny conditions.
Author contributions
RV and YCG designed the experiment. YCG performed the anatomical and statistical analysis. RV and YCG collected leaf samples, analyzed and interpreted the data and wrote the manuscript.
Acknowledgments
We acknowledge the Fundación La Tortuga for the travel support in the collecting of the plant material. We also thank the professors José A. Véliz and Víctor Franco-Salazar for their collaboration in the collection of plant material and for their valuable suggestions throughout the completion of this work.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Royer DL, McElwain JC, Adams JM, et al. Sensitivity of leaf size and shape to climate within Acer rubrum and Quercus kelloggii. New Phytol. 2008;179(3):808–817.
- Tian M, Yu G, He N, et al. Leaf morphological and anatomical traits from tropical to temperate coniferous forests: mechanisms and influencing factors. Sci Rep. 2016;6(1):19703.
- Schlichting CD. The evolution of phenotypic plasticity in plants. Annu Rev Ecol Evol S. 1986;17(1):667–693.
- Dong N, Prentice IC, Wright IJ, et al. Components of leaf‐trait variation along environmental gradients. New Phytol. 2020;228(1):82–94.
- Matteucci SD. Las zonas áridas y semiáridas de Venezuela [The arid and semi-arid zones of Venezuela]. Zonas áridas 1986; 4:39–48. Spanish.
- Vila MS. Las sequías en Venezuela [Droughts in Venezuela]. Caracas: Fondo Editorial Común; 1975. ( Spanish).
- Cervigón F. Las Dependencias Federales [The Federal Dependencies]. Caracas: Exlibris; 1992. Spanish.
- Pérez L. Data from: corografía municipal del estado Sucre[dataset]. 2012. In: Cumaná, Repositorio institucional de la Universidad de Oriente [Internet]. [cited 2018 Mar 12]. Available from: http://ri2.bib.udo.edu.ve:8080/jspui/handle/123456789/184 2006
- Cumana LJ. Caracterización de las formaciones vegetales de la península de Araya, Estado Sucre, Venezuela [Characterization of the plant formations of the Araya peninsula, Sucre State, Venezuela]. Saber. 1999;11(1): 7–16. Spanish.
- Valerio R, De Franco I, Cumana J. Anatomía foliar comparada de dos subespecies de Capparis flexuosa L. (Capparidaceae) [Comparative leaf anatomy of two subspecies of Capparis flexuosa L. (Capparidaceae).]. Saber. 1998;10(2): 7–13. Spanish.
- García M, Lapp M. Morfoanatomía foliar en tres biotipos de Pithecellobium unguis-cati (L.) Bentham creciendo en distintas comunidades vegetales [Leaf morphoanatomy in three biotypes of Pithecellobium unguis-cati (L.) Bentham growing in different plant communities]. Phyton. 2001;2001: 147–158. Spanish.
- Torrecilla P, Castro M, Lapp M. Morfoanatomía foliar en especímenes de Capparis flexuosa (L.) L. (Capparaceae) creciendo en tres localidades distintas del estado Aragua (Venezuela) [Leaf morphoanatomy in specimens of Capparis flexuosa (L.) L. (Capparaceae) growing in three different localities of the Aragua state (Venezuela)]. Ernstia. 2008;19: 35–54. Spanish.
- Hoyos J. Flora de la Isla Margarita, Venezuela [Flora of the Margarita Island, Venezuela]. Caracas: Sociedad y Fundación La Salle de Ciencias Naturales; 1985. Spanish.
- Ståhl B. On the identity of Jacquinia armillaris (Theophrastaceae) and related species. Brittonia. 1992;44(1):54–60.
- Delascio F, González A. Flórula del monumento natural Tetas de María Guevara. Isla de Margarita, Estado Nueva Esparta [Florule of the natural monument Tetas of María Guevara. Margarita Island, Nueva Esparta State]. Caracas: Instituto Nacional de Parques, Jardín Botánico de Caracas, LITOPAR, C.A; 1988. Spanish.
- Steyermark J. Dicotiledóneas: theophrastaceae. In: Manara B, editor. Flora del Parque Nacional Morrocoy. Caracas: Fundación Instituto Botánico de Venezuela-Agencia Española de Cooperación Internacional; 1994. p. 357–359.
- Valerio R, Franco-Salazar V, Véliz J. Adaptaciones epidérmicas foliares de cuatro especies siempreverdes, isla La Tortuga, Venezuela [Leaf epidermal adaptations of four evergreen species, La Tortuga Island, Venezuela]. Acta Bot Venez. 2013;36(1):39–59.
- Kuster VC, Da Silva Campos L, Possatti L, et al. Leaf morphology and anatomy of Jacquinia armillaris Jacq. (Primulaceae) from two coastal restinga environments. Iheringia Ser Bot. 2018;73(3):240–249.
- Gong H, Gao J. Soil and climatic drivers of plant SLA (specific leaf area). Glob Ecol Conserv. 2019;20:e00696.
- De Luna BN, Freitas MDF, da Silva KMM, et al. Are the glandular trichomes in Jacquinia armillaris (Theophrastoideae—Primulaceae) salt glands? Protoplasma. 2020;257(3):863–870.
- Kuster VC, da Silva LC, Meira RMSA. Anatomical and histochemical evidence of leaf salt glands in Jacquinia armillaris Jacq. (Primulaceae). Flora. 2020;262:151493.
- Gilabert de Brito J, López I, Pérez R. Manual de métodos y procedimientos de referencias [Reference methods and procedures manual]. Maracay: Fondo Nacional de Investigaciones Agropecuarias; 1990. ( Spanish).
- Roth I. Microtecnia vegetal [Plant micro-technique]. Caracas: Ediciones de la Biblioteca, Imprenta Universitaria, Universidad Central de Venezuela; 1964. ( Spanish).
- Dilcher DL. Approaches to the identification of angiosperm leaf remains. Bot Rev. 1974;40(1):1–157.
- Hickey L. Classification of architecture of dicotyledonous leaves. Am J Bot. 1973;60(1):17–33.
- Ram M, Nayyar V. A rapid method of obtaining epidermal peel in plants treatment with cupric sulphate and hydrochloric acid. Stain Technol. 1974;49(2):114–116.
- Diane N, Jacob C, Hilger HH. Leaf anatomy and foliar trichomes in Heliotropiaceae and their systematic relevance. Flora. 2003;198(6):468–485.
- Pinto TM, Anjos MR, Martins NM, et al. Structural analysis of Castanea sativa Mill. leaves from different regions in the tree top. Braz Arch Biol Techn. 2011;54(1):117–124.
- Dörken VM, Lepetit B. Morpho-anatomical and physiological differences between sun and shade leaves in Abies alba Mill (Pinaceae, Coniferales): a combined approach. Plant Cell Environ. 2018;41(7):1683–1697.
- Lemos VDT, de Lucena EMP, Bonilla OH, et al. Ecological anatomy of Eugenia luschnathiana (O.Berg) Klotzsch ex B.D.Jacks. (Myrtaceae) leaves in the Restinga region, state of Ceara. Rev Bras Frutic. 2018;40(4):e–696.
- Wang M, Zheng Q, Shen Q, et al. The critical role of potassium in plant stress response. Int J Mol Sci. 2013;14(4):7370–7390.
- Hasanuzzaman M, Bhuyan MHMB, Nahar K, et al. Potassium: a vital regulator of plant responses and tolerance to abiotic stresses. Agronomy. 2018;8(3):31.
- Lee K, Nah SY, Kim ES. Micromorphology and development of the epicuticular structure on the epidermal cell of ginseng leaves. J Ginseng Res. 2015;39(2):135–140.
- Kosma DK, Bourdenx B, Bernard A, et al. The impact of water deficiency on leaf cuticle lipids of Arabidopsis. Plant Physiol. 2009;151(4):1918–1929.
- Reina-Pinto JJ, Yephremov A. Surface lipids and plant defenses. Plant Physiol Bioch. 2009;47(6):540–549.
- Onoda Y, Richards L, Westoby M. The importance of leaf cuticle for carbon economy and mechanical strength. New Phytol. 2012;196(2):441–447.
- Bourgault R, Matschi S, Vasquez M, et al. Constructing functional cuticles: analysis of relationships between cuticle lipid composition, ultrastructure and water barrier function in developing adult maize leaves. Ann Bot. 2020;125(1):79–91.
- Gibson AC. Structure-function relations of warm desert plants. Berlin: Spring-Verlag; 1996.
- Cuttler JM, Rains DW, Loomis RS. The importance of cell size in the water relations of plants. Physiol Plant. 1977;40(4):255–260.
- Boughalleb F, Denden M, Tiba BB. Anatomical changes induced by increasing NaCl salinity in three fodder shrubs, Nitraria retusa, Atriplex halimus and Medicago arborea. Acta Physiol Plant. 2009;31(5):947–960.
- De Luna BN, Freitas MF, Baas P, et al. Leaf Anatomy of Five Neotropical Genera of Primulaceae. Int J Plant Sci. 2017;178(5):362–377.
- Kulkarni M, Borse T, Chaphalkar S. Mining anatomical traits: a Novel modelling approach for increased water use efficiency under drought conditions in plants. Czech J Genet Plant. 2008;44: 11–21.
- García M, Lapp M. Anatomía foliar en especimenes de Oyedaea verbesinoides dc. (Asteraceae) creciendo en tres ambientes distintos [Leaf anatomy in specimens of Oyedaea verbesinoides dc. (Asteraceae) growing in three different environments]. Ernstia. 2005;15: 129–143. Spanish.
- Xi JJ, Chen HY, Bai WP, et al. Sodium-Related Adaptations to Drought: new Insights From the Xerophyte Plant Zygophyllum xanthoxylum. Front Plant Sci. 2018;9:1678.
- Çavuşoğlu K, Kılıç S, Kabar K. Some morphological and anatomical observations during alleviation of salinity (NaCl) stress on seed germination and seedling growth of barley by polyamines. Acta Physiol Plant. 2007;29(6):551–557.
- Hameed M, Ashraf M, Ahmad MSA, et al. Structural and functional adaptations in plants for salinity tolerance. In: Ashraf M, Ozturk M, Amhad MSAeditors. Plant adaptation and phytoremediation. Netherlands: Springer Science+Business Media B.V; 2010. p. 151–170.
- Ely F, Torres F, Gaviria J. Relación entre la morfoanatomia foliar de tres especies de Miconia (Melastomataceae) con su hábitat y distribución altitudinal en el Parque nacional Sierra Nevada de Mérida, Venezuela [Relationship between the foliar morphoanatomy of three Miconia species (Melastomataceae) with their habitat and altitudinal distribution in the Sierra Nevada de Mérida National Park, Venezuela]. Acta Bot Venez. 2005;28:275–299.
- Du ZC, Yang Z. Comparative study on the characteristics of photosynthesis and transpiration in Aneurolepidium chinense of different soil types. Acta Bot Sin. 1995;37:66–73.
- Jaramillo-Pérez AT, Quintanar-Isaías A, Fraile-Ortega ME, et al. Morfoanatomía foliar de Alvaradoa amorphoides Liebm. del estado de Morelos, México [Leaf morphoanatomy of Alvaradoa amorphoides Liebm. from the state of Morelos, Mexico]. Polibotánica. 2015;40:79–98, Spanish
- Jáuregui D, Castro M, Ruiz-Zapata T, et al. Anatomía de los órganos vegetativos de dos especies de Atriplex (Chenopodiaceae) de Venezuela [Anatomy of the vegetative organs of two species of Atriplex (Chenopodiaceae) from Venezuela]. Rev Biol Trop. 2014;62(4):1625–1636. Spanish.
- Balsamo RA, Bauer AM, Davis SD, et al. Leaf biomechanics, morphology, and anatomy of the deciduous mesophyte Prunus serrulata (Rosaceae) and the evergreen sclerophyllous shrub Heteromeles arbutifolia (Rosaceae). Am J Bot. 2003;90(1):72–77.
- Rhizopoulou S, Meletiou-Christou MS, Diamantoglou S. Water relations for sun and shade leaves of four Mediterranean evergreen sclerophylls. J Exp Bot. 1991;42(238):627–635.
- Ragonese AM. Caracteres xeromorfos foliares de Nassauvia langascae (Compositae) [Xeromorphic foliar characters of Nassauvia langascae (Compositae)]. Darwiniana. 1990;30: 1–10. Spanish.
- Leroux O. Collenchyma: a versatile mechanical tissue with dynamic cell walls. Ann Bot. 2012;110(6):1083–1098.
- Jáuregui D, Cardozo A. Anatomía foliar de dos especies de Chrysobalanaceae presentes en el Parque Nacional Henry Pittier [Leaf anatomy of two species of Chrysobalanaceae present in Henry Pittier National Park]. Acta Bot Venez. 2000;23:9–18. Spanish.