ABSTRACT
Introduction
This review examines the impact of allergic rhinitis (AR) on health, emphasizing precision medicine as a transformative approach, with AR treatments to individual factors and explores allergen immunotherapy (AIT) as a disease-modifying strategy, stressing adherence to 2019 ARIA care pathways. The role of digital technologies and mobile health tools in supporting patient-centered care is emphasized. Challenges and future prospects, including improved AIT adherence and novel biomarkers, are addressed.
Area covered
This article provides a comprehensive overview of personalized medicine, encompassing its definition, key components, and transformative impact on healthcare. It explores the role of next-generation sequencing in advancing personalized medicine, highlighting potential benefits and challenges in healthcare system implementation. Additionally, the article explores the underlying mechanisms of allergic rhinitis, the efficacy of allergen immunotherapy, and the utilization of mobile apps to improve adherence and self-care.
Expert opinion
It also delves into the pathophysiology of AR and the use of innovative technologies and advancements in the AR to improve patient care. The insights presented in this article are valuable for understanding the evolving landscape of precision medicine and its impact on AR.
1. Introduction
In precision medicine, patient data is analyzed individually in order to ensure that treatments are tailored specifically to the unique characteristics of that particular individual. It is also referred to as PM or P4 medicine (predictive, personalized, participatory, and preventive) [Citation1]. This approach aims to optimize treatment effectiveness while minimizing risks, morbidity, mortality, and overall healthcare costs by delivering the most suitable intervention at the right time [Citation2]. New developments such as next-gene sequencing propelled PM’s emergence after the Human Genome Project. The idea gained popularity with the 2011 U.S. National Research Council report ‘Towards Precision Medicine,’ which promoted an extensive ‘Knowledge Network.’ Finding the essential individual characteristics that are relevant to particular diseases is the difficult part [Citation3]. In recent years, terms such as Stratified Medicine, Precision Medicine and Personalized Medicine, have been employed with the intention of directing attention toward the individual patient and their unique and variably expressed molecular composition [Citation4]. The distinction between precision medicine and personalized medicine lies in their approaches to tailoring healthcare to individual patients. Precision medicine typically focuses on using measurable biomarkers, often including genomic data, to predict disease risk, prognosis, and treatment response with high specificity [Citation5]. For instance, precision medicine may involve using genetic testing to identify specific gene mutations in cancer patients to predict response to targeted therapies, such as HER2-targeted therapies in breast cancer [Citation6]. On the other hand, personalized medicine takes a broader approach, considering not only genomic data but also environmental exposures and social determinants of health to tailor interventions to individual patients’ unique circumstances. An example of personalized medicine could be tailoring asthma management plans to individual patients’ environmental exposures, such as allergen avoidance strategies based on specific allergen sensitivities identified through environmental assessments and allergy testing. These examples illustrate how precision medicine primarily relies on molecular and genomic data to guide treatment decisions, while personalized medicine takes a more holistic approach by considering broader factors such as environmental exposures and social determinants of health in tailoring patient care [Citation7]. Personalized medicine is the process of tailoring medical interventions to each patient’s specific needs. Using this method, people are categorized into discrete groups based on differences in their susceptibility to contract certain illnesses or react differently to different therapies. By customizing interventions to a person’s genetic composition, way of life, and other pertinent variables, personalized medicine seeks to maximize healthcare outcomes while maximizing the effectiveness of treatments and reducing the possibility of side effects [Citation8]. Personalized care based on the patient’s genetic, immunological, and bio-psycho-social profile needs to be viewed as a dynamic process with clinical stratification, individual endotyping, and deep phenotyping serving as key components. Using personalized diagnostic and therapeutic tools, individual care seeks to successfully treat patients with customized therapy, prevent disease relapse, and forecast the clinical course of the illness [Citation9].
Allergic rhinitis (AR), characterized by diverse nasal inflammation, encompasses three primary clinical phenotypes: allergic rhinitis, infectious rhinitis, and non-allergic forms, occasionally exhibiting partial overlap [Citation10]. More people are expected to develop allergies like hay fever soon because of changes in the environment and how we live [Citation11]. The ICAR-Allergic Rhinitis 2023 stands as a crucial resource, offering extensive insights and approaches to navigate and comprehend the complexities of allergic rhinitis within the dynamic healthcare domain [Citation12]. AR constitutes a substantial global health issue, standing among the most prevalent diseases worldwide and significantly affecting patients’ quality of life. Although guideline-based treatments successfully address the needs of most AR patients, up to 20% may persistently encounter symptoms despite receiving appropriate multiple therapies. In childhood, chronic rhinitis persisting for more than 10 days primarily presents as AR, with a prevalence ranging from 10% to 20%, depending on the geographic location of reference [Citation13]. From a clinical perspective, AR manifests through symptoms such as rhinorrhea, itchy nose and eyes, sneezing, and nasal congestion, displaying variations in both severity and duration [Citation14]. Additionally, AR is linked to disrupted sleep patterns, fatigue, decreased concentration, and diminished productivity, collectively exerting a substantial impact on the quality of life for those affected [Citation15]. Furthermore, AR often coexists with various upper airway comorbidities, including adenoiditis, which is notably prevalent in preschool children, as well as chronic rhinosinusitis [Citation16–18].
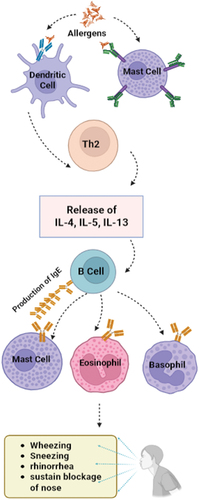
The pathophysiology of AR involves the initial uptake of an allergen by a dendritic cell, initiating the activation of Th2 cells. These Th2 cells, pivotal in defending against extracellular pathogens, generate inflammatory cytokines such as IL-4, IL-5, and IL-13. This sequence promotes the transformation of B-cells into plasma cells, leading to the production of IgE antibodies. Subsequently, these antibodies bind to mast cells, basophils, and eosinophils, triggering the release of histamine, an inflammatory signaling molecule. Histamines play a role in expelling allergens from the body, resulting in allergic rhinitis symptoms such as sneezing and nasal congestion, as depicted in [Citation19].
Precision medicine holds the promise to transform the care of allergic rhinitis by customizing treatment based on individual genetic, environmental, and lifestyle considerations. By employing genetic profiling, allergen sensitivity testing, and pharmacogenomics, healthcare providers can craft tailored treatment plans that may involve targeted immunotherapy and optimized medication choices. Identification of biomarkers facilitates the monitoring of disease progression and treatment response. Additionally, the integration of environmental data and artificial intelligence contributes to a better understanding of individualized triggers. This patient-centric approach empowers individuals to actively engage in their healthcare decisions, representing a significant step toward more effective and personalized allergic rhinitis care.
2. Genetic basis of allergic rhinitis
The development of AR involves a complex interplay between genetic predisposition and exposure to environmental factors, particularly allergens. Although AR does not adhere to a straightforward Mendelian hereditary pattern, research on twins has indicated a hereditary component. Monozygous twins demonstrate a 45–60% concordance for AR, while dizygous twins exhibit a lower concordance of 25%. These findings imply an estimated inheritability ranging from 0.33 to 0.75 for AR. Notably, AR is frequently associated with other atopic diseases that have a genetic basis, such as allergic asthma and atopic dermatitis. However, genetic studies in AR encounter challenges due to various complexities. Understanding the intricate relationship between genetics and environmental factors is essential for unraveling the mechanisms that underlie allergic rhinitis [Citation20]. Critical genetic factors that influence the development of AR involve the Human Leukocyte Antigen (HLA) genes and interleukins. The HLA gene complex, crucial for immune system functionality, encompasses variants such as HLA-DQB1, HLA-DRB1, and HLA-DRA, which are associated with an increased susceptibility to AR. Interleukins, a group of cytokines that regulate immune responses, play a significant role in this context. Variants in IL4 and IL4R, which promote T-helper 2 (Th2) cell differentiation, have been identified as contributors to the susceptibility of AR. Similarly, variants in IL13, which functions similarly to IL4, and IL10, an anti-inflammatory cytokine, have also been associated with an elevated risk of allergic rhinitis, influencing the modulation of immune responses.
3. Traditional therapies of AR
Traditional medicines, including traditional Chinese medicine (TCM), Ayurvedic, Kampo, and African traditional medicine, have been extensively studied for their potential in managing allergic rhinitis (AR) symptoms. Studies within the past five years have consistently demonstrated that therapies targeting antioxidant and anti-inflammatory pathways, hold promise in reducing the risk of developing AR and providing therapeutic relief for affected individuals [Citation21]. Similarly, Ayurvedic and Kampo medicines, originating from India and Japan respectively, have also been explored for their therapeutic potential in alleviating AR symptoms. In Kampo therapy, prescriptions of herbal blends like Mao-to or Byakko-ka-ninjin-to are often recommended to address the fundamental imbalances believed to contribute to allergic symptoms [Citation22]. These holistic medical systems often incorporate a combination of herbal remedies, dietary modifications, nasal irrigation techniques such as saline solutions or herbal decoctions (known as nasya), as well as lifestyle adjustments to address the underlying inflammatory processes contributing to AR. Commonly used herbs in these traditions, such as turmeric, ginger, and licorice, are revered for their potent anti-inflammatory properties, which can help mitigate allergic reactions [Citation23]. Furthermore, African traditional medicine has also demonstrated its efficacy in managing AR symptoms through the utilization of various indigenous plants and herbs. For instance, remedies like rooibos tea, African geranium (umckaloabo), and honey are believed to possess anti-inflammatory and soothing properties, offering relief from allergic rhinitis symptoms. Among these herbal remedies, P. sidoides root extract EPs 7630, commonly referred to as Umckaloabo, has garnered particular interest for its potential therapeutic effects in treating acute respiratory infections, including acute rhinosinusitis and the common cold in adults. While numerous studies have investigated the efficacy of P. sidoides in alleviating symptoms of respiratory tract infections, including AR, some uncertainty remains regarding its overall effectiveness, necessitating further research and clinical validation [Citation24].
4. Diagnostic tests for allergic rhinitis
4.1. Routinary diagnostic tests
4.1.1. Skin Prick Test (SPT)
SPTs are a common and easy technique for determining allergens in a clinical setting as well as for diagnosing IgE-mediated allergic disease. It is considered the first-line diagnostic test for AR. In diagnosing AR, the SPT is a quick and cost-effective procedure. It involves applying small drops of allergen extracts to the skin and lightly pricking the area to observe for immediate reactions like redness and swelling. A positive reaction indicates sensitization to the tested allergen, aiding in the identification of specific triggers for AR. While invasive, SPT offers valuable insights for targeted treatment plans, including allergen avoidance and immunotherapy [Citation25].
4.1.2. Blood Tests for Specific IgE (sIgE)
It is used as a second-line diagnostic tool when SPT results are inconclusive or when certain medical conditions or medications prevent skin testing. In the diagnosis of AR, blood tests measuring specific IgE (sIgE) levels offer a noninvasive alternative to skin tests. This method involves collecting a blood sample to quantitatively assess IgE antibodies for various allergens. Positive results, indicative of heightened sIgE levels, assist in pinpointing specific triggers for allergic rhinitis. While results may take longer and can be influenced by certain factors, blood tests prove especially valuable when skin tests are impractical or inconclusive. They play a crucial role in guiding personalized treatment plans, including allergen avoidance strategies and immunotherapy [Citation26].
4.2. Tests for research use
4.2.1. Nasal Provocation Test (NPT)
NPT is a precise diagnostic tool considered as a third-line diagnostic test for studying AR pathophysiology by inducing controlled nasal mucosal reactions to specific allergens. It reveals characteristic responses, such as mucosal edema, paleness, itching, salivation, and sneezing, signifying a positive reaction [Citation27]. While highly sensitive, it has limitations such as time constraints and potential nonspecific reactions. A novel NPT protocol with multiple aeroallergens in one session addresses these issues, proving specific, sensitive, reproducible, and significantly reducing testing time. This approach offers efficiency and precision in diagnosing Late Asthmatic Reactions (LAR) and occupational rhinitis, complementing the comprehensive evaluation of detailed clinical history and nasal exploration [Citation28].
4.2.2. Pollen challenge chambers
A pollen challenge chamber is a specialized facility designed to expose individuals to pollen allergens, validating their inhalation responses. Advantages include the ability to conduct assays outside natural pollination periods, controlled and uniform exposure for participants, immunity to weather conditions, and minimal impact on personal activities. The chamber comprises a chamber room and a control room with viewing windows, audio-video systems for communication, and emergency supplies. The air-handling system is computer-controlled, maintaining positive pressure to reduce outside contaminants. Pollen delivery is precise, facilitated by a computer-controlled feeder and adjustable blowers [Citation29].
4.2.3. Allergen microarrays
While SPT is widely used for allergen sensitization, its invasiveness and lack of quantitative data prompt exploration of alternatives. Molecular-based allergy diagnostics, like the ImmunoCAP, utilize allergen microarray technology. Introduced in 2002, this chip-based platform simultaneously assesses over 100 allergens with minimal serum volume. However, incidental IgE detection poses challenges, requiring careful clinical history correlation. Despite covering 112 allergen molecules, ImmunoCAP ISAC’s limited sensitivity hinders routine use [Citation30]. Advances like the MeDALL allergen-chip, with 170 allergen molecules, aim to improve sensitivity and overall efficacy compared to traditional ImmunoCAP systems [Citation31].
4.2.4. Basophil and mast cell activation test
Basophils and mast cells (MCs) have pivotal roles in immediate-type allergic reactions, releasing histamine through degranulation. The functional responses of these cells are assessed through the Basophil Activation Test (BAT) as well as from the Mast Cell Activation Test (MAT). When there is incongruence between patient history and sIgE test results or when measuring sIgE is impractical, BAT and MAT become valuable considerations [Citation32].
4.2.5. Nasal allergen challenge test (NAC)
NAC is a gold standard for diagnosing Local Allergic Rhinitis (LAR), a phenotype deviating from traditional classifications. Unlike Allergic Rhinitis (AR), LAR patients test negative for skin prick tests and serum sIgE, necessitating NAC for diagnosis. Despite being time-consuming, recent studies support the safety and reliability of NAC, with a proposed streamlined protocol, allowing multiple allergen provocations in a single session. Oral antihistamines and nasal steroids, commonly used in AR, are presumed effective for LAR based on shared clinical features. Allergen Immunotherapy (AIT) has shown promise in treating AR, with studies demonstrating positive outcomes, improved symptom scores, medication-free days, and increased allergen tolerance in NAC tests.
5. Allergen immunotherapy
One important strategy that is presently used in conjunction with pharmaceutical therapies is AIT, which has various benefits over conventional management. The choice to prescribe AIT must be tailored, considering the clinical relevance of allergens, the persistence of symptoms even after taking guidelines-based treatments, and the accessibility of efficient and superior extracts. As of now, no verified biomarkers exist to forecast AIT success [Citation33].
In the Molecular Allergology Users Guide (PAI 2022), several examples demonstrate the utility of Component-Resolved Diagnostics (CRD) in the care of allergic rhinitis (AR) patients with sensitization to indoor and outdoor aeroallergen sources. CRD enables the identification of specific allergen components, aiding in the differentiation between sensitization to indoor allergens like house dust mites (e.g. Dermatophagoides pteronyssinus, Dermatophagoides farinae) and outdoor allergens such as pollen (e.g. grass pollen, tree pollen) [Citation34]. Additionally, CRD can help identify sensitization patterns associated with different clinical phenotypes of AR, distinguishing between sensitization to perennial allergens like pet dander (e.g. cat, dog) and seasonal allergens like tree or grass pollen, thereby guiding personalized management strategies including allergen avoidance, pharmacotherapy, or allergen immunotherapy [Citation35]. When administering AIT based on Component-Resolved Diagnosis (CRD), doctors compare the level of IgE antibodies to different allergens. They assess how someone reacts to common allergens compared to less common ones. Traditional tests for pollen allergies utilize whole pollen extracts, particularly for patients who are monosensitized or oligosensitized to pollens, even when the pollen seasons overlap. However, in areas where individuals react to less common allergens, like specific proteins in pollen, doctors may need to conduct more advanced tests to pinpoint these specific triggers accurately [Citation36]. This is crucial because AIT might not contain enough of these less common allergens also called as tiny allergens to be effective, or they could potentially worsen allergy symptoms. Therefore, having precise information about what someone is allergic to helps doctors recommend the most appropriate treatment, which may include AIT [Citation37,Citation38]. displays new ways to give AIT, along with their pros and cons. AIT aims to make your body tolerate allergens better by changing how your immune cells work. shows that the main goal of AIT is to build up this tolerance to allergens. When AIT works well, it could mean needing fewer allergy treatments, feeling better in daily life, and having fewer allergy symptoms like sneezing or itching [Citation39]. Ongoing research on allergen immunotherapy (AIT) delivery methods explores various approaches to administer allergens, including subcutaneous injections, sublingual tablets or drops, oral immunotherapy, and novel delivery systems like nanoparticles or microneedles. These methods aim to improve efficacy, safety, and patient adherence in managing allergic diseases [Citation40].
Table 1. Different routes of administration for Allergen Specific Immunotherapy.
6. Biological agents
Although a number of biological agents have recently been studied for the treatment of AR, none of them are presently licensed specifically for this use. Omalizumab is a humanized anti-IgE monoclonal antibody that can decrease IgE receptors on effector cells and prevent free IgE from attaching to mast cells and basophil receptors. Omalizumab significantly minimizes the need for crisis medication, improves the standard of life, and alleviates symptoms in poorly managed allergic rhinitis, according to a meta-analysis of 11 studies including 2870 patients [Citation41]. Omalizumab is generally not advised as monotherapy for AR due to its high cost; however, in individuals with highly sensitive poly-allergic rhinitis who are more susceptible to anaphylaxis, it may be taken into consideration in conjunction with allergen immunotherapy. Omalizumab is one of the monoclonal antibodies that has demonstrated potential in the treatment of chronic rhinosinusitis with nasal polyps (CRSwNP). Anti-IgE, anti-IL-5, and anti-IL-4/IL-13 therapies were shown to be successful in lowering polyp scores, enhancing olfaction, type 2 related biomarkers, nasal airflow, and the standard of life as well as boosting computed tomography outcomes [Citation42].
The addition of Dupilumab to inhaled corticosteroids has been shown in recent investigations to greatly enhance asthma, clinical, and radiologic, outcomes in patients with CRSwNP [Citation43]. There are now studies being conducted on other prospective biological medicines that target IL-33 and Siglec-8 [Citation44]. Furthermore, because of their anti-inflammatory and immunomodulatory qualities, which include the suppression of pro-inflammatory cytokines like IL-8 and tumor necrosis factor-α (TNF-α), macrolide antibiotics have demonstrated noteworthy advantages in chronic rhinosinusitis without nasal polyps (CRSsNP) [Citation45].
7. Tailored therapies for neurogenic allergic rhinitis: a personalized medicine approach
Avoiding the causative agent is the preferred course of action in the management of diseases involving neurogenic inflammation-driven endotypes (gustatory rhinitis and occupational rhinitis) and neuronal imbalance (rhinitis medicamentosa). It is advised that all patients with rhinitis avoid irritants and stop smoking [Citation46]. Similar to this, the main tactic for drug-induced forms of nonallergic rhinitis (NAR) is to stay away from the medicine that triggers it, while aspirin desensitization may be helpful for those who are aspirin intolerant [Citation47]. Anti-inflammatory medications such as nasal corticosteroids and nasal antihistamines may be beneficial for treating inflammatory subtypes such as drug-induced rhinitis and occupational rhinitis. Treatment strategies for NAR noninflammatory phenotypes differ according to the assumed etiology. For instance, the first line of treatment for rhinitis in the elderly is ipratropium bromide, an anticholinergic medication [Citation48]. Transient receptor potential cation channel subfamily V, receptor 1 (TRPV1) expression has been successfully reduced in individuals with irritant-induced rhinitis when capsaicin is used as a targeted intervention for the neurogenic noninflammatory endotype. Remarkably, in cultured human nasal epithelial cells and mast cells, capsaicin administration did not cause apoptosis or necrosis or change the shape of nasal epithelial cells. It has recently been discovered that zelastine, a well-known medication for NAR sufferers, exhibits TRPV1 channel activity by modifying Ca2+ signaling in nasal epithelial cells and sensory neurons [Citation49].
8. Other personalized approaches to target allergic rhinitis
The inflammatory response triggered by Th2 in AR has revealed possible molecular targets for medication development. Th2 cells, T cytotoxic type 2 cells, eosinophils, and basophils all express the chemoattractant receptor homologous molecule (CRTH2), which is interacted with by prostaglandin D2 (PGD2) [Citation50]. BI671800 and OC000459, two CRTH2 antagonists, have shown promise in treating seasonal AR [Citation51]. Another CRTH2 antagonist, setipiprant (ACT-129,968), demonstrated promise in phase II studies but did not achieve the same level of success in phase III trials. A Th2 cytokine inhibitor called saplotast tosilate demonstrated poor therapeutic efficacy in treating persistent AR, although it was able to efficiently decrease the production of IL-4 and IL-5 in vitro and in animal models. Phosphodiesterase 4 inhibitor roflumilast reduced AR symptoms in a limited trial, but larger trials are needed to confirm this treatment’s effectiveness. Seasonal itching did not improve when nasal neuronal hyperresponsiveness was addressed with a TRPV1 antagonist (SB705,498) [Citation52]. By encouraging a transition from Th2 to Th1 response, allergen coupling to toll-like receptor (TLR) agonists, such as TLR4 and TLR9, has been shown to have favorable immunomodulatory effects when used in subcutaneous immunotherapy formulations. Moreover, allergens bound to bacterial or viral DNA sequences through TLR pathways have the potential to elicit Th1 responses [Citation53].
9. Pharmacogenomics in allergic rhinitis
Pharmacogenomics in AR refers to the study of how a particular individual’s genetic composition affects how they respond to AR treatments. This field aims to identify genetic variations that can impact drug metabolism, efficacy, and potential adverse reactions in individuals with AR. Through an understanding of the genetic factors influencing drug response, health care providers can personalize treatment regimens to optimize therapeutic outcomes and minimize the risk of adverse effects. This personalized approach to medication management holds promise for improving the effectiveness of treatments for AR [Citation54].
Pharmacogenomic information can help personalize treatment approaches for AR by considering genetic variations that influence the efficacy and safety of medications commonly used to manage this condition. For example, variations in the genes that encode drug-metabolizing enzymes, such cytochrome P450 (CYP), isoenzymes, can affect how the body processes medications like antihistamines, decongestants, and corticosteroids. In the case of corticosteroids, which are often prescribed for AR, pharmacogenomic biomarkers can help predict a patient’s response to these drugs. For example, polymorphisms in the TBX21, FCER2, and CRHR1 genes have been associated with responses to inhaled corticosteroids in asthma patients, although their specific role in AR is not yet fully understood [Citation55]. Recent studies have shed light on the potential role of specific biomarkers and genomic markers in allergic rhinitis. For instance, examined the association between genetic variants of the SERPINB10 gene and allergic rhinitis susceptibility in Chinese populations, identifying several genetic variants significantly associated with allergic rhinitis susceptibility. These studies highlight the importance of specific biomarkers and genomic markers in understanding the underlying mechanisms of allergic rhinitis and their potential implications for diagnosis, treatment, and personalized medicine. Another study by Li et al. (2016) explored the impact of genetic variations in histamine receptors (HRH1 and HRH4) on treatment response to antihistamines in allergic rhinitis patients, identifying certain single nucleotide polymorphisms (SNPs) in the HRH1 gene associated with variations in treatment response [Citation56]. Additionally, Bossley et al. (2014) investigated the association between serum periostin levels and type-2 inflammation in children with asthma and allergic rhinitis, revealing elevated periostin levels in children with allergic sensitization [Citation57]. Gao et al. (2013) found that genetic variation in superoxide dismutase 1 (SOD1) modulates the association between cadmium exposure and allergic airway disease incidence, including allergic rhinitis. This highlights the role of genetic factors in the susceptibility to allergic airway diseases in response to environmental exposures [Citation58]. Moreover, the pharmacogenomic information can be used to guide dosing decisions. For instance, patients who are poor metabolizers of certain drugs due to genetic variations may require lower doses to achieve the same therapeutic effect, potentially reducing side effects. Conversely, those who are rapid metabolizers may need higher doses to be effective. In the context of AR, this means that by understanding a patient’s genetic profile, clinicians can tailor the medication and dose to the individual, improving the likelihood of a successful treatment outcome and reducing the risk of adverse reactions. This personalized approach to treatment can lead to better symptom control and quality of life for patients with AR [Citation59]. Genetic variations can influence the response to specific AR in several ways. Firstly, they can impact the immune system’s recognition and response to allergens, affecting the severity and duration of allergic reactions. Additionally, genetic variations may influence the production and function of immunoglobulin E (IgE), which plays a crucial role in allergic responses. Furthermore, variations in genes related to inflammation and airway hyperresponsiveness can affect the development and persistence of AR. Finally, genetic variations may also influence the effectiveness of specific treatments for AR, potentially impacting individual responses to medications and immunotherapies [Citation50]. Pharmacogenomics, which focuses on how an individual’s genetic makeup influences their response to drugs, holds promise in personalized medicine for allergic rhinitis (AR) treatment. By understanding genetic variations that affect drug metabolism, efficacy, and adverse reactions, healthcare providers can tailor treatment strategies to maximize effectiveness and minimize side effects. Here are a couple of case studies demonstrating the potential of pharmacogenomics in AR treatment as shown in .
Table 2. Case studies related to pharmacogenomic practices in precision medicines for AR.
10. Environmental and lifestyle consideration
Environmental factors have significant role in the development and exacerbation of AR. For individuals who are susceptible, exposure to allergens such mold spores, dust mites, animal dander, and pollen can result in allergic reactions. Other environmental factors such as air pollution, climate change, ozone, smoking, and viral infections can also have contribution to the development and severity of AR. Additionally, an increased risk of AR has been linked to exposure to environmental toxicants, such as industrial chemicals and pesticides. Depending on an individual’s susceptibility and exposure levels, the impact of environmental factors on AR can be complex and varied [Citation60].
11. Personalized recommendations for lifestyle modifications to manage AR symptoms may include
11.1. Identify allergens
Conduct allergen testing to pinpoint precise triggers, then develop a plan to minimize exposure in daily life.
11.2. Customize indoor air quality
Implement personalized indoor air strategies based on identified sensitivities, such as using specific air purifiers.
11.3. Tailored outdoor activities
Customize outdoor plans according to individual sensitivity to pollen and other triggers, potentially using allergy forecast apps.
11.4. Personalized stress management
Tailor stress reduction techniques to the individual’s lifestyle, incorporating mindfulness or relaxation activities.
11.5. Individualized dietary adjustments
Collaborate with a healthcare provider or nutritionist to identify dietary modifications that align with the individual’s allergies and nutritional needs.
11.6. Custom exercise plans
Develop a personalized exercise routine considering indoor vs. outdoor activities and potential allergen exposure.It is important to note that these recommendations should be personalized based on individual triggers, sensitivities, and overall health status. Consulting with a healthcare professional or allergist for personalized lifestyle modifications is recommended [Citation50].
12. Digital health tools and precision medicine
Utilizing the digital health tools in the management of AR is a relatively new and rapidly evolving field. These tools, such as mobile apps and wearable devices, offer several novel approaches to the diagnosis, treatment, and monitoring of AR. One of the main advantages of digital health solutions that the ability to provide real-time data on patients’ symptoms and treatment response. This can help healthcare professionals to tailor treatments to individual patients depending on their unique needs and characteristics, resulting in better outcomes and increased patient satisfaction. For example, a mobile app-based intervention was found to improve adherence to intranasal corticosteroids and reduce symptoms in patients with AR [Citation61]. The ability of digital health technologies to provide remote monitoring and reduce the need of in-person visits is an additional advantage. This can be particularly useful for patients with AR who may have difficulty accessing healthcare services due to geographic or financial barriers. Digital health tools additionally offer patients access to educational resources and self-management tools, including symptom trackers and medication reminders. This capability enhances overall health outcomes and empowers individuals to actively engage in their own treatment [Citation62].
However, there are also challenges associated with the use of digital health tools in AR management, such as data privacy concerns and the need for effective integration with existing healthcare systems. Overall, the use of digital health tools in AR management represents a promising new approach to improving patient outcomes and reducing healthcare costs. The use of digital health tools in AR management has the potential to enhance patient care and improve outcomes.
12.1. Potential applications of digital health tools in AR management include
12.1.1. Allergen tracking apps
These apps can help individuals track local pollen counts, air quality, and other environmental factors that may trigger AR symptoms. They can provide personalized alerts and recommendations based on individual sensitivities and environmental conditions.
12.1.2. Symptom tracking and management apps
Digital tools can enable individuals to track their symptoms, medication usage, and treatment responses. This data can be valuable for both patients and healthcare providers in understanding symptom patterns and treatment effectiveness.
12.1.3. Telemedicine and virtual consultations
Digital platforms offer the opportunity for remote consultations with allergists and other healthcare providers, providing convenient access to specialized care. This is particularly beneficial for individuals in remote or underserved areas.
12.1.4. Education and self-management resources
Digital platforms can provide access to educational materials, self-management strategies, and lifestyle recommendations for individuals with AR. These resources can empower patients to take an active role in managing their condition.
12.1.5. Wearable devices
Wearable technology, such as smartwatches and activity trackers, can potentially monitor environmental factors, physical activity, and physiological parameters that may impact AR symptoms.
12.1.6. Personalized treatment algorithms
Digital health tools have the capability to amalgamate patient data, encompassing genetic information, environmental exposures, and treatment responses, in order to formulate personalized treatment algorithms and recommendations.In summary, incorporating digital health tools into AR management holds the promise of enhancing patient engagement, delivering personalized care, and improving overall condition management. Nevertheless, it is crucial to ascertain that these tools are grounded in evidence, user-friendly, and accessible to diverse patient populations [Citation63].
13. Currently in progress precision medicine research on AR
The condition known as AR is one of the most common illnesses in the world and has a major negative influence on the health of those who suffer from it. Most AR patients with guideline-based treatment have control over their symptoms; however, up to 20% of patients may still experience symptoms even after taking many prescriptions for proper treatment. Advance customized or personalized therapies aim to minimize side effects and improve therapeutic outcomes by considering the patient’s phenotypic, endotype, and genetic, immunological, and biopsychosocial characteristics. Novel targeted treatments for AR include biologics, small compounds, and AIT. The most compelling data now supports the combination of omalizumab with AIT, with trials showing a positive synergistic impact [Citation64,Citation65].
13.1. AIT a prototype personalized therapy for AR
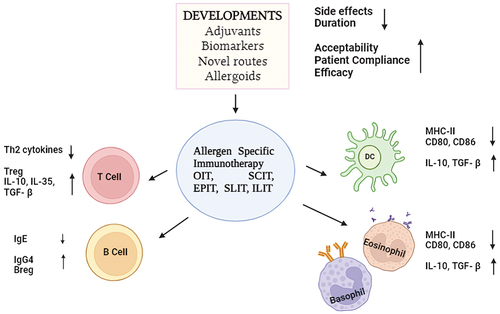
Patients with AR are known to respond exceptionally well in the treatment of allergen immunotherapy, or AIT. Therefore, in compliance with the 2019 ARIA (Allergic Rhinitis and its Impact on Asthma) care pathways, healthcare professionals managing patients with AR should carefully evaluate the use and application of AIT [Citation66]. It produces enduring positive outcomes through significant alterations in the gene and protein expression patterns of allergen-specific T lymphocytes and plasma cells in nasal tissues. Acknowledged as an exceptionally effective treatment for individuals with AR, clinicians overseeing patients with this condition should give serious consideration to the application and administration of AIT following the guidelines outlined in the 2019 ARIA care pathways [Citation67]. It is characterized as an extended therapeutic approach wherein individuals with allergies are repetitively administered extracts containing allergens. The principal objective is to promote tolerance to allergens by impeding the immune-pathogenic pathways that give rise to allergic reactions. In the realm of IgE-mediated inhalant allergies, AIT stands as the exclusive disease-modifying treatment, distinguishing itself from symptomatic medications. Rooted in its distinct immunological rationale, AIT serves as a therapeutic paradigm within the framework of precision medicine, aspiring to tailor treatment strategies in accordance with the unique immunological and clinical attributes specific to each patient [Citation68].
13.2. Clinical decision support in the digital realm for AR
The diverse pathogenic nature and intricate clinical and immunological characteristics of allergic conditions make it challenging to establish an evidence-based clinical diagnosis. Additionally, there is generally a limited number of allergy professionals in proportion to the population of individuals with allergies, and this ratio varies significantly among different nations [Citation69]. Clinical practice often entails demanding situations, with physicians managing a diverse patient population, each presenting unique diseases, comorbidities, and treatment plans. Similar to any medical device, a supportive tool should possess a high degree of user-friendliness, ideally exhibiting adaptability to particular settings, showcasing extensive interoperability, and encompassing a comprehensive risk management framework for enhanced robustness. Various challenges, beyond technical issues such as optimizing the human-computer interface, need to be addressed within different approaches [Citation70]. The emergence of computerized decision assistance concepts presents a potentially valuable tool for aiding physicians in analyzing intricate recommendations and extensive datasets. Technologies in the realm of electronic health (eHealth), especially mobile health (mHealth) solutions, are gaining popularity, providing valuable clinical data on patients. However, it’s crucial to note that a Computerized Decision Support System (CDSS) should not merely serve as an information tool but should also provide specific recommendations for diagnostic and treatment decisions. This discourse centers on the examination of various software tools and mobile solutions that hold the potential to significantly improve and augment the process of clinical decision-making directly at the point-of-care. This improvement can be achieved through (A) targeted patient information, (B) clinical knowledge rooted in guidelines and evidence, and (C) prospectively gathered data, either generated by patients or collected through sensors [Citation71]. The potential of digital technologies in the hands of qualified professionals for preventing, diagnosing, treating, and monitoring allergy diseases is considerable. Several concepts in different stages of development and validation offer a promising outlook for the upcoming year. However, to achieve medical product registration, extensive evaluations are necessary, and as of now, there are no commercially available instruments. Additional challenges in achieving successful adoption include the integration into healthcare practices and ensuring interoperability with existing software systems. Enhanced understanding and applicability can be achieved through increased clinical research and real-world experiences with digital assistance tools in clinical routines [Citation72].
13.3. mHealth in AR
There have recently been few mHealth tools available for the diagnosis of AR, and there is little impact of mHealth on rhinitis diagnosis. The evolution of an innovative wave of point-of-care devices is unfolding, propelled by advancements in integrated biosensors, wireless communication, and power harvesting techniques. Despite the widespread utilization of digital platforms, it is essential to underscore that cost-effectiveness stands as a fundamental prerequisite for ensuring accessibility to any diagnostic tool seamlessly connecting to a smartphone, such as peak nasal inspiratory flow meters or intranasal biosensors. On the other hand, a number of apps have addressed the monitoring of AR control. Allergymonitor, for example, makes it possible to monitor drug intake and symptoms and correlates them with local pollen concentrations [Citation73,Citation74]. The Visual Analog Scale (VAS) is used by the MASK (Mobile Airways Sentinel Network) MASK‐Air, formerly known as Allergy Diary, to evaluate symptoms associated with the eyes, nose, and asthma in addition to job impairment and a general assessment. Through the use of this software, user-generated data has yielded fresh insights into the characteristics of allergic disorders, treatment regimens, and work productivity [Citation75]. By compiling large datasets, mobile apps can also reveal novel trends in allergic disorders. As an illustrative instance, the Mobile Airways Sentinel Network (MASK) identified distinctive patterns of allergic multimorbidity that had not been previously observed in the Mechanisms of the Development of Allergy (MeDALL) study. Subsequently, leveraging this newfound knowledge, a subsequent reanalysis of the MeDALL data substantiated additional patterns associated with asthma, rhinitis, and conjunctivitis [Citation31].
14. Obstacles and prospects for the future
Although the use of both medication and AIT has historically been successful in treating AR, there is still much room for improvement. It’s true that the developments made possible by cutting-edge precision medicine have raised the bar for AIT, medication therapy, and diagnostics. But it’s clear that the road ahead will present more obstacles than the progress made thus far. Especially since AIT is meant to be used for three years in a row, it is crucial to address the serious problem of poor adherence. Otherwise, the efficacy of AIT might be compromised [Citation76]. The importance of delving into distinct biomarkers, characterized as molecular mechanisms serving as diagnostic tools, predictors of response, and parameters for follow-up, becomes apparent within the framework of targeted allergen immunotherapy (AIT). Concerning potential serological biomarkers, there is substantial evidence indicating that immunotherapy initiates the synthesis of immunoglobulin A (IgA), particularly notable in the context of sublingual immunotherapy (SLIT). Moreover, diverse subclasses of immunoglobulin G (IgG), with a pronounced emphasis on subcutaneous immunotherapy (SCIT), are stimulated by immunotherapy. Significantly, the emergence of an IgG2 response plays a pivotal role in competing with immunoglobulin E (IgE) specific to a particular allergen [Citation77]. Several emerging biomarkers hold promise for personalized medicine approaches in allergic rhinitis. Periostin, thymic stromal lymphopoietin (TSLP), eosinophil-derived neurotoxin (EDN), cysteinyl leukotrienes (CysLTs), and eosinophil cationic protein (ECP) have been implicated in allergic inflammation and are associated with disease severity, symptom exacerbation, and treatment response in allergic rhinitis patients [Citation78]. While periostin and TSLP are extensively studied, EDN, CysLTs, and ECP offer additional insights into the pathogenesis of allergic rhinitis and may aid in the development of targeted therapies and personalized treatment strategies [Citation79].
Furthermore, ongoing research is exploring novel methods of administration that have the potential to significantly address the needs and preferences of patients, enhancing therapy adherence; however, these methods still necessitate more comprehensive studies [Citation80]. For instance, intralymphatic immunotherapy (ILIT) has the potential to reduce the frequency of administration, while the anti-inflammatory impact of local nasal immunotherapy (LNIT) utilizing FIP-fve peptide and denatured mite Tyrophagus putrescentiae could be leveraged for the treatment of respiratory allergic conditions [Citation81,Citation82].
15. Conclusion
The review provides a comprehensive overview of the advancements in precision medicine for allergic rhinitis (AR). It discusses the application and administration of allergen immunotherapy (AIT) as a disease-modifying treatment for AR, emphasizing the importance of adhering to the 2019 ARIA care pathways. Additionally, it highlights the potential of digital technologies, mobile health (mHealth) tools, and clinical decision support systems in aiding physicians in diagnosing, treating, and monitoring allergic conditions. The document also addresses the obstacles and prospects for the future, emphasizing the need for improved adherence to AIT and the exploration of novel biomarkers and methods of administration. Overall, it provides valuable insights into the current state and future directions of precision medicine in the management of AR. However, it also acknowledges the significant challenges that must be overcome, such as the need for more robust data infrastructure, increased collaboration among stakeholders, and greater public awareness and education. Overall, this concludes that personalized medicine has the potential to transform the healthcare industry and improve patient outcomes, but it will require continued investment, innovation, and collaboration to achieve its full potential.
16. Expert opinion
In the context of precision medicine in allergic rhinitis, advances in AR including the use of allergen immunotherapy (AIT), have significantly improved the understanding and management of this condition. AIT, involving the gradual administration of allergens to desensitize the immune system, has been pivotal in providing long-term relief from symptoms. Additionally, personalized therapies tailored to individual genetic and environmental factors have emerged as a promising approach. The integration of digital technologies, mobile health tools, and clinical decision support systems has enhanced the monitoring and treatment of allergic rhinitis, offering potential for more personalized and effective care. Ongoing research has provided valuable insights into the characteristics of allergic disorders, leading to a deeper understanding of the condition and potential treatment strategies. Overall, these advancements collectively contribute to the evolving landscape of allergic rhinitis management, offering new opportunities for improved patient care and outcomes.
Increased utilization of digital health tools: With the growing popularity of mobile health technologies, it is likely that more patients will be using apps and other digital tools to monitor their symptoms, track their medication intake, and communicate with their healthcare providers. This could lead to more personalized and effective treatment plans, as physicians will have access to more accurate and up-to-date patient data. Greater emphasis on biomarkers and personalized therapies: As precision medicine continues to evolve, there may be a greater focus on identifying specific biomarkers that can be used to tailor treatment plans to individual patients. This could lead to more personalized and effective therapies, as physicians will be able to target the underlying causes of allergic rhinitis more precisely.
Increased collaboration and data sharing: With the growing emphasis on precision medicine, it is likely that there will be increased collaboration among researchers, healthcare providers, and patients. This could lead to more comprehensive data sharing and analysis, which could help to identify new treatment approaches and improve patient outcomes.
Greater public awareness and education: As precision medicine becomes more widely adopted, there may be a greater emphasis on public awareness and education. This could include initiatives to educate patients about the benefits of precision medicine, as well as efforts to increase public funding for research and development in this field. Overall, it is likely that precision medicine will continue to play an increasingly important role in the management of allergic rhinitis over the next five years. With continued investment, innovation, and collaboration, it is possible that precision medicine could revolutionize the healthcare industry and improve patient outcomes in a significant way. Overall, this review provides valuable insights into the present status and future prospects of precision medicine in managing allergic rhinitis (AR). It acknowledges the significant challenges that need to be addressed, such as improving adherence to treatment and exploring novel biomarkers and administration methods. Additionally, this review recognizes the potential for precision medicine in AR to transform the healthcare industry and enhance patient outcomes. It emphasizes that achieving this potential will require sustained investment, innovation, and collaboration among various stakeholders in the healthcare sector.
Article highlights
Personalized medicine, driven by individual genetic, lifestyle, and relevant factors, has the potential to transform healthcare. Next-generation sequencing advancements enable the identification of genetic variations impacting drug responses.
Allergic rhinitis, affecting patients’ quality of life, can benefit from personalized therapies like allergen immunotherapy, considering various patient characteristics.
Innovations like AIT, mHealth, etc offer potential improvements in treatment adherence for those with chronic respiratory conditions.
Integrating personalized medicine into healthcare systems presents challenges, including data privacy, evidence-based guidelines, and routine clinical practice integration.
In essence, personalized medicine can revolutionize healthcare by tailoring efficient treatments to individual needs, particularly in conditions like allergic rhinitis, utilizing innovative technologies while addressing associated challenges and opportunities in implementation.
Declaration of interests
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Authors contributions
All authors have substantially contributed to the conception and design of the review article and interpreting the relevant literature and have been involved in writing the review article or revised it for intellectual content.
Acknowledgments
The authors express their gratitude to the Department of Pharmaceutical Sciences, School of Health Sciences and Technologies, Dr. Vishwanath Karad, MIT World Peace University in Pune, BHARAT, for their ongoing support and motivation.
Additional information
Funding
References
- Wang X, Du K, She W, et al. Recent advances in the diagnosis of allergic rhinitis. Expert Rev Clin Immunol. 2018;14(11):957–964. doi: 10.1080/1744666X.2018.1530113
- Proper SP, Azouz NP, Mersha TB. Mersha TBJFiI. Achieving precision medicine in allergic disease: progress and challenges. Front Immunol. 2021;12:720746. doi: 10.3389/fimmu.2021.720746
- Council NR, Earth Do, Studies L, et al. Toward precision medicine: building a knowledge network for biomedical research and a new taxonomy of disease. 2011.
- Rubin MAJN. Health: make precision medicine work for cancer care. Nature. 2015;520(7547):290–291. doi: 10.1038/520290a
- Khoury MJ, Iademarco MF, WTJAjopm R. Precision public health for the era of precision medicine. 2016;50(3):398. doi: 10.1016/j.amepre.2015.08.031
- Slamon DJ, Leyland-Jones B, Shak S, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344(11):783–792. doi: 10.1056/NEJM200103153441101
- Flory JH, Sleiman PM, Christie JD, et al. 17q12-21 variants interact with smoke exposure as a risk factor for pediatric asthma but are equally associated with early-onset versus late-onset asthma in North Americans of European ancestry. J Allergy Clin Immunol. 2009;124(3):605–607. doi: 10.1016/j.jaci.2009.05.047
- Beccia F, Causio FA, Hoxhaj I, et al. Integrating China in the international consortium for personalised medicine. A position paper on healthcare professionals’ education and citizens’ empowerment in personalised medicine. 2023;23(1):438. doi: 10.1186/s12909-023-04420-z
- König IR, Fuchs O, Hansen G, et al. What is precision medicine? Eur Respir J. 2017;50(4):1700391. doi: 10.1183/13993003.00391-2017
- Montella S, Baraldi E, Cazzato S, et al. Severe asthma features in children: a case–control online survey. Ital J Pediatr. 2016;42(1):1–9. doi: 10.1186/s13052-016-0217-z
- von Mutius E. von Mutius EJJoa, immunology c. Influences in allergy: epidemiology and the environment. 2004;113(3):373–379. doi: 10.1016/j.jaci.2003.12.040
- Wise SK, Damask C, Roland LT, et al., editors. International consensus statement on allergy and rhinology: allergic rhinitis–2023. In International forum of allergy & rhinology. Wiley Online Library; 2023.
- Bauchau V, Sjerj D. Prevalence and rate of diagnosis of allergic rhinitis in Europe. Eur Respir J. 2004;24(5):758–764. doi: 10.1183/09031936.04.00013904
- Licari A, Caimmi S, Bosa L, et al. Rhinosinusitis and asthma: a very long engagement. Int J Immunopathol Pharmacol. 2014;27(4):499–508. doi: 10.1177/039463201402700405
- Bousquet J, Pfaar O, Agache I, et al. ARIA‐EAACI care pathways for allergen immunotherapy in respiratory allergy. 2021;11(4):e12014.
- Cirillo I, Marseglia G, Klersy C, et al. Allergic patients have more numerous and prolonged respiratory infections than nonallergic subjects. Allergy. 2007;62(9):1087–1090. doi: 10.1111/j.1398-9995.2007.01401.x
- Brambilla I, Pusateri A, Pagella F, et al. Adenoids in children: advances in immunology, diagnosis, and surgery. 2014;27(3):346–352. doi: 10.1002/ca.22373
- Marseglia GL, Pagella F, Klersy C, et al. The 10-day mark is a good way to diagnose not only acute rhinosinusitis but also adenoiditis, as confirmed by endoscopy. 2007;71(4):581–583. doi: 10.1016/j.ijporl.2006.12.003
- Hoyte FC, Hsjf N. Recent advances in allergic rhinitis. F1000Res. 2018;7:1333. doi: 10.12688/f1000research.15367.1
- Ortiz RA, Kcji B, Clinics A. Genetics of allergic diseases. Immunol Allergy Clin North Am. 2015;35(1):19–44. doi: 10.1016/j.iac.2014.09.014
- Qin Z, Xie L, Li W, et al. New Insights into Mechanisms Traditional Chinese Medicine for Allergic Rhinitis by Regulating Inflammatory and Oxidative Stress Pathways. J Asthma Allergy. 2024;Volume 17:97–112. doi: 10.2147/JAA.S444923
- Watanabe K, Matsuura K, Gao P, et al. Traditional Japanese Kampo medicine: clinical research between modernity and traditional medicine—the state of research and methodological suggestions for the future. Evidence-Based Complementary Altern Med. 2011;2011(1). doi: 10.1093/ecam/neq067
- Dahanayake JM, Perera PK, Galappaththy P, et al. Efficacy and safety of two Ayurvedic dosage forms for allergic rhinitis: Study protocol for an open-label randomized controlled trial. Trials. 2020;21(1):1–8. doi: 10.1186/s13063-019-4004-1
- Mahomoodally MFJE-bc, medicine a. Traditional medicines in Africa: an appraisal of ten potent African medicinal plants. 2013;
- Rasool R, Shera IA, Nissar S, et al. Role of skin prick test in allergic disorders: a prospective study in kashmiri population in light of review. 2013;58(1):12–17. doi: 10.4103/0019-5154.105276
- Ansotegui IJ, Melioli G, Canonica GW, et al. IgE allergy diagnostics and other relevant tests in allergy. World Allergy Organ Position Pap. 2020;13(2):100080. doi: 10.1016/j.waojou.2019.100080
- Agache I, Bilò M, Braunstahl GJ, et al. In vivo diagnosis of allergic diseases-allergen provocation tests. Allergy. 2015;70(4):355–365. doi: 10.1111/all.12586
- González-Pérez R, El-Qutob D, Letrán A, et al. Precision medicine in mite allergic rhinitis Front. Allergy. 2021;2:724727. doi: 10.3389/falgy.2021.724727
- Angjeli E, Gomes P, Lane KJ, et al. Technical and clinical validation of the Allergen BioCube® for Timothy grass. 2017;5(1):78–84. doi: 10.1002/iid3.143
- Jeon H, Jung JH, Kim Y, et al. Allergen microarrays for in vitro diagnostics of allergies: comparison with ImmunoCAP and AdvanSure. 2018;38(4):338. doi: 10.3343/alm.2018.38.4.338
- Anto JM, Bousquet J, Akdis M, et al. Mechanisms of the Development of Allergy (MeDALL): Introducing novel concepts in allergy phenotypes. 2017;139(2):388–399. doi: 10.1016/j.jaci.2016.12.940
- Hoffmann H, Santos A, Mayorga C, et al. The clinical utility of basophil activation testing in diagnosis and monitoring of allergic disease. 2015;70(11):1393–1405. doi: 10.1111/all.12698
- Bousquet J, Pfaar O, Togias A, et al. 2019 ARIA Care pathways for allergen immunotherapy. Alergologia 2019;4(3):2087–2102. doi: 10.26416/Aler.3.4.2019.2749
- Allen K, Canonica GW, Ansotegui IJ, et al. A WAO-ARIA-GA2LEN consensus document on molecular-based allergy diagnostics. 2013;6(1).
- Matricardi P, Kleine‐Tebbe J, Hoffmann H, et al. EAACI molecular allergology user’s guide. 2016;27:1–250. doi: 10.1111/pai.12563
- Luengo O, Cardona VJC, allergy t. Component resolved diagnosis: when should it be used? Clin Transl Allergy. 2014;4(1):1–9. doi: 10.1186/2045-7022-4-28
- Barber D, Moreno C, Ledesma A, et al. Degree of olive pollen exposure and sensitization patterns. Clin Implic. 2007;17(Suppl 1):11–16.
- Douladiris N, Savvatianos S, Roumpedaki I, et al. A molecular diagnostic algorithm to guide pollen immunotherapy in southern Europe: towards component-resolved management of allergic diseases. 2013;162(2):163–172. doi: 10.1159/000353113
- Kucuksezer UC, Ozdemir C, Cevhertas L, et al. Mechanisms of allergen-specific immunotherapy and allergen tolerance. 2020;69(4):549–560. doi: 10.1016/j.alit.2020.08.002
- Johnson L, Duschl A, Himly MJV. Nanotechnology-based vaccines for allergen-specific immunotherapy: Potentials and challenges of conventional and novel adjuvants under research. 2020;8(2):237. doi: 10.3390/vaccines8020237
- Tsabouri S, Tseretopoulou X, Priftis K, et al. Omalizumab for the treatment of inadequately controlled allergic rhinitis: a systematic review and meta-analysis of randomized clinical trials. J Allergy Clin Immunol Pract. 2014;2(3):332–340. e1. doi: 10.1016/j.jaip.2014.02.001
- Tsetsos N, Goudakos JK, Daskalakis D, et al. Monoclonal antibodies for the treatment of chronic rhinosinusitis with nasal polyposis: a systematic review. 2018;56(1):11–21. doi: 10.4193/Rhin17.156
- Khwaja S, Kumar B, Philpott C, et al. Efficacy and safety of dupilumab in patients with severe chronic rhinosinusitis with nasal polyps (LIBERTY NP SINUS-24 and LIBERTY NP SINUS-52): results from two multicentre, randomised, double-blind, placebo-controlled, parallel-group phase 3 trials. 2019.
- Ghadersohi STan BKJOCoNA. Contemporary pharmacotherapy for allergic rhinitis and chronic rhinosinusitis. Otolaryngol Clin North Am. 2017;50(6):1135–1151. doi: 10.1016/j.otc.2017.08.009
- Oakley GM, Harvey RJ, VJJCa L, et al. The role of macrolides in chronic rhinosinusitis (CRSsNP and CRSwNP). Curr Allergy Asthma Rep. 2017;17(5):1–10. doi: 10.1007/s11882-017-0696-z
- Hellings PW, Klimek L, Cingi C, et al. Non‐allergic rhinitis: position paper of the European Academy of Allergy and Clinical Immunology. 2017;72(11):1657–1665. doi: 10.1111/all.13200
- Klimek L, Dollner R, Pfaar O, et al. Aspirin desensitization: useful treatment for chronic rhinosinusitis with nasal polyps (CRSwNP) in aspirin-exacerbated respiratory disease (AERD)? Curr Allergy Asthma Rep. 2014;14(6):1–9. doi: 10.1007/s11882-014-0441-9
- Malmberg H, Grahne B, Holopainen E, et al. Ipratropium (Atrovent) in the treatment of vasomotor rhinitis of elderly patients. 1983;8(4):273–276. doi: 10.1111/j.1365-2273.1983.tb01441.x
- Van Gerven L, Steelant B, Hellings PJA. Nasal hyperreactivity in rhinitis: A diagnostic and therapeutic challenge. 2018;73(9):1784–1791. doi: 10.1111/all.13453
- Agache I, CAJTJoci A. Precision medicine and phenotypes, endotypes, genotypes, regiotypes, and theratypes of allergic diseases. J Clin Investigation. 2019;129(4):1493–1503. doi: 10.1172/JCI124611
- Shiraishi Y, Takeda K, Domenico J, et al. Role of prostaglandin D2 and CRTH2 blockade in early- and late-phase nasal responses. Clin Exp Allergy. 2014;44(8):1076–1082. doi: 10.1111/cea.12280
- Krug N, Gupta A, Badorrek P, et al. Efficacy of the oral chemoattractant receptor homologous molecule on TH2 cells antagonist BI 671800 in patients with seasonal allergic rhinitis. J Allergy Clin Immunol. 2014;133(2):414–419. e8. doi: 10.1016/j.jaci.2013.10.013
- Ratner P, Andrews CP, Hampel FC, et al. Efficacy and safety of setipiprant in seasonal allergic rhinitis: results from Phase 2 and Phase 3 randomized, double-blind, placebo-and active-referenced studies. Allergy, Asthma & Clin Immunol. 2017;13(1):1–15. doi: 10.1186/s13223-017-0183-z
- Voukantsis D, Berger U, Tzima F, et al. Personalized symptoms forecasting for pollen-induced allergic rhinitis sufferers. Int J Biometeorol. 2015;59(7):889–897. doi: 10.1007/s00484-014-0905-6
- Meurs H, Zaagsma J, Maarsingh H, et al. Recent patents in allergy/immunology: use of arginase inhibitors in the treatment of asthma and allergic rhinitis. 2019;74(6):1206. doi: 10.1111/all.13770
- Liu AH, Anderson WC III, Dutmer CM, et al. Advances in asthma 2015: Across the lifespan. 2016;138(2):397–404. doi: 10.1016/j.jaci.2016.06.013
- Brockow KJA. Dilemmas of allergy diagnosis in perioperative anaphylaxis. 2014;69(10):1265–1266. doi: 10.1111/all.12485
- Vidal C, Porras-Hurtado L, Cruz R, et al. Association of thromboxane A1 synthase (TBXAS1) gene polymorphism with acute urticaria induced by nonsteroidal anti-inflammatory drugs. 2013;132(4):989–991. doi: 10.1016/j.jaci.2013.04.045
- Khan DA, EjjjoA P, Immunology C. Pharmacogenomic biomarkers in allergy and immunology practice. J Allergy Clin Immunol. 2020;146(3):509–512. doi: 10.1016/j.jaci.2020.05.050
- Murrison LB, Brandt EB, Myers JB, et al. Environmental exposures and mechanisms in allergy and asthma development. 2019;129(4):1504–1515. doi: 10.1172/JCI124612
- Bousquet J, Devillier P, Arnavielhe S, et al. Treatment of allergic rhinitis using mobile technology with real‐world data: the MASK observational pilot study. 2018;73(9):1763–1774. doi: 10.1111/all.13406
- Munteanu LA, Frandes M, Timar B, et al. The efficacy of a mobile phone application to improve adherence to treatment and self-management in people with chronic respiratory disease in Romanian population–a pilot study. BMC Health Serv Res. 2020;20(1):1–11. doi: 10.1186/s12913-020-05340-0
- Nebeker C, Parrish EM, Graham S. The AI-powered digital health sector: ethical and regulatory considerations when developing digital mental health tools for the older adult demographic. In: Artificial Intelligence in Brain and Mental Health: Philosophical, Ethical & Policy Issues. Springer; 2022. p. 159–176.
- Breiteneder H, Diamant Z, Eiwegger T, et al. Future research trends in understanding the mechanisms underlying allergic diseases for improved patient care. 2019;74(12):2293–2311. doi: 10.1111/all.13851
- Licari A, Castagnoli R, Tosca MA, et al. Personalized therapies for the treatment of allergic rhinitis. 2019;4(5):275–281. doi: 10.1080/23808993.2019.1681896
- Zhang Y, Lan F, Zhang LJA. Advances and highlights in allergic rhinitis. 2021;76(11):3383–3389. doi: 10.1111/all.15044
- Li H, Chen S, Cheng L, et al. Chinese guideline on sublingual immunotherapy for allergic rhinitis and asthma. 2019;11(12):4936.
- Dhami S, Nurmatov U, Arasi S, et al. Allergen immunotherapy for allergic rhinoconjunctivitis: a systematic review and meta‐analysis. 2017;72(11):1597–1631. doi: 10.1111/all.13201
- Rudin RS, Fischer SH, Y SHI, et al. Trends in the use of clinical decision support by health system–affiliated ambulatory clinics in the united states. 2019;7(4):4–10.
- Sutton RT, Pincock D, Baumgart DC, et al. An overview of clinical decision support systems: benefits, risks, and strategies for success. 2020;3(1):17. doi: 10.1038/s41746-020-0221-y
- Prgomet M, Li L, Niazkhani Z, et al. Impact of commercial computerized provider order entry (CPOE) and clinical decision support systems (CDSSs) on medication errors, length of stay, and mortality in intensive care units: a systematic review and meta-analysis. 2017;24(2):413–422. doi: 10.1093/jamia/ocw145
- Dramburg S, Marchante Fernández M, Potapova E, et al. The potential of clinical decision support systems for prevention, diagnosis, and monitoring of allergic diseases. 2020;11:575892. doi: 10.3389/fimmu.2020.02116
- Costa C, Menesatti P, Brighetti M, et al. Pilot study on the short-term prediction of symptoms in children with hay fever monitored with e-Health technology. Eur Ann Allergy Clin Immunol. 2014;46(6):216–225.
- Matricardi PM, Dramburg S, Alvarez‐Perea A, et al. The role of mobile health technologies in allergy care: An EAACI position paper. 2020;75(2):259–272. doi: 10.1111/all.13953
- Correia de Sousa J. ARIA 2016: care pathways implementing emerging technologies for predictive medicine in rhinitis and asthma across the life cycle. 2016.
- Ridolo E, Incorvaia C, Pucciarini F, et al. Current treatment strategies for seasonal allergic rhinitis: where are we heading? 2022;20(1):9. doi: 10.1186/s12948-022-00176-x
- Shamji MH, Valenta R, Jardetzky T, et al. The role of allergen‐specific IgE, IgG and IgA in allergic disease. 2021;76(12):3627–3641. doi: 10.1111/all.14908
- Kauppinen Z, Rautiainen T, Mäntyjärvi V, et al. Mutant derivatives of the main respiratory allergen of cow are less allergenic than the intact molecule. Clin & Exp Allergy. 1999;29(7):989–996. doi: 10.1046/j.1365-2222.1999.00605.x
- Shen L, Kapsogeorgou EK, Yu M, et al. Evaluation of salivary gland protein 1 antibodies in patients with primary and secondary Sjogren’s syndrome. 2014;155(1):42–46. doi: 10.1016/j.clim.2014.08.009
- Yen C-Y, Yu C-H, Tsai J-J, et al. Effects of local nasal immunotherapy with FIP-fve peptide and denatured Tyrophagus putrescentiae for storage mite-induced airway inflammation. 2022;70(1):6. doi: 10.1007/s00005-022-00645-w
- Kasemsuk N, Ngaotepprutaram P, Kanjanawasee D, et al., editors. Local nasal immunotherapy for allergic rhinitis: a systematic review and meta‐analysis. In International Forum of Allergy & Rhinology. Wiley Online Library; 2022.
- Ridolo E, Incorvaia C, Heffler E, et al. The present and future of allergen immunotherapy in personalized medicine. 2022;12(5):774.
- Dhami S, Kakourou A, Asamoah F, et al. Allergen immunotherapy for allergic asthma: a systematic review and meta‐analysis. 2017;72(12):1825–1848. doi: 10.1111/all.13208
- Larenas‐Linnemann D, Antolín‐Amérigo D, Parisi C, et al. National clinical practice guidelines for allergen immunotherapy: an international assessment applying AGREE‐II. 2018;73(3):664–672. doi: 10.1111/all.13316
- Huang Y, Wang C, Wang X, et al. Efficacy and safety of subcutaneous immunotherapy with house dust mite for allergic rhinitis: a meta‐analysis of randomized controlled trials. 2019;74(1):189–192. doi: 10.1111/all.13583
- Jarkvist J, Salehi C, Akin C, et al. Venom immunotherapy in patients with clonal mast cell disorders: IgG4 correlates with protection. 2020;75(1):169–177. doi: 10.1111/all.13980
- Tam H, Calderon MA, Manikam L, et al. Specific allergen immunotherapy for the treatment of atopic eczema. Database Systematic Rev. 2016 2;2016(2). doi: 10.1002/14651858.CD008774.pub2
- Reier‐Nilsen T, Michelsen MM, Lødrup Carlsen KC, et al. Feasibility of desensitizing children highly allergic to peanut by high‐dose oral immunotherapy. 2019;74(2):337–348. doi: 10.1111/all.13604
- Chu DK, Wood RA, French S, et al. Oral immunotherapy for peanut allergy (PACE): a systematic review and meta-analysis of efficacy and safety. 2019;393(10187):2222–2232. doi: 10.1016/S0140-6736(19)30420-9
- Senti G, Freiburghaus AU, Larenas-Linnemann D, et al. Intralymphatic immunotherapy: update and unmet needs. 2019;178(2):141–149. doi: 10.1159/000493647
- Witten M, Malling H-J, Blom L, et al. Is intralymphatic immunotherapy ready for clinical use in patients with grass pollen allergy? J Allergy And Clin Immunol. 2013;132(5):1248–1252. e5. doi: 10.1016/j.jaci.2013.07.033
- Jones SM, Sicherer SH, Burks AW, et al. Epicutaneous immunotherapy for the treatment of peanut allergy in children and young adults. J Allergy Clin Immunol. 2017;139(4):1242–1252. e9. doi: 10.1016/j.jaci.2016.08.017
- Pongracic JA, Gagnon R, Sussman G, et al. Safety of epicutaneous immunotherapy in peanut-allergic children: REALISE randomized clinical trial results. J Allergy Clin Immunol Pract. 2022;10(7):1864–1873. e10. doi: 10.1016/j.jaip.2021.11.017
- Mondoulet L, Dioszeghy V, Busato F, et al. Gata3 hypermethylation and Foxp3 hypomethylation are associated with sustained protection and bystander effect following epicutaneous immunotherapy in peanut‐sensitized mice. 2019;74(1):152–164. doi: 10.1111/all.13479
- Spergel JM, Elci OU, Muir AB, et al. Efficacy of epicutaneous immunotherapy in children with milk-induced eosinophilic esophagitis. Clin Gastroenterol Hepatol. 2020;18(2):328–336. e7. doi: 10.1016/j.cgh.2019.05.014
- Jeny F, Uzunhan Y, Lacroix M, et al. Predictors of mortality in fibrosing pulmonary sarcoidosis. 2020;169:105997. doi: 10.1016/j.rmed.2020.105997
- Fricke-Galindo I, Ortega-Vázquez A, Monroy-Jaramillo N, et al. Allele and genotype frequencies of genes relevant to anti-epileptic drug therapy in Mexican-Mestizo healthy volunteers. 2016;17(17):1913–1930.
- AJIrop G. Complexities of CYP2D6 gene analysis and interpretation. Int Rev Psychiatry. 2013;25(5):534–553. doi: 10.3109/09540261.2013.825581
- Sim S, Kacevska M, Ingelman-Sundberg MJTpj. Pharmacogenomics of drug-metabolizing enzymes: a recent update on clinical implications and endogenous effects. Pharmacogenomics J. 2013;13(1):1–11. doi: 10.1038/tpj.2012.45