Abstract
The rapid decrease of natural resources and generation of huge amount of metallic wastes from mining industries has led to the focus of researchers to shift to alternative methods of waste benefaction and resource recycling. This study aims at the development of an eco friendly technique to recover Manganese (Mn) from mining waste residues using Acinetobacter sp. Bioleaching experiments were conducted in shake flasks at initial pH 6.5, 5% w/v inoculums and 2% pulp density at 30 °C with agitation speed 200 RPM and Acinetobacter sp. as inoculum. Mn recovery of 76% was recorded in 20 days. The analysis of the changes in cellular protein expression and conformation was carried out through sodium dodecyl sulphate polyacrylamide gel electrophoresis (SDS PAGE) and fourier transform infrared spectroscopy. The results reveal that bioleaching can alter protein expression and also result in conformational changes in protein structure. The present study sheds light on the greener alternative to recover and recycle manganese from wastes native bacteria. Exciting prospects for the utilization of mining wastes are in store in the near future; providing an economic and ecologically sound alternative to pyrometallurgical processes.
1. Introduction
The alignment of the present research focus on newer methods of metal extraction from secondary sources is rooted to the ever-growing global demand for metals (Mohanty et al., Citation2016a). Due to the limitation of natural mineral sources and also the huge wastage of minerals in form of mining wastes, the industries are in the pursuit of embracing newer, efficient and eco friendly alternatives for resource recycling (Das & Mishra, Citation2010). Bioleaching is now being accepted to be one of the efficient metal extraction processes by the mining industry. In comparison to the traditional metallurgical methods, bioleaching is less energy intensive and is environmentally friendly. It uses the inherent ability of microorganisms that enables them to convert insoluble conjugated minerals into their soluble and extractable forms (Chen et al., Citation2011; Das, Ghosh, Mohanty, & Sukla, Citation2015a). Micro-organisms can solubilize metals both directly and indirectly (Acharya, Kar, & Sukla, Citation2003). Direct mechanism involves the obtaining of electrons directly from the reduced elements. In this case, cell adhesion to the mineral surface is an absolute essential prerequisite. The indirect mechanism is mainly based on the production of metabolites like organic acid which in turn causes metal solubilization (Das, Pradhan, & SuklaCitation, 2012; Das, Swain, Panda, Pradhan, & Sukla, Citation2012; Ghosh, Mohanty, Akcil, Sukla, & Das, Citation2016).
Manganese is used in varied industries like steel, alloys, batteries, and chemical reagents (Das et al., Citation2015b; Ghosh & Das, Citation2015). Naturally occurring ores with manganese concentrations beyond 30% are preferred industrially while the leaner deposits are usually ignored due to the lack of economic viability of the traditional extraction processes. India has manganese ore reserves of around 176.5 million tons (Das, Sukla, Pradhan, & Nayak, Citation2011). The Keonjhar – Bonai region of Odisha boasts a high amount of ore reserves of the country. Almost half of the natural reserves of Mn consists of low-grade ores. Several studies have been reported on bioleaching of Mn using micro-organisms, but most of these are restricted to use a few well-known bacterial strains (Sanket, Ghosh, Sahoo, Nayak, & Das, Citation2016).
However, the identification and culturing of native microbial strains from Mn mining site has become an essential step for biorecovery of Mn due to their inherent tolerance and solubilization potential of Mn. Studies on Mn solubilization using site-specific bacterial isolates from the mining deposits of Odisha, India are scanty. Enhancing solubilization potential through Mn-tolerant bacterial diversity present in the mining waste has not been explored in detail till now. The present investigation endeavours to shed light on the outcomes of Mn exposure on a native Mn solubilizing bacterial strain and also on the development of site-specific solubilization as well as bioremediation process for the metal-contaminated sites in Odisha, India, which may well be contextually applied to other Mn-contaminated sites.
2. Materials and methods
2.1. Sample collection and characterization
The mining waste samples (low-grade ores) from a ferro manganese mining deposits in Odisha, India, were collected and stored at sterile conditions at 4 °C until further studies were carried out. The metal content in the sample was analysed by acid digestion using aqua regia. Scanning electron microscope (S-400, Hitachi, Tokyo, Japan) was used to observe the surface morphology of the collected ore sample that was fixed with glutaraldehyde.
2.2. Microorganism
Previously isolated and identified strain Acinetobacter sp. MSB 5 (KP635224) was used in the present investigation. Aseptic techniques were used for handling and inoculation of the culture.
The maximum tolerable concentration (MTC) of the bacterial strain was determined by the well diffusion method in nutrient agar medium with MnCl2 concentrations ranging from 100 to 1200 mM. The maximum concentration of Mn in the medium in which visible growth was observed was taken as the MTC.
2.3. Bioleaching studies
Modified K medium having a composition of (g/l): MnSO4 0.3, yeast extract 0.7 and peptone 2.2 was used as the bleaching medium. 2% (w/v) low-grade Mn ore sample and 2% (w/v) dextrose were further supplemented into the medium. The experiments were carried out in duplicates and at an initial pH of 6.5 and temperature 30 °C for 20 days. Ten millilitres of bacterial innoculums was added to experimental flasks containing 90 ml media. The flask containing media without the bacterial culture was maintained as a control. To determine the manganese leaching percentage, each of the above flasks contents were centrifuged at 15,000 rpm for 10 min at 4 °C and the supernatant were analysed for manganese in the solution by ICP AES analysis.
2.4. Whole cell protein extraction and profiling by SDS-PAGE
Bacterial culture was grown in sterile nutrient broth. Five millilitres of the bacterial culture was centrifuged at 10,000 rpm at 4 °C for 10 min followed by the discarding of the supernatant.1 ml lysis buffer (50 mM Tris HCl; 100 mM NaCl; 1 mM Tween 20; 5% Gycerol;1 mM EDTA) was added to the pellet and centrifuged at 5000 rpm at 5 °C for 2 min. Two hundred microlitres of this supernatant was incubated with chilled 1600 μL acetone for 30 min at 5 °C. The collected protein was dried. 100 μL PBS was added to this precipitated protein for bringing it into solution. The protein estimation was carried out using Bradford’s method taking BSA as standard, having a concentration ranging from 0 to 100 mg/ml (Sawhney & Singh, Citation2001).
Bacterial cell pellets of the Mn-treated micro-organism was obtained from the leached liquor by centrifugation at 10000 rpm for 15 min followed by washing with phosphate buffer (pH 7.0) to remove the traces of remaining media. The cell pellet obtained was mixed with 1 ml of 2X sample buffer (0.5% SDS; 1.25% β mercaptoethanol; 0.03% Bromophenol Blue; 2.5% glycerol; 15 mM TrisCl; pH-6.8) and incubated in a boiling water bath for 30 min. This was further used for protein profiling using SDS-PAGE (BIO-RAD Mini-PROTEAN Tetra System). Fifteen microlitres of the protein sample was used for SDS-PAGE holding 2.5% stacking and 12.5% of resolving gels. A 200 KDa protein marker (Sigma) was used to identify the protein bands of the Mn treated as well as untreated micro-organisms. After electrophoresis for 4 h at 100 V, the gel was stained with Coomassie brilliant blue in 45% (v/v) methanol and 10% (v/v) acetic acid for one hour. Destaining was carried out using 20% (v/v) methanol and 10% (v/v) acetic acid for 12 h. The gel was rinsed in distilled water to obtain a clear background. Finally, the gel was viewed and photographed with Bio rad gel doc system. The same protocol of SDS PAGE was followed for the untreated bacterial cultures.
2.5. Fourier transform infrared spectroscopy (FTIR) measurements
The functional group characterization of the bacterial strain in presence and absence of Mn was carried out by Fourier transform-infrared spectrometer (Nicolet 6700, Thermo Scientific Instruments). One milligram of lyophilized bacterial sample was mixed with 100 mg KBr and a pellet was formed by vacuum pressure technique. The pellets were then used for recording the spectra from 4000 to 400 cm−1.
3. Result and discussion
3.1. Sample characterization
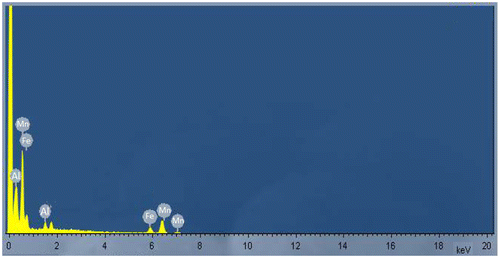
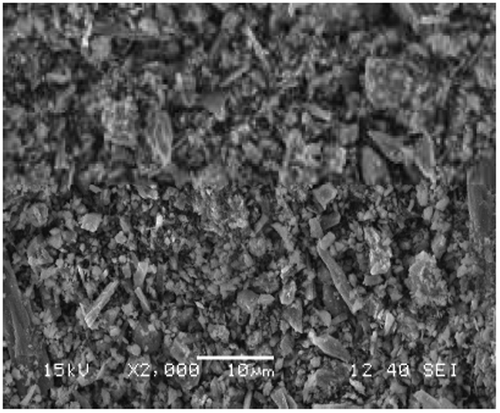
The sample was found to contain about 22% manganese with iron being the other major component as estimated through acid digestion technique. The surface morphology was observed through SEM (Figure ). Chemical and metallic constituents were determined by using an EDX spectrometer in Figure , the detection limit was 0.01%. Presence of Iron and Aluminium was observed in the sample apart from Manganese.
3.2. Mn tolerance study
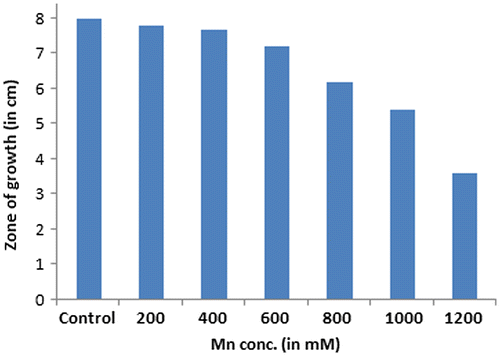
Acinetobacter sp. was found to be well viable in a Mn concentration up to 600 mM. The zone of growth was spanned throughout the plate and had a diameter of 7.2 cm. Beyond that, the diameter of the zone of growth of the bacterial strain kept decreasing and no growth was observed on plates with a Mn concentration of 1200 mM. The maximum tolerable concentration was therefore found to be 1000 mM. Figure shows the graph of decreasing length of the zones.
3.3. Bioleaching of Mn
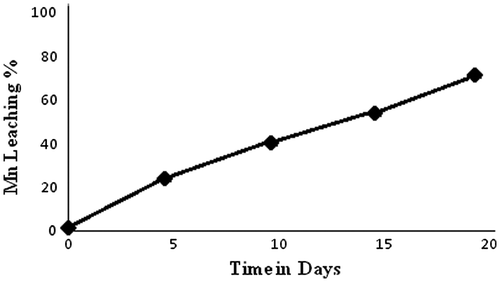
The experimental results for bioleaching showed that 76% of Mn was recovered by Acinetobacter sp. MSB 5 after 20 days (Figure ). In the first days, the rate of bioleaching was slower as compared to the rate of bioleaching acquired after tenth day. The variation of pH was in agreement to the rate of bioleaching and the lowest pH of 3.8 and highest percentage of bioleaching was observed on the twentieth day. The high percentage of Mn bio recovery can be attributed to the fact that the strain has been isolated from a mining environment and native strains have been known to demonstrate high tolerance and solubilization potential due to the stress coping mechanism that is inherently present in them (Ghosh, Mohanty, Nayak, Sukla, & Das, Citation2015; Mohanty et al., Citation2016b; Sanket et al., Citation2016). Negligible Mn recovery was observed in the sterile control flasks.
Figure 4. Mn leaching under optimized conditions. 76% of Mn was recovered by Acinetobacter sp. after 20 days at pH 6.5, pulp density 2% and temperature 30 °C.
Different bioleaching mechanisms are found in different strains, however direct mechanism of metal solubilization is most commonly used by bacteria in which there is intracellular solubilization due to the presence of enzymes like multi copper oxidase, manganese reductase, etc. Some part of the recovery however occurs by indirect mechanism in which different organic acids like oxalic, citric and maleic acid induce the metal solubilization. Similar studies on Mn bio recovery have been reported earlier in which Mn has been recovered from spent Zn-Mn batteries by Alicyclobacillus sp and Sulfobacillus sp, from low-grade ores by Staphylococcus epidermis and from electronic wastes by A. ferrooxidans and A. thiooxidans with a Mn biorecovery percentage of 60, 80, and 75, respectively. (Das, Pradhan et al. Citation2012; Wang, Bai, Xu, & Liang, Citation2009; Xin et al., Citation2012).
3.4. Effect of bioleaching on expression of cellular proteins
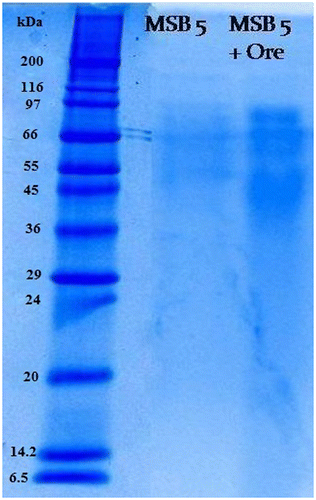
The total protein content of Acinetobacter sp. MSB 5 was found to be 27.20 g/l. Protein analysis was carried out for Acinetobacter sp. MSB 5 using SDS PAGE analysis and the adaptive effect of metal stress on the bacterial strain was investigated. The electrophoresis analysis in 12.5% SDS-PAGE of whole cells lysate protein is summarized in Figure . On exposure of Acinetobacter sp to Mn ore, there were certain protein bands which were upregulated and certain bands were lost. The thickening of a protein band indicates that the protein has increased its concentration in the cell in presence of Mn. Protein bands of molecular weight of 97, 66, 55, and 36 kD were seen. 97 and 66 kD protein bands were seen to be more thickened in the presence of Mn indicating more of that protein has been produced in the cell. It was obvious that some sets of proteins were induced under 2% Mn ore. This group of proteins are characterized with high intensity and may be responsible for Mn reduction.
3.5. Analysis of chemical functional groups
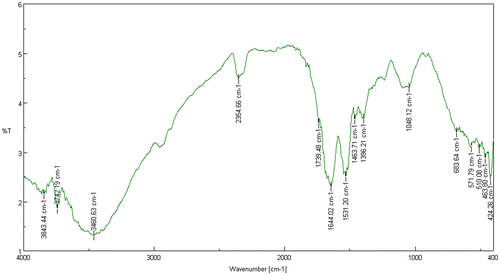
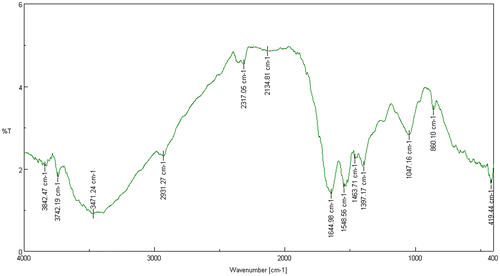
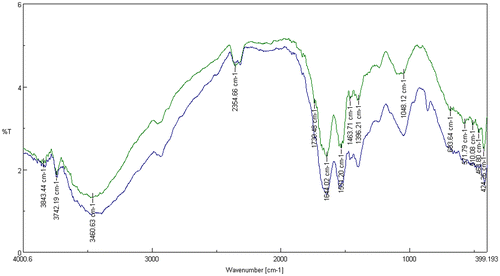
FT-IR spectroscopy is a molecular vibrational spectroscopy analysis that analyses chemical functional groups in absorbance regions ranging between 14,000 to 20 cm−1, with the maximum applicable frequencies of nearly all molecules resembled to the standard infrared spectrum (4000 and 400 cm−1) (Parikh & Chorover, Citation2005; Prakash, Marimuthu, Antonisamy, & Jeeva, Citation2011). The IR spectra of Acinetobacter sp. MSB 5 (Figure ) and Mn-treated Acinetobacter sp. MSB 5 were obtained (Figure ). The spectra of Acinetobacter sp. MSB 5 showed peaks like 3460.63, 1739.48, 1644.02, 1531.20, 1463.71, and 1048.12 cm−1. These peaks code for alcohols, phenols, carboxylic acids, Amide I: C O, C N, N H, nitro compounds, alkanes and aliphatic amines, respectively. While the spectra of Mn-treated Acinetobacter sp. MSB 5 showed peaks like 3471.24, 2931.27, 2134.81, 1644.98, 1548.56, and 1463.71 cm−1. These peaks represent alcohols, phenols, Alkanes, alkynes, alkenes, Amide II: N H, C N, and alkanes, respectively (Table ). In addition to the formation of newer carbon bonds in Mn-treated Acinetobacter sp. MSB 5, there is a change in its protein conformations as well. While Acinetobacter sp. MSB 5 is seen to have a protein peak at 1644.02 representing Amide I: C O, C N, N H, Mn-treated Acinetobacter sp. MSB 5 has a peak at 1548.56 representing Amide II: N H, C N. A peak at 1645 cm−1 corresponds to random coils. The change in the appearance of peaks suggests a protein conformational alteration due to Mn solubilization. The peak shift from 1644.02 represents a decrease in randomly coiled proteins after Mn-solubilization. Figure shows an overlap of the IR spectra of Acinetobacter sp. MSB and Mn treated Acinetobacter sp. MSB 5.
Table 1. IR peaks of Acinetobacter sp. and Mn treated Acinetobacter sp.
3.5. Possible mechanism for variation in protein expression
The bacterial cells directly interact with the metal surfaces of the ore by using an enzymatic type of mechanism during the process of bioleaching. Multicopper oxidase family enzymes are now known to be involved in Mn(II) oxidation. MCOs from bacterial strain are responsible to activate Mn solubilization in bacterial strains. There is enough evidence to support the fact that proteins play an integral role in the process of Mn solubilization (Abhilash, Ghosh, & Pandey, Citation2015; Dick, Justin, Terry, & Bradley, Citation2008). High metal resistance through horizontal gene transfer is a familiar feature of metal solubilizing bacterial strains (Rawlings, Citation2005). A varied response in metal stress conditions have been reported between Mn-treated and untreated strains in this study. The tolerance limits of bacterial strains can be stretched through repeated subculturing in metal-rich conditions (Mohapatra, Bohidar, Pradhan, Kar, & Sukla, Citation2007) while some indigenous strains show naturally elevated metal resistance and elicit variable response. Toxic effects of heavy metals occur due to protein repression (Felicioa, Garcia, Bertolinia, Ottobonib, & Novoa, Citation2003) although cellular toxicity can also occur due to interference with membrane integrity or damage to nucleic acid through generation of Reactive Oxygen Species (Li, Zuo, Li, & Wang, Citation2012). Therefore, it is quite obvious that the process of Mn solubilization will be accompanied by the upregulation and downregulation of proteins in the bacterial cells. Metal stress can also induce changes in conformational structures of protein moieties. It can be concluded that the observed variation in bacterial proteins before and after bioleaching is due to the mineral–microbe interaction which finally leads to metal solubilization.
4. Conclusion
Bioleaching is an economic process of recovering minerals from low- to medium-grade ores and waste residues using micro-organisms. The absence of a suitable substitute for manganese in its major applications, rapid industrialization, gradual depletion of high-grade ore and the rising insufficiency of natural deposits has lead to the inflation of its demand. The conventional pyrometallurgical methods used for extraction of manganese from are very expensive, requires lots of energy and additionally causes environmental pollution problems Bioleaching is a greener alternative and is a persuading solution to the arising problems. In the present investigation, bioleaching of (Mn) from mining waste deposits using indigenous bacterial strain Acinetobacter sp. was investigated. Mn recovery of 76% was recorded in 20 days at initial pH 6.5, 2% w/v inoculums and 2% pulp density at 30 °C with agitation speed 200 RPM. On exposure of Acinetobacter sp. to Mn, 97 and 66 kDa bands appeared to be more thickened in SDS PAGE gel. Therefore, the induction of some sets of proteins under the influence of 2% Mn ore is very obvious. These groups of proteins might find their role in Mn solubilization. Examination of the changes in cellular functional group before and after Mn bioleaching was accomplished by FTIR spectroscopy. Protein–Mn interaction results in the alteration of amide regions and also results in conformational changes due to Mn solubilization. Therefore, the role of proteins in microbial interaction with the ore is very evident. To accomplish the present industrial demand for manganese, it is necessary to develop alternative processes of manganese recovery from low-grade ore deposits and bioleaching seems to be that alternative approach which tackles the problems associated with conventional recovery techniques.
Disclosure statement
No potential conflict of interest was reported by the authors.
Funding
This work was supported by Department of Biotechnology, Government of India [BT/PR7454/BCE/8/949/2012], Department of Science and Technology (DST), Government of India [SP/YO/031/2016] and Ministry of Mines, Government of India [07/46SSAG/CAT].
Acknowledgement
The authors are thankful to the Department of Biotechnology, Government of India for extending funding support for carrying out studies on Mn bioleaching.
References
- Abhilash, Ghosh, A., & Pandey, B.D. (2015). Bioleaching of low grade granitic chalcopyrite ore by hyperthermophiles: Elucidation of kinetics-mechanism. Metallurgical Research and Technology, 112 (506), 1–14.
- Acharya, C., Kar, R.N., & Sukla, L.B. (2003). Studies on reaction mechanism of bioleaching of manganese ore. Minerals Engineering, 16, 1027–1030.10.1016/S0892-6875(03)00239-5
- Chen, P., Yan, L., Leng, F., Nan, W., Yue, X., Zheng, Y., … Li, H. (2011). Bioleaching of realgar by Acidithiobacillus ferrooxidans using ferrous iron and elemental sulphur as the sole and mixed energy sources. Bioresource Technology, 102, 3260–3267.10.1016/j.biortech.2010.11.059
- Das, A.P., Sukla, L.B., Pradhan, N., & Nayak, S. (2011). Manganese biomining: A review. Bioresource Technology, 102, 7381–7387.10.1016/j.biortech.2011.05.018
- Das, A.P., Ghosh, S., Mohanty, S., & Sukla, L.B. (2015a). Advances in manganese pollution and its bioremediation In A. Singh, R. Kuhad, C. Ward, & P. Owen (Eds.), Environmental Microbial Biotechnology Soil biology (p. 45). Berlin: Springer.
- Das, A.P., Ghosh, S., Mohanty, S., & Sukla, L.B. (2015b). Consequences of manganese compounds: A review. Toxicol. Environ. Chem, 96, 981–997.
- Das, A.P., Pradhan, N., & Sukla, L.B. (2012). Microbial recovery of manganese using Staphylococcus epidermidis. International Journal of Nonferrous Metallurgy, 1, 9–12.10.4236/ijnm.2012.12002
- Das, A.P., Swain, S., Panda, S., Pradhan, N., & Sukla, L.B. (2012). Reductive acid leaching of low grade manganese ores. Geomaterials, 2, 70–72.10.4236/gm.2012.24011
- Das, A.P., & Mishra, S. (2010). Biodegradation of the metallic carcinogen hexavalent chromium Cr(VI) by an indigenously isolated bacterial strain. Journal of carcinogenesis, 9, 6.
- Dick, G.J., Justin, W., Terry, J., & Bradley, M.T. (2008). Direct identification of a bacterial manganese (II) oxidase, the multicopper oxidase MnxG, from spores of several different marine Bacillus species. Applied and Environmental Microbiology, 74, 1527–1534.10.1128/AEM.01240-07
- Felicioa, A.P., Garcia, O., Bertolinia, C., Ottobonib, L.M.M., & Novoa, M.T.M. (2003). The effects of copper ions on the synthesis of periplasmic and membrane proteins in Acidithiobacillus ferrooxidans as analyzed by SDS-PAGE and 2D-PAGE. Hydrometallurgy, 71, 165–171.10.1016/S0304-386X(03)00153-1
- Ghosh, S., Mohanty, S., Akcil, A., Sukla, L.B., & Das, A.P. (2016). A greener approach for resource recycling: Manganese bioleaching. Chemosphere, 154, 628–639.10.1016/j.chemosphere.2016.04.028
- Ghosh, S., Mohanty, S., Nayak, S., Sukla, L.B., & Das, A.P. (2015). Molecular identification of indigenous manganese solubilising bacterial biodiversity from manganese mining deposits. Journal of Basic Microbiology, 55, 1–9.
- Ghosh, S., & Das, A.P. (2015). Modified titanium oxide (TiO2) nano-composites and its array of applications: A review. Toxicological & Environmental Chemistry, 97, 491–514.10.1080/02772248.2015.1052204
- Li, Y., Zuo, W., Li, Y., & Wang, X. (2012). Cloning of multicopper oxidase gene from Ochrobactrum sp. 531 and characterization of its alkaline laccase activity towards phenolic substrates. Advances in Biological Chemistry, 2, 248–255.10.4236/abc.2012.23031
- Mohanty, S., Ghosh, S., Nayak, S., & Das, A.P. (2016a). Bioleaching of manganese by Aspergillums oryzae isolated from mining deposits. Chemosphere, 172, 302–309.
- Mohanty, S., Ghosh, S., Nayak, S., & Das, A.P. (2016b). Isolation, identification and screening of manganese solubilizing fungi from low grade manganese ore deposits. Geomicrobiology Journal, 34, 309–316. doi:10.1080/01490451.2016.1189016
- Mohapatra, S., Bohidar, S., Pradhan, N., Kar, R.N., & Sukla, L.B. (2007). Microbial extraction of nickel from Sukinda chromite overburden by Acidithiobacillus ferrooxidans and Aspergillus strains. Hydrometallurgy, 85(1), 1–8.10.1016/j.hydromet.2006.07.001
- Prakash, J.W., Marimuthu, J., Antonisamy, & Jeeva, S. (2011) Antimicrobial activity of certain fresh water microalgae from Thamirabarani River, Tamil Nadu, South India. Asian Pacific Journal of Tropical Biomedicine, 1, S170–S173.10.1016/S2221-1691(11)60149-4
- Parikh, S.J., & Chorover, J. (2005). FTIR spectroscopic study of biogenic Mn-Oxide formation by Pseudomonas putida GB-1. Geomicrobiology Journal, 22, 207–218.10.1080/01490450590947724
- Rawlings, D.E. (2005). Characteristics and adaptability of iron and sulfur-oxidizing microorganisms used for the recovery of metals from minerals and their concentrates. Microbial Cell Factories, 4, 13.10.1186/1475-2859-4-13
- Sanket, A.S., Ghosh, S., Sahoo, R., Nayak, S., & Das, A.P. (2016). Molecular identification of acidophilic Manganese (Mn) solubilizing bacteria from mining effluents and their application in mineral beneficiation. Geomicrobiology Journal, 34, 71–80. doi:10.1080/01490451.2016.1141340
- Sawhney, S.K., & Singh, R. (2001). Introductory practical biochemistry. Mumbai: Narosa Publishing House.
- Wang, J., Bai, J., Xu, J., & Liang, B. (2009). Bioleaching of metals from printed wire boards by Acidithiobacillus ferrooxidans and Acidithiobacillus thiooxidans and their mixture. Journal of Hazardous Materials, 172, 1100–1105.10.1016/j.jhazmat.2009.07.102
- Xin, B., Jiang, W., Li, X., Zhang, K., Liu, C., Wang, R., & Wang, Y. (2012). Analysis of reasons for decline of bioleaching efficiency of spent Zn–Mn batteries at high pulp densities and exploration measure for improving performance. Bioresource Technology, 112, 186–192.10.1016/j.biortech.2012.02.133