Abstract
Acetaminophen toxicity remains a leading cause of acute liver failure in the United States and world-wide. Fomepizole is a cytochrome P450 inhibitor that may inhibit production of toxic metabolites of acetaminophen. However there are limited clinical data of its use for this purpose in the literature. Here we present a case series of six patients treated with fomepizole for acute acetaminophen toxicity who sustained no substantial liver injury.
Introduction
Acetaminophen (APAP) is a commonly used analgesic and antipyretic. Its toxicity is a common cause of acute liver failure in the United States [Citation1, Citation2]. After therapeutic dosing, approximately 5% of APAP undergoes metabolism by cytochrome P450 2E1 (CYP2E1) to N-Acetyl-p-benzoquinone imine (NAPQI), a highly hepatotoxic metabolite. Acetaminophen overdose may overwhelm normal detoxification pathways ed, and the conversion of APAP to NAPQI by CYP2E1 drives hepatotoxicity, which we define here as elevations in ALT greater than 5 times the upper limit of normal for our reference laboratory (>230 U/L). Standard therapy with IV N-acetylcysteine (NAC) is effective in most cases although higher doses may be necessary for larger ingestions [Citation3]. Additionally, there are reports of hepatotoxicity despite early use of IV NAC [Citation4]. This may exceed 5% of cases in some reports of early administration <8 h post ingestion including death and need for liver transplant to as high as 25% in one large Australian study when IV NAC was administered >8 h post ingestion [Citation4, Citation5].
Fomepizole is a potent inhibitor of CYP2E1 [6]. Fomepizole issafey and effective in humans for inhibition of alcohol dehydrogenase in toxic alcohol and glycol ingestion. The kinetics and metabolism of fomepizole has also been well studied [Citation6]. The role of fomepizole as an inhibitor of CYP2E1 to reduce the conversion of APAP to NAPQI has been postulated and studied in animal models as well as human hepatocytes [Citation7–9], however there are limited clinical data [Citation10].
Here we present a case series of patients treated with fomepizole for persistently elevated APAP concentrations who sustained no substantial liver injury. Our study spans cases from 2017 to 2019 encountered at free-standing pediatric and adult tertiary care hospitals within the same medical center. A one-bag method for IV NAC (30,000 mg in 1 Liter IV fluid) to administer the loading dose of 150 mg/kg over 1 h followed by a continuous infusion of 12.5 mg/kg/hour used in our institution over the last 2 years has been validated by colleagues at another academic center in our area and used in the US and Canada [Citation11, Citation12].
Case series
Case 1: A 49-year-old woman with history of depression presented for acute encephalopathy following ingestion of benzodiazepines (temazepam and lorazepam) and unknown co-ingestants. Initial work up, estimated to be 4 h after ingestion, was significant for an APAP concentration of 140.8 µg/mL, as well as salicylate concentration of 45 mg/dL. Serum chemistries, including transaminase concentrations, INR, and total bilirubin, were within normal ranges. We administered a single dose of activated charcoal and IV N-acetylcysteine (NAC) at 150 mg/kg load over one hour followed by 12.5 mg/kg/hour continuous infusion. APAP remained significantly elevated (see ), prompting us to continue NAC at 12.5 mg/kg/hour beyond the initial scheduled duration. In addition, we administered fomepizole 15 mg/kg IV over 30 min followed by 10 mg/kg every 12 h. Due to persistently elevated APAP concentrations and concern for co-ingestants including salicylates, we performed one session of hemodialysis approximately 36 h after ingestion. Serum APAP and salicylate concentrations checked immediately after dialysis were undetectable, and we discontinued IV NAC and fomepizole. During this hospitalization, her transaminases peaked at marginally above the upper limit of normal (see ), and she demonstrated no biochemical or clinical evidence of liver failure.
Figure 1. Acetaminophen concentrations (µg/mL) plotted over time (hours after ingestion) – Time either confirmed or estimated on clinical history. Concentrations plotted on a standard Rumack Matthew nomogram with Rumack-Matthew line and treatment line shown. All laboratory studies were performed at a single institution.
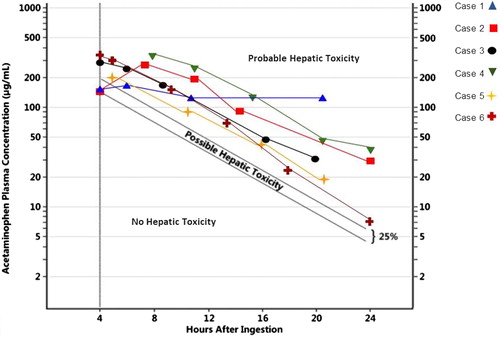
Table 1. AST and ALT over time from estimated or confirmed ingestion.
Case 2: A 14-year-old girl presented with acute encephalopathy and lethargy following an ingestion of unknown quantities of acetaminophen and diphenhydramine. Her initial APAP concentration four hours after ingestion was 135 µg/mL. We initiated treatment with IV NAC 150 mg/kg loading dose over one hour followed by a 12.5 mg/kg/hour continuous infusion. The APAP concentration six hours after ingestion returned at 251.7 µg/mL, prompting us to administer fomepizole at 15 mg/kg IV over 30 min followed by 10 mg/kg every 12 h until serum APAP concentrations cleared. During this hospitalization, there was no biochemical evidence of hepatocellular injury (see ), or clinical signs of liver failure.
Case 3: A 9-year-old boy presented with ingestion of a large quantity of acetaminophen. His serum APAP concentration four hours after ingestion was 281.7 µg/mL for which we initiated treatment with IV NAC 150 mg/kg loading dose over one hour followed by 12.5 mg/kg/hour continuous infusion. A repeat APAP concentration at 6 h was 239.7 µg/mL for which we administered fomepizole 15 mg/kg IV over 30 min followed by 10 mg/kg 12 h later. Despite his persistently elevated APAP concentration (see ), his ALT remained within normal limits (see ), and he demonstrated no evidence of liver failure.
Case 4: A 15-year-old girl with a history of depression with prior suicide attempts presented to the hospital with severe nausea and emesis after admitting to intentional polysubstance ingestion with an estimated 100 tablets of 500 mg of APAP. She was initially seen at a referral hospital where an APAP concentration obtained two hours after ingestion was 236.1 µg/mL. She received an unknown dose of activated charcoal and started on IV NAC 150 mg/kg over one hour followed by a continuous infusion of 12.5 mg/kg/hour. She was transferred to our hospital where an APAP concentration taken 8.5 h after ingestion was to 311.9 µg/mL. We increased the dose of her NAC to 18.75 mg/kg/hour and administered fomepizole 15 mg/kg over one hour followed by 10 mg/kg every 12 h until APAP concentration was undetectable. Throughout this hospitalization, despite persistently elevated APAP concentrations (see ), the patient developed no biochemical evidence of hepatocellular injury (see ) or clinical signs of liver failure.
Case 5: A 42-year-old woman presented to the hospital with nausea, lethargy and tachycardia following reported ingestion of an estimated 200 tablets of 500 mg of APAP, 200 tablets of 200 mg of ibuprofen, and 200 tablets of 2 mg of loperamide. Her APAP concentration obtained an estimated five hours after ingestion was 201.8 µg/mL, for which we started IV NAC at 150 mg/kg over one hour followed by 12.5 mg/kg/hour continuous infusion. Due to elevated APAP concentrations and concern that co-ingestants may delay absorption of APAP and prolong elimination, we administered fomepizole at 15 mg/kg over 30 min followed by 10 mg/kg every 12 h. Despite persistently elevated APAP concentrations, her ALT remained within normal limits (see ), and she demonstrated no clinical signs of liver failure.
Case 6: A 15-year-old girl presented to the hospital following a reported intentional ingestion of an estimated 100 to 125 tablets of 500 mg APAP in a suicide attempt. She initially arrived at a community hospital where an APAP concentration obtained approximately one to two hours after ingestion was 210 µg/mL and which rose to 361 µg/mL four hours after ingestion. She received IV NAC at 150 mg/kg loading dose over one hour followed by 12.5 mg/kg/hour. The community hospital called our center where we recommended administering fomepizole 15 mg/kg infused over 30 min prior to transfer to our center. Fomepizole began approximately 5.5 h after ingestion. On arrival to our center, patient had persistently elevated APAP concentrations (see ). Her symptoms included nausea and abdominal tenderness without right upper quadrant pain. We continued IV NAC for a standard 21 h infusion. Throughout the hospitalization, she demonstrated no evidence of hepatocellular damage (see ) or clinical evidence of liver failure.
Discussion
We describe patients with persistently elevated APAP concentrations managed with fomepizole as an inhibitor of CYP2E1 in order to prevent conversion of APAP to the hepatotoxic metabolite NAPQI. Our use of fomepizole is off-label and is an adjunct to standard therapy with N-acetylcysteine. All calculations were based on estimated and/or confirmed ingestion times for APAP. All six patients received IV NAC within 8 h of ingestion.
Approximately 5–6% of patients treated with IV NAC within 8 h of ingestion develop acute liver injury or failure [Citation2, Citation4]. Risk factors for developing liver injury in these patients include APAP concentrations above 200 µg/mL four hours after ingestion with higher concentrations with persistent elevations likely conferring higher risk. Decision to utilize fomepizole in these patients occurred due to APAP concentrations similar those seen in patients who developed liver injury despite early IV NAC therapy. Justification for use of fomepizole included the potential benefits as well as the ease of dosing and generally favorable adverse effect profile of fomepizole. All patients in this case series tolerated all therapy and survived to discharge without significant liver injury.
Limitations of our report are the retrospective nature of the series, and the lack of control group. We cannot say what would have occurred without fomepizole. It is possible that IV NAC therapy alone was entirely successful in preventing hepatic injury. In one case, hemodialysis was used in part due to co-ingestion of salicylate, and the beneficial effect of our use of fomepizole may have been partly overshadowed. Other case reports describe hemodialysis in conjunction with IV NAC and fomepizole [Citation13], although extracorporeal removal of acetaminophen is necessary only in rare cases [Citation14].
Conclusions
These cases demonstrate the safety and potential benefit to using fomepizole in conjunction with standard therapy for APAP overdose. Further study remains to determine the timing of and indications for the use of fomepizole as adjunctive therapy in acetaminophen poisoning.
References
- McGill MR, Jaeschke H. Metabolism and disposition of acetaminophen: recent advances in relation to hepatotoxicity and diagnosis. Pharm Res. 2013; 30(9):2174–2187.
- Heard KJ. Acetylcysteine for acetaminophen poisoning. N Engl J Med. 2008;359(3):285–292.
- Rumack BH, Bateman DN. Acetaminophen and acetylcysteine dose and duration: past, present and future. Clin Toxicol. 2012;50(2):91–98.
- Doyon S, Klein-Schwartz W. Hepatotoxicity despite early administration of intravenous N-acetylcysteine for acute acetaminophen overdose. Acad Emerg Med. 2009;16(1):34–39.
- Whyte IM, Francis B, Dawson AH. Safety and efficacy of intravenous N-acetylcysteine for acetaminophen overdose: analysis of the Hunter Area Toxicology Service (HATS) database. Curr Med Res Opin. 2007;23(10):2359–2368.
- McMartin KE, Sebastian CS, Dies D, et al. Kinetics and metabolism of fomepizole in healthy humans. Clin Toxicol. 2012;50(5):375–383.
- Hazai E, Vereczkey L, Monostory K. Reduction of toxic metabolite formation of acetaminophen. Biochem Biophys Res Commun. 2002;291(4):1089–1094.
- Akakpo JY, Ramachandran A, Kandel SE, et al. 4-Methylpyrazole protects against acetaminophen hepatotoxicity in mice and in primary human hepatocytes. Hum Exp Toxicol. 2018;37(12):1310–1322.
- Akakpo JY, Ramachandran A, Duan L, et al. Delayed treatment with 4-methylpyrazole protects against acetaminophen hepatotoxicity in mice by inhibition of c-Jun n-Terminal Kinase. Toxicol Sci. 2019;170(1):57–68.
- Yip L, Heard K. Potential adjunct treatment for high-risk acetaminophen overdose. Clin Toxicol. 2016;54(5):459–459. [Epub 2016 Feb 26].
- Johnson MT, McCammon CA, Mullins ME, et al. Evaluation of a simplified N-acetylcysteine dosing regimen for the treatment of acetaminophen toxicity. Ann Pharmacother. 2011;45(6):713–720.
- Mullins ME, Yarema MC, Sivilotti MLA, et al. Comment on “transition to two-bag intravenous acetylcysteine for acetaminophen overdose”. Clin Toxiocol. 2019:1–3. doi: 10.1080/15563650.2019.1649418
- Kiernan EA, Fritzges JA, Henry KA, et al. A case report of massive acetaminophen poisoning treated with a novel “triple therapy: N-acetylcysteine, 4-methypyrazole and hemodialysis”. Case Rep Emerg Med. 2019;2019:1–4.
- Gosselin S, on behalf of the Extrip Workgroup, Juurlink DN, Kielstein JT, Ghannoum M, Laverge V, Nolin TD, Hoffman RS, Extrip W. Extracorporeal treatment of acetaminophen poisoning: recommendations from the EXTRIP workgroup. Clin Toxicol. 2014;52(8):856–867.
