Abstract
A substantial amount of research has been dedicated to aquatic biofilms, but unlike the influence of macrograzers, that of microscopic herbivores (2 μm to 2 mm) has been largely disregarded. The selective pressure that they may exert remains unexplored, despite its potential impact on microbial community structure and ultimately, function. We performed in situ experiments to assess the abundance of microflora and micro-meiofauna in mature biofilms, in four rivers located in southwest France, stressing their quantitative importance (averaging 50% of the total number of organisms counted). We highlighted that the balance between microflora and micro-meiofauna depends on local environmental conditions, and specific taxon selection is likely to provide information on river pollution. Indeed, microalgae were directly favoured by nutrients and indirectly by biocides targeting micrograzers. In contrast, micro-meiofauna was predominant in sites where herbicides were found, in particular photosynthesis inhibitors (e.g. in the River Ponteils, 97% micro-meiofauna). Although the prey–predator relationships in the laboratory experiment appeared to depend on the initial pools of microorganisms during the first days, they reached stable states within a week. Then, all cultures stabilized around a ratio of 20 ± 6 : 1 prey/potential predator. Further research is required to better understand the abiotic (water quality, habitat) and biotic (traits of the organisms present) drivers governing the coexistence of microalgae and their predators across the microbial landscape, and its consequences on the structure and function of the aquatic ecosystem.
Keywords:
Introduction
In freshwater streams, key biological processes are ensured by biofilms (primary production, nutrient cycling, provision of food resources), which are at the basis of ecosystem functioning. Aquatic biofilms are complex microscale ecosystems composed of microscopic algae, bacteria, protozoans and other micrograzers in constant interaction together, and with their environment. The close spatial proximity between these very diverse life-strategy organisms engenders many biotic interactions: competition for resources, facilitation, protection (providing refuge), syntrophism (e.g. algal exudates released during photosynthesis are metabolised by bacteria, inorganic nutrients produced by heterotrophic mineralization are taken up by algae), and predation by micrograzers. Moreover, the biofilm organisms develop under environmental constraints that control their development. Hence, biofilms are interfaces that integrate a variety of responses to environmental changes and chemical stressors; their rapid interaction with dissolved substances results in functional and structural responses, endorsing them as ‘early warning systems’ of disturbances to the ecosystem (Sabater et al. Citation2007). In these microecosystems, the responses to environmental stressors vary depending on the target organisms, and the overall impacts of pollutants resulting from both direct and indirect effects (Proia et al. Citation2012). Unlike diatoms (brown microalgae) or bacteria that have received great attention over recent decades, the micro-meiofauna has been overlooked. Its biodiversity in river biofilms remains almost totally unexplored, despite its potential influence on algal composition, as also exerted by macrofauna (more extensively studied; e.g. Bott and Borchardt Citation1999; Lange et al. Citation2011). Micro-meiofauna is composed of microscopic heterotrophs – generally defined in a size range of 2 μm to 2 mm (e.g. Artois et al. Citation2011). In attached communities such as biofilms, prey species (microalgae) may have difficulty avoiding their predators, and must coexist with them, at the cost of high predation risk. Hence, understanding prey–predator relationships in biofilms under natural, complex environmental conditions, is a topical question, just as it is for higher organisms (Hammill et al. Citation2015), and requires dedicated investigations. Few data are available on the quantitative importance of micro-meiofauna in fluvial biofilms. However, algal ingestion by microconsumers, reviewed by Bott (Citation1996), can reach hourly rates of 13.5 diatoms per predator, suggesting significant potential top–down control of microalgal abundance by micro-meiofauna. Precise quantification and characterization of the biofilm micro-meiofauna would consequently increase our understanding of the river microbial ecosystem and of the relationships between microalgae and micrograzers. Predators are expected to be more vulnerable to environmental changes than their prey, due to their longer life cycles and dependence on lower trophic levels (Petchey et al. Citation1999). Prey–predator coexistence may consequently be shaped by environmental (abiotic) fluctuations, and indicate greater quality (in terms of abiotic stability) of the sites colonized.
In this context, we performed complementary field and laboratory experiments. We analysed the microflora and micro-meiofauna abundances in mature biofilms collected in four rivers in southwest France. The numbers of micro-meiofauna and microflora individuals in natural biofilms were quantified in upstream and downstream sections of the rivers, with the objective to determine how environmental stressors (such as toxic contamination) shape, directly and indirectly, periphytic community (biofilm) structure. Large-scale studies demonstrate a decrease in the specific diversity of benthic microorganisms with environmental pollution (Tison et al. Citation2005; Donohue et al. Citation2009). Therefore, we expected that upstream species composition would be more diversified and reflect the local pool of species, from which would stem the downstream community affected by degraded water quality. Species selection was anticipated to be linked with possible pollution sources along the gradients. The sites selected exhibited different types of contamination, and we predicted that herbicide-impacted sites would be dominated by micro-meiofauna, whereas insecticide contamination would rather target fauna and favour microalgae. Field characterization was complemented with simplified laboratory experiments, under controlled uncontaminated conditions, to assess the joint growth dynamics of microalgae and micro-meiofauna depending on their respective initial densities. We expected to experimentally determine the co-evolution of prey and predator numbers over time, at three different prey–predator ratios (starving, intermediate and excess). Specific aims were to confirm the quantitative impact of grazing pressure in biofilms, and to demonstrate that prey–predator relationships at the microbial scale fit commonly used models for higher organisms.
Materials and methods
Sampling natural biofilm communities: field study
Sampling sites
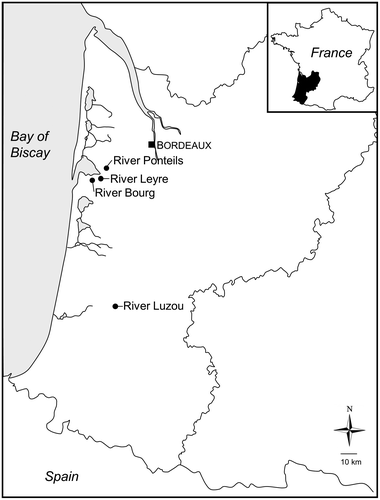
We selected four rivers in southwest France (Figure ). All presented a sandy substrate and slightly acidic water typical of the Landes hydroecoregion (Wasson, Chandesris, and Pella Citation2002), with pine riparian cover. In 2014 in each river, artificial substrates (unpolished glass slides, 300 cm2 total area) were immersed for 1 month of colonization to collect mature biofilms (Peterson and Stevenson Citation1990), following the sampling method detailed in Morin et al. (Citation2007). The substrates were placed in openwork plastic boxes, preventing grazing by larger predators (e.g. fish) and ensuring comparable water current conditions. Using a buoy, they were immersed 10 cm below the water surface, under similar light penetration conditions. Triplicate glass slides were placed upstream (reference sites) and downstream, in three tributaries of Arcachon Bay (River Leyre, River Bourg, River Ponteils) and a river from the Landes area (River Luzou), about 90 km south. Their watersheds are characterized by contrasting land uses: agricultural (Leyre, Bourg), urban (Bourg) and industrial (Ponteils, Luzou).
More specifically, the Leyre is the main tributary of Arcachon Bay and drains a large (2000 km2) watershed mainly occupied by pine forest, with a 13% agricultural area predominantly used for maize production. The Bourg watershed is characterized by market gardening (mostly carrots) and peri-urban weeding. The Ponteils and Luzou receive passive landfill inputs and complex industrial discharge, respectively.
Sample collection and analysis
After a 1-month immersion, all substrates except those of the Ponteils upstream site (due to substrate loss) were recovered. Water physicochemistry (pH, dissolved oxygen, conductivity and temperature) was measured in situ using appropriate WTW probes. One-litre water samples were collected, stored at 4°C and analysed in the laboratory for nutrients.
The mature biofilms were carefully scraped off each artificial substrate with a razor blade, suspended in a standard volume (200 mL) of Evian mineral water (Danone, 1.5-l PET bottles) and stored at 4°C in the dark until microscopic observation (up to 48 h). A 20-ml aliquot of each triplicate suspension was used to quantify dry weight following the European standard NF EN 872. Then, replicate suspensions from each sampling site were pooled for taxonomic analyses, to counter possible heterogeneity in species colonization of the slides. Microalgae (distinguishing between diatoms, cyanobacteria and green algae) and micro-meiofauna were quantified and identified simultaneously from fresh material, using a Bürker counting chamber under light microscopy with 40× magnification (Leica DMLS phase-contrast microscope, Germany). Briefly, the suspension was gently homogenized and a 15-ml sample was collected. The suspension was decanted for 16 h to concentrate the organisms, and 2 ml of the pellet was dropped onto the Bürker chamber. For each sample, all organisms in all fields of the chamber (1.8 μm3 in total) were counted to calculate organism densities. For each taxonomic group, the densities were then recorded as individuals per cm2 of glass substrate. The specifically identified micro-meiofauna taxa were protozoa (flagellates, heliozoans, sarcodinae – amoebae, thecamoebians – and ciliates) and metazoans (rotifers, nematodes, oligochaetes, gastrotrichs and dipteran larvae). They were identified to the lowest taxonomic level possible following de Puytorac, Grain, and Mignot (Citation1987), Foissner (Citation1996), Vedry (Citation1996), Canler et al. (Citation1999), Lynn (Citation2010) and Tachet et al. (Citation2010).
Coexistence of predators and prey under simplified conditions: laboratory experiment
Collection of natural communities
Triplicate glass slides were implemented with the same protocols at the three sites studied in Lainé, Morin, and Tison-Rosebery (Citation2014) along the River Luzou, for 30 days in June 2014. A third site, 5 km further downstream, was added to those described above. At this site, the industrial contaminants are diluted (Lainé, Morin, and Tison-Rosebery Citation2014) and the ratio between microalgae and micro-meiofauna was expected to differ slightly from the two sites upstream. The glass slides were individually scraped and diluted in Evian water to give triplicate 200-ml suspensions per treatment, called Up (inoculum from upstream), Dw1 (downstream) and Dw2 (far downstream).
Experimental set-up
Nine independent experimental units (triplicate suspensions of Up, Dw1 and Dw2) were incubated in batch mode under the same ambient conditions (uncontaminated, without nutrient addition), in thermoregulated chambers (20°C) with a light : dark cycle of 14 : 10 h. The experimental units were not agitated, to allow the microorganisms to settle. Over a 3-week experimental period, samples were collected every day under flame to prevent contamination. Algal composition was checked daily for the proportions of diatoms, green algae and cyanobacteria by means of a PhytoPAM fluorimeter (Heinz Walz, Germany) in all experimental units. The microbial community was described in vivo on days 0, 2, 7, 10, 15 and 18, following the counting method detailed above.
Data analyses
In the field study, the different classes of organisms were grouped as follows: diatoms, green algae, cyanobacteria, flagellates, protozoa other than flagellates, and metazoans based on the microscopic counts. Their relative abundances were log transformed and analysed by principal components analysis (PCA) with PC-Ord software (version 6.08; MjM Software, Gleneden Beach, OR, USA), to identify patterns in community composition related with sampling sites.
The abundances of the taxonomic groups were used as a measure of their specific growth over the 1-month immersion period, and to estimate the potentially grazed numbers of diatoms, as most of the literature data refer to diatoms as prey. Our calculations used averaged values of previously reported grazing rates, based on the hourly consumption of 3–6 diatoms/ciliate (McCormick Citation1991) and 0–13 diatoms/rotifer (Borchardt and Bott Citation1995).
The abundances of diatoms and of potential predators during the laboratory experiment were plotted over time. Linear regressions were performed to assess the dependence between abundances of prey and predators.
Results and Discussion
Field abundances of predators and prey are determined by environmental conditions
Between-river differences in microbial biodiversity
As shown in Figure , taxonomic compositions diverged between rivers. Axis 1, accounting for almost 67% of the total variance, discriminated rivers where micro-meiofauna was more abundant, on the right hand panel (Rivers Ponteils and Leyre), from the Rivers Bourg and Luzou dominated by autotrophs. Micro-meiofauna was, however, quantitatively important in all cases (> 20% of the individuals counted) and reached 97% of the organisms inventoried at Ponteils downstream. In relation to the high abundances of larger organisms, i.e. protozoa and metazoans (Table , Figure ), the River Ponteils was much more productive than the others, with higher biofilm dry weight. In all cases, flagellates were the most numerous of the micro-meiofauna.
Figure 2. Principal Components Analysis (PCA): distribution of sampling sites based on transformed (log(x + 1)) relative abundances of the major components of biofilms. Close to each sampling site location, the proportions of autotrophs (light background) and micro-meiofauna (dark background) are described (pie charts). The corresponding periphytic groups are labelled accordingly in the PCA.
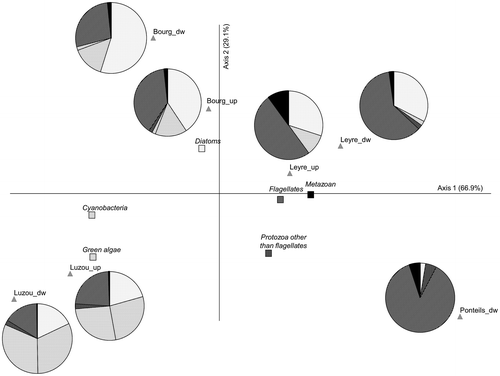
Table 1. Water and biofilm characteristics after a 1-month immersion of artificial substrates in situ. Biofilm dry weights are means ± SE (n=3).
Differences in species composition depending on the rivers may reflect local pools of species determined by small-scale environmental drivers. Species coexistence is allowed by their differences in ecological or functional features (traits), to achieve the highest fitness outcomes under context-dependent conditions (e.g. Descamps-Julien and Gonzalez Citation2005; Loza, Perona, and Mateo Citation2014). Differences in microalgal fitness may reflect trade-offs between physiological tolerance of environmental conditions (and extremes) and competitive ability, e.g. nutrient acquisition by periphytic diatoms depending on the position of cells within the biofilm (Burkholder, Wetzel, and Klomparens Citation1990). The contrasting communities found in the rivers (Figure ) can therefore be explained by differences in environmental conditions. Although all rivers had slightly acidic waters with low conductivity and orthophosphate concentrations (Table ), high nitrate concentrations were recorded in the Bourg and downstream in the Leyre, as a probable consequence of agricultural land use of their watersheds. Nutrient availability, which is known to greatly influence community composition in biofilms, was associated here with higher abundances of diatoms. In the Luzou, periphytic communities were more diversified, as a probable result of warmer temperatures (sampling in spring versus in winter in Arcachon Bay tributaries) and/or highlighting seasonality in community successions.
Potential drivers of the upstream to downstream changes in species distributions
Variations in global biomass (biofilm dry weight) along the rivers were observed. In all rivers but the Luzou, biofilm biomass increased from upstream to downstream, as did the total numbers of organisms (microalgae, protozoa, metazoans) per unit area colonized (Table ). In Figure , upstream sites were grouped around the origin of the biplot, and the corresponding downstream sections spread over but still remained in the same quarter of the PCA. This spread showed increasing differences from reference conditions, highlighting specific changes in taxonomic composition, i.e. species selection from their upstream pool of species. Along the Leyre, the relative abundance of micro-meiofauna increased slightly, whereas it decreased in the Bourg and Luzou. The biofilms sampled in the River Ponteils were dominated by heterotrophs (mainly protozoa). The changes in communities in the downstream sections of the rivers studied probably reflected changes in water quality such as nutrient availability and contamination. In particular, coexistence between microorganisms may be modulated by diverse environmental conditions, including toxic compounds with distinct modes of action, targeting prey or predators. Indeed, different patterns in community composition changes from upstream to downstream (Figure ) may result from the type of contamination present, impacting the balance between periphytic autotrophs and heterotrophs. Although not measured specifically in this work, data were available about the kind of pollutants present downstream in the rivers studied (Fauvelle Citation2012; Lainé, Morin, and Tison-Rosebery Citation2014).
The relative increase in microalgae in the Rivers Bourg and Luzou may result from direct and indirect impacts of toxic inputs. Increasing concentrations of insecticides (e.g. chlorpyrifos, which is frequently measured in the sediments of the Bourg; Fauvelle Citation2012) and complex industrial releases (mixtures of metals and aromatic compounds in the Luzou; Lainé, Morin, and Tison-Rosebery Citation2014) were recorded. Micro-meiofauna, directly targeted by such contaminations, decreased in abundance probably as a consequence of higher mortality and/or reduced fitness. The resulting lower grazing pressure indirectly favoured microalgal development. In contrast, a decrease in microalgal densities associated with higher abundances of micro-meiofauna was observed in the Ponteils and Leyre. In these rivers, pesticides were monitored continuously for 20 months in 2010/11 by Fauvelle (Citation2012), revealing chronic contamination by herbicides. Direct inhibitors of photosynthesis such as isoproturon and diuron in the Ponteils (average concentrations measured by Fauvelle Citation2012: 380 ± 50 ng/l and 37 ± 7 ng/l, respectively) almost eliminated the microalgal component of the biofilm and the community was dominated by heterotrophs (> 90% of individuals). The Leyre was mostly contaminated by chloroacetanilides (acetochlor, metolachlor and their metabolites oxanilic acid and ethane sulphonic acid). The chloroacetanilide gradient was quite weak (2.1 ± 0.2 μg/l in total chloroacetanilides including metabolites; Fauvelle Citation2012), and the highest concentrations recorded downstream (~ 4 μg/l) were far below the acute toxicity thresholds determined by Roubeix et al. (Citation2012). The relative decrease in autotrophs (in proportion but not in cell numbers) probably resulted from both the low toxicity of herbicides towards microalgae at the concentrations occurring and the increase of the numbers of flagellates, whose growth was potentially less detrimentally affected by the pesticides.
Potential grazing activity
In many systems, the most important factors determining species distribution relate to differences in the ability to coexist with predators (Paine Citation1976; Chase et al. Citation2002). Among the micro-meiofauna inventoried, 40–60% of the taxa have already been classified as predators of microalgae (de Puytorac, .Grain, and Mignot Citation1987; Vedry Citation1996; Bertrand et al. Citation2011; Majdi et al. Citation2012) and/or were observed here with microalgal content. Indeed, at the abundances we found in situ, micro-meiofauna may exert a strong selection pressure in the ecosystems in which they exist, thereby altering the structure of the biological communities. Based on the abundances of diatoms and micro-meiofauna counted in the field samples, we calculated the potentially grazed numbers of diatoms over the 1-month immersion period (Table ). These values clearly exceed the numbers actually recorded by several orders. This suggests high top–down control of periphytic predators on microalgal densities, even for taxa with elevated reproduction rates (up to two divisions/day; Baars Citation1983). However, the mature biofilms were still composed of high numbers of both microalgae and micro-meiofauna, denoting complex coexistence relationships that were therefore investigated over time under simplified conditions (see next section).
Coexistence of prey and predator rapidly reaches a stable state under simplified conditions
The abundances of both predators and prey were followed over time under laboratory conditions. Our streamlined experiment was performed using suspensions of batch-cultured biofilms taken directly from river sites, involving a simplification of natural conditions due to the transitory destructuring of the biofilm. Despite the change in community structure (not a biofilm anymore but rather a suspension allowed to settle down), the percentage of crawling micro-meiofauna did not significantly decrease (with values of 48.4 ± 8.9% on day 0 and of 42.7 ± 0.7% on day 18). This experiment aimed to characterize the long-term coexistence between microalgae and micro-meiofauna under three different treatments (variable prey–predator proportions). Exhaustive information on the taxonomic composition of micro-meiofauna is detailed in Table .
Table 2. Temporal variation of the micro-meiofauna, including non-predatory taxa (laboratory).
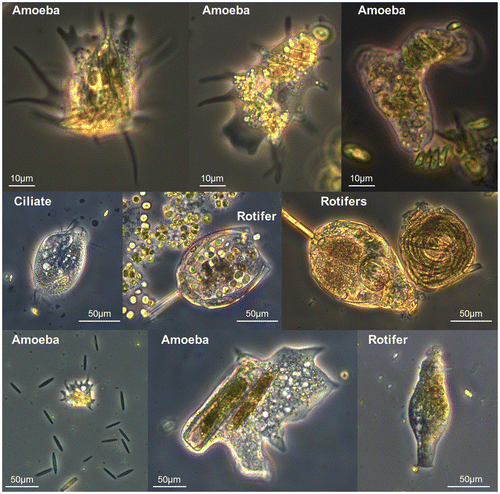
At the beginning of the experiment, the autotrophic component of the inocula was composed of about 38 ± 1% green algae, 36 ± 2% diatoms and 26 ± 2% cyanobacteria (PhytoPAM measurements). The proportion of green algae increased over time in all treatments and represented 78 ± 4% on day 18, as a consequence of more favourable conditions for their growth and/or preferential grazing of diatoms (whose abundance decreased to 7 ± 2% at the end of the experiment). Mutual exclusion may have occurred between microalgae; hence green algae may have taken competitive advantage of slightly higher temperatures, or of the light conditions in the chambers. Besides, it has to be noted that nutrient loads were very low as we used mineral water to suspend the organisms, such conditions usually not being optimal for green algae (e.g. Sigee Citation2005). Depending on the site of origin of the communities, different trends were observed in the experimental units (Figure ). Throughout the experiment, predator densities clearly followed the trends of diatom abundances in Up (R2 = 0.45, p = 0.002) and Dw1 (R2 = 0.36, p = 0.008) treatments, whereas they diverged in Dw2 (R2 = 0.002, p = 0.85) during the first week (day 0 to day 7). In Dw2 where the initial pools of micrograzers were higher, their abundances decreased three-fold during the first days, due to the change of environment (culture conditions) and/or to a lower balance between prey and predators in this treatment. In all treatments, however, the patterns of prey and predator densities developed similarly from the second week. Our data followed prey-density-dependent patterns (Type II or Type III, Holling Citation1959): over the second experimental period (from day 7) when the densities stabilized. We calculated a ratio of 20 ± 6 : 1 prey/potential predator (n = 36, R2 = 0.26, p = 0.001, pooling all treatments). This positive correlation confirms the findings of Kanavilil and Kurissery (Citation2013) in Canadian freshwaters (creeks and lakes). Classical predator models assume that predators capture and consume prey singly; however, protozoa have the ability to consume high numbers of individuals simultaneously (over 100 diatoms at the same time, as mentioned by McCormick Citation1991). In our samples too, amoebae, ciliates and rotifers were occasionally found to contain numerous microalgal cells (Figure ).
Figure 3. Temporal dynamics of diatoms (open diamonds) and potential micro-meiofauna predators (dark circles) in the three experimental treatments (laboratory). Densities are average (cell/cm²) ± SE values (n = 3 replicates per date). Linear correlations calculated between abundances of preys and potential predators are mentioned for each treatment (n = 18).

Moreover, non-predatory micro-meiofauna (in particular, flagellates) were not taken into account even though they were 8- to 10-fold more abundant than the predators. Although not directly involved in the microalgal density count, these organisms coexisted and probably competed for resources such as nutrients and space, and may have played an indirect role in structuring the community.
On the other hand, prey can reduce predator consumption rates by exhibiting active or passive defences. Interspecific variations in constitutive (permanently expressed) or induced defences may lead to differences in prey susceptibility to predation, partitioning the species along a gradient of predation risk (Stoks and McPeek Citation2003). Passive ability to make capture harder – such as microalgal size, shape, presence of spines –,would allow larger species to coexist with predators at a higher density than smaller ones. Nevertheless, Bott (Citation1996) reported cases of micrograzers ingesting microalgae larger than themselves, by body distension. Our observations (e.g. Figure ) provided experimental evidence of such grazing activity for amoebae. Among the features that may actively help the prey to avoid predators, we can mention: escape behaviour (motility and velocity, e.g. Coquillé et al. Citation2015), and/or the excretion of allelopathic substances (Leflaive et al. Citation2008). In this study, we observed that in situ abundance of microalgae and micro-meiofauna are driven by local conditions, and that prey–predator dynamics under controlled conditions are likely to follow classical models established for higher organisms, irrespective of the different forms of prey defence. These results are a first step in elucidating the link between interspecific differences in defence, functional responses and environmental conditions.
Conclusions
Given the fact that the same environmental factors (light, temperature, dissolved substances including contaminants) structure the communities of microalgae and micro-meiofauna (de Puytorac, Grain, and Mignot Citation1987), their joint dynamics are likely to be affected by ambient changes. Even though the periphytic prey–predator relationships seem to rapidly reach a stable state under uncontaminated conditions (laboratory), this equilibrium may be impacted by various disturbances, such as punctual toxic pollution. Thereafter, the periphytic ecosystem would either reach another stable state with community functions maintained, or could dysfunction.
The ubiquity of micro-meiofauna and its high ingestion capacity for microalgae suggest a substantial qualitative and quantitative impact on periphyton. Further work is required to better understand whether prey–predator relationships and stability of the communities within biofilms are modified by changes in environmental conditions. How natural or anthropic environmental factors shape periphytic biodiversity (in species and traits), both directly and indirectly, is a topic that requires further investigation. To that aim, determining whether micro-meiofauna has prey preferences is a prerequisite. Does the choice of grazed microalgae depend on their morphological attributes and/or on the feeding mode of the predator (capture and ingestion modes, for example)? What is the impact of grazing selectivity on the overall vulnerability of biofilm communities?
Notes on contributors
Julie Neury-Ormanni, master student. Contribution: participated in field work, conducted laboratory work, contributed to analyses.
Jacky Vedrenne, senior technician. Contribution: participated in field work, conducted laboratory work, contributed to analyses and co-wrote the manuscript.
Soizic Morin, research scientist. Contribution: planned the study, participated in field and laboratory work, contributed to analyses and wrote the manuscript.
Acknowledgements
This study was carried out with the financial support of the French National Research Agency (ANR) in the framework of the ‘Investments for the Future’ programme, within the Cluster of Excellence COTE (ANR-10-LABX-45) and from the Adour-Garonne Water Agency (convention 310 33 0075, 2012–2014). The authors wish to thank Juliette Rosebery and Vincent Fauvelle (Irstea) for their help in field sampling, as well as Mélissa Eon and Karine Madarassou from the Chemistry Laboratory at Irstea Bordeaux for performing nutrient analyses and dry weight determinations. Benoît Schoefs (Université du Maine à Le Mans) and the two anonymous reviewers of our manuscript are thanked for their constructive comments that greatly helped to improve the text. Peter Winterton is warmly acknowledged for English corrections and suggestions on the manuscript.
References
- Artois, T., D. Fontaneto, W. D. Hummon, S. J. Mcinnes, M. A. Todaro, M. V. Sørensen, and A. Zullini. 2011. “Ubiquity of microscopic animals? Evidence from the morphological approach in species identifi cation.” In Biogeography of Microscopic Organisms: Is Everything Small Everywhere?, edited by D. Fontaneto, 244–83. Cambridge, U.K.: Cambridge University Press.
- Baars, J. W. 1983. “Autoecological investigations on freshwater diatoms. 1. Generation times of some species.” Archiv für Hydrobiologie - Supplement 67: 11–18.
- Bertrand, J. C., P. Caumette, P. Lebaron, R. Matheron, and P. Normand. 2011. Écologie microbienne : Microbiologie des Milieux Naturels et Anthropisés. Pau: Presses Universitaires de Pau et des Pays de l'Adour.
- Borchardt, M. A., and T. L. Bott. 1995. “Meiofaunal grazing of bacteria and algae in a piedmont stream.” Journal of the North American Benthological Society 14: 278–298.
- Bott, T.L. 1996. “Algae in microscopic food webs.” In Algal Ecology: Freshwater Benthic Ecosystems, edited by R.J. Stevenson, M.L. Bothwell, and R.L. Lowe, p. 574–608 Academic Press, San Diego.
- Bott, T. L., and M. A. Borchardt. 1999. “Grazing of protozoa, bacteria, and diatoms by meiofauna in lotic epibenthic communities.” Journal of the North American Benthological Society 18: 499–513.
- Burkholder, J. M., R. G. Wetzel, and K. L. Klomparens. 1990. “Direct comparison of phosphate uptake by adnate and loosely attached microalgae within an intact biofilm matrix.” Applied and Environmental Microbiology 56: 2882–2890.
- Canler, J. P., E. Cotteux, P. Duchène, and J. M. Perret. 1999. Aide au Diagnostic des Stations d'Épuration par l'Observation Microscopique des Boues Activées. Cahors: CEMAGREF editions.
- Chase, J. M., P. A. Abrams, J. P. Grover, S. Diehl, P. Chesson, R. D. Holt, S. A. Richards, R. M. Nisbet, and T. J. Case. 2002. “The interaction between predation and competition: a review and synthesis.” Ecology Letters 5: 302–315.
- Coquillé, N., G. Jan, A. Moreira, and S. Morin. 2015. “Use of diatom motility features as endpoints of metolachlor toxicity.” Aquatic Toxicology 158: 202–210.
- De Puytorac, P., J. Grain, and J. P. Mignot. 1987. Précis de protistologie. Paris: Société Nouvelle des Editions Boubée.
- Descamps-Julien, B., and A. Gonzalez. 2005. “Stable coexistence in a fluctuating environment: An experimental demonstration.” Ecology 86: 2815–2824.
- Donohue, I., A. L. Jackson, M. T. Pusch, and K. Irvine. 2009. “Nutrient enrichment homogenizes lake benthic assemblages at local and regional scales.” Ecology Letters 90: 3470–3477.
- Fauvelle, V. 2012. “Évaluation de la contamination en pesticides des tributaires du bassin d’Arcachon et développement d’un échantillonneur passif spécifique des herbicides anioniques.” Doctorat en chimie environnementale. PhD thesis - Univ. Bordeaux I Ecole Doct. Sciences chimiques (Bordeaux).
- Foissner, W. 1996. “A user-friendly guide to the ciliate (protozoa, ciliophora) commonly used by hydrobiologists as bioindicators in rivers, lakes, and waste waters, with notes on their ecology.” Freshwater Biology 35: 375–482.
- Hammill, E., T. B. Atwood, P. Corvalan, and D. S. Srivastava. 2015. “Behavioural responses to predation may explain shifts in community structure.” Freshwater Biology 60: 125–35.
- Holling, C. S. 1959. “The components of predation as revealed by a study of small-mammal predation of the European Pine Sawfly.” The Canadian Entomologist 91: 293–320.
- Kanavillil, N., and S. Kurissery. 2013. “Dynamics of grazing protozoa follow that of microalgae in natural biofilm communities.” Hydrobiologia 718: 93–107.
- Lainé, M., S. Morin, and J. Tison-Rosebery. 2014. “A multicompartment approach – diatoms, macrophytes, benthic macroinvertebrates and fish – to assess the impact of toxic industrial releases on a small French river.” PLoS ONE 9 (7): e102358.
- Lange, K., A. Liess, J. J. Piggott, C. R. Townsend, and C. D. Matthaei. 2011. “Light, nutrients and grazing interact to determine stream diatom community composition and functional group structure.” Freshwater Biology 56: 264–278.
- Leflaive, J., S. Boulêtreau, E. Buffan-Dubau, and L. Ten-Hage. 2008. “Temporal patterns in epilithic biofilm – relation with a putative allelopathic activity.” Fundamental and Applied Limnology 173: 121–134.
- Loza, V., E. Perona, and P. Mateo. 2014. “Specific responses to nitrogen and phosphorus enrichment in cyanobacteria: factors influencing changes in species dominance along eutrophic gradients.” Water Research 48: 622–631.
- Lynn, D. H. 2010. The Ciliated Protozoa: Characterization, Classification, and Guide to the Literature. 3rd ed. Dordrecht, NLD: Springer.
- Majdi, N., M. Tackx, W. Traunspurger, and E. Buffan-Dubau. 2012. “Feeding of biofilm-dwelling nematodes examined using HPLC-analysis of gut pigment contents.” Hydrobiologia 680: 219–232.
- Mccormick, P. V. 1991. “Lotic protistan herbivore selectivity and its potential impact on benthic algal assemblages.” Journal of the North American Benthological Society 10: 238–250.
- Morin, S., M. Vivas-Nogues, T. T. Duong, A. Boudou, M. Coste, and F. Delmas. 2007. “Dynamics of benthic diatom colonization in a cadmium/zinc-polluted river (Riou-mort, France).” Fundamental and Applied Limnology / Archiv für Hydrobiologie 168: 179–187.
- Paine, R. T. 1976. “Size-limited predation: an observational and experimental approach with the Mytilus-Pisaster interaction.” Ecology 57: 858–873.
- Petchey, O. L., P. T. Mcphearson, T. M. Casey, and P. J. Morin. 1999. “Environmental warming alters food-web structure and ecosystem function.” Nature 402 (6757): 69–72.
- Peterson, C. G., and R. J. Stevenson. 1990. “Post-spate development of epilithic algal communities in different current environments.” Canadian Journal of Botany 68: 2092–2105.
- Proia, L., F. Cassió, C. Pascoal, A. Tlili, and A. M. Romaní. 2012. “The use of attached microbial communities to assess ecological risks of pollutants in river ecosystems: the role of heterotrophs.” In Handbook of Environmental Chemistry, edited by H. Guasch, A. Ginebreda and A. Geiszinger, 55–83. Heidelberg: Springer.
- Roubeix, V., V. Fauvelle, J. Tison-Rosebery, N. Mazzella, M. Coste, and F. Delmas. 2012. “Assessing the impact of chloroacetanilide herbicides and their metabolites on periphyton in the Leyre river (SW France) via short term growth inhibition tests on autochthonous diatoms.” Journal of Environmental Monitoring 14 (6): 1655–1663.
- Sabater, S., H. Guasch, M. Ricart, A. Romaní, G. Vidal, C. Klünder, and M. Schmitt-Jansen. 2007. “Monitoring the effect of chemicals on biological communities. The biofilm as an interface.” Analytical and Bioanalytical Chemistry 387 (4): 1425–1434.
- Sigee, D. C. 2005. Freshwater Microbiology: Biodiversity and Dynamic Interactions of Microorganisms in the Aquatic Environment. Chichester, UK: John Wiley & Sons.
- Stoks, R., and M. A. Mcpeek. 2003. “Antipredator behavior and physiology determine lestes species turnover along the pond-permanence gradient.” Ecology 84: 3327–3338.
- Tachet, H., P. Richoux, M. Bournaud, and P. Usseglio Polatera. 2010. Invertébrés d'eau douce : Systématique, biologie, écologie. Paris: CNRS Editions.
- Tison, J., Y. S. Park, M. Coste, J. G. Wasson, L. Ector, F. Rimet, and F. Delmas. 2005. “Typology of diatom communities and the influence of hydro-ecoregions: a study on the French hydrosystem scale.” Water Research 39: 3177–3188.
- Vedry, B. 1996. Les biomasses épuratrices. Nanterre: Agence de l'eau Seine-Normandie.
- Wasson, J.G., A. Chandesris, and H. Pella. 2002. Définition des hydro-écorégions de France métropolitaine. Approche régionale de typologie des eaux courantes et éléments pour la définition des peuplements de référence d'invertébrés, 190: Technical report, Cemagref Lyon BEA/LHQ.