 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Assessment of biomass and carbon stock (CS) of trees was carried out in three selected study sites (four 0.25 ha [50 m × 50 m] plots in each site) in East Godavari region of Eastern Ghats. Aboveground biomass (AGB) was estimated by the non-harvest method by using allometric equations. The AGB ranged from 58.04 (site I) to 368.39 (site III) Mg/ha and the total CS of trees ranged from 44.51 to 218.84 Mg/ha. The highest CS accumulation in site III could be due to more soil moisture and abundant large diameter trees while the least was obtained in the site I could be due to relatively high human disturbance. Xylia xylocarpa was a dominant biomass and carbon assimilator in the site I and II, while Terminalia arjuna was the highest contributor in site III. The present study revealed that T. arjuna and X. xylocarpa are the vital tree species to endure and sink more carbon in tropical dry forest of East Godavari region under various disturbances.
Introduction
Climate change, biodiversity loss, and global warming are some of the key environmental issues present-day society is facing. By increasing carbon storage and decreasing greenhouse gas (GHG) emissions, conserved forests can bring a drastic change in the composition of global atmospheric GHGs (Lung & Espira, Citation2015). Biodiversity and its connection with the carbon cycle have become current research interests for mitigating climate change (Midgley et al., Citation2010). In the Conference of Parties meeting (COP 21) held in 2015 in Paris, France, 195 countries agreed “to keep a global temperature rise this century well below 2°C.” All the member countries of UNFCCC have acknowledged the crucial role that the resilient forests play in both climate change mitigation and development (COP21, Citation2015; UNFCC, Citation2014). The afforestation and reforestation programs in the damaged forest areas or in the barren lands can now be recognized as carbon sinks under Kyoto Protocol. Forests are important to mitigate carbon and well-managed forests increase the resilience of ecosystem services, particularly trees absorb and store large quantities of carbon (UNFAO, Citation2010, Citation2015). REDD (Reducing emissions from deforestation and forest degradation) and REDD+ mechanisms enable countries or projects that avoid forest loss by compensating financial rewards through quantified carbon estimates and in order to quantify carbon benefits, assimilation of carbon has to be quantified from the forests (Ebeling & Yasue, Citation2008; Vieilledent et al., Citation2012).
Geographically tropical forests cover between 7% and 10% of land area of the planet (Lewis et al., Citation2009) which consists of 471 ± 93 pg of stored carbon (Pan et al., Citation2011). Hence, tropical forest ecosystems are essential for maintaining both carbon cycling and biodiversity (Midgley et al., Citation2010). Annual carbon sink in tropical forests at a global level is approximately 1.3 pg (Lewis et al., Citation2009; Lung & Espira, Citation2015). Tropical forests are clearing at an approximate rate of 15–17 M ha/y (FAO, Citation1995; Naveenkumar, Arunkumar, & Sundarapandian, Citation2017). If the same situation pertains, the carbon sink of terrestrial constituent of the planet declines while emission of GHGs will increase and thereby resulting in drastic climatic events.
In tropical forests, the quantitative studies on accumulated carbon pools, carbon fixation, and net primary production are well addressed in global scale but the regional dilemma with research gaps has been well noticed (Achard, Eva, Mayaux, Stibig, & Belward, Citation2004; Djomo, Knohl, & Gravenhorst, Citation2011; Hertel et al., Citation2009). Several researchers indicated that there is an uncertainty in carbon sink and stocks in spatial scale of tropical forest ecosystems which may be due to variation in topography, forest types, elevation, level of anthropogenic pressures, and microclimate (Becknell, Kucek, & Powers, Citation2012; Gandhi & Sundarapandian, Citation2017; Naveenkumar et al., Citation2017). In general, carbon stock (CS) was assessed in woody vegetation which has >10 cm DBH (Diameter at Breast Height); however, only a few studies were focused on juvenile population of woody species contribution in carbon sink and stocks of tropics (Chaturvedi, Raghubanshi, & Singh, Citation2012a; Gandhi & Sundarapandian, Citation2017; Mohanraj, Saravanan, & Dhanakumar, Citation2011; Naveenkumar et al., Citation2017). Generally, small stem circumference class (<10 cm) individuals in the tree population have grown rapidly than the large-diameter class trees and comprise a significant proportion in the understory woody plant biomass (Chaturvedi et al., Citation2012a). The requisite for estimating biomass in forest ecosystems is supported by the necessity of information on the forest carbon stocking capacity.
National level biomass inventories are carried on the basis of remote sensing. However, the uncertainty in biomass estimates persisted because CS and sequestration potential varied due to numerous factors. Biomass and CSs varied at a spatial scale in any forest type even in a small patch (1 km2) due to variation in microclimate. Accurate estimation method through harvesting is not practically feasible because most of the forest ecosystems are declared as protected in several countries. Allometric equation provides an alternative estimation of biomass accepted by the scientific community and UNFCCC (Chave et al., Citation2003).
The Eastern Ghats are located on India’s eastern coast and they form several discontinuous hill ranges from the northern Odisha to Tamil Nadu covering Andhra Pradesh. The Eastern Ghats with their geographical diversity host four major rivers (Godavari, Mahanadi, Krishna, and Kaveri) of peninsular India. Biomass and carbon assessment studies were mainly focused in the southernmost part of Eastern Ghats (Gandhi & Sundarapandian, Citation2017; Mohanraj et al., Citation2011; Naveenkumar et al., Citation2017; Pragasan, Citation2014, Citation2015; Sundarapandian, Dar, Gandhi, Srinivas, & Subashree, Citation2013) but little is known from the northern part (Behera & Misra, Citation2006; Sahu, Sharma, & Ravindranath, Citation2015; Sahu, Suresh, & Ravindranath, Citation2016). However, quantitative CS assessment studies in East Godavari region of Eastern Ghats are still lacking. According to Sheikh, Kumar, Bussman, and Todaria (Citation2011), the biomass reserves in the Eastern Ghats are being depleting continuously from 2003. Hence, the present study was a preliminary work undertaken with the objectives to assess the biomass and CS of trees in three dry tropical forest sites of the Eastern Ghats in East Godavari District, Andhra Pradesh.
Study area
The field study was performed in forest sites of East Godavari region situated in the northern part of Eastern Ghats ranged between 17°33′N and 81°45′E (). Average annual precipitation (for a decade) was 109 ± 6.6 cm with a peak was observed in the month of October (22.2 ± 2.9 cm) and a minimum in the month of March (0.29 ± 0.02 cm). Average monthly maximum temperature ranged from 28 to 38°C and a mean monthly minimum temperature ranged from 18 to 27°C ().
Figure 1. Location of the study sites in East Godavari region of Eastern Ghats, Andhra Pradesh, India.
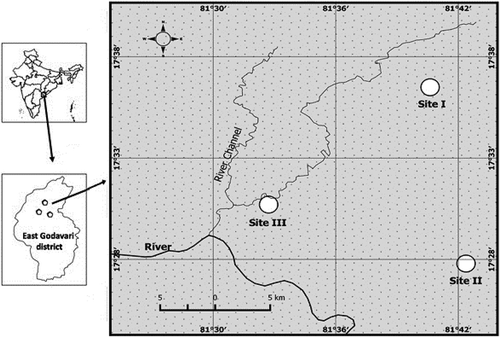
Figure 2. Rainfall and temperature patterns of the study areas in tropical dry forest of East Godavari region, Andhra Pradesh, India (source: http://www.indiawaterportal.org/met_data/).
Methods
Three study sites (each 1 ha [4 square plots of 0.25 ha with an interval of 500 m]) were selected in reserve forest of East Godavari region for the present study. Each plot has been further sub-gridded into 25 (10 m × 10 m) quadrats as workable units. The site I is nearer to the human settlements. Illegal tree felling and grazing are frequent in study site I even if it was demarcated as a part of the reserve forest. Site II was positioned far from the human settlements which receive relatively less disturbance than site I, and site III is nearer to rivulet which joins the tributaries of river Godavari and also experiences minor disturbances. All the live tree individuals above 1 cm GBH (Girth at Breast Height) were enumerated and its diameter at 1.37 m height was measured. The diameter of each stem was measured and summed up for multistemmed trees. Plant samples were collected, preserved, and identified with “Flora of Eastern Ghats: Hill ranges of South East India” by Pullaiah, Rao, Ramamurthy, Karuppusamy, and Rani (Citation2011). The AGB of adults (≥10 cm DBH) and juvenile population (≥3–<10 cm DBH) of trees was estimated with allometric equation (1) given by Brown, Gillespie, and Lugo (Citation1989).
To estimate the AGB of saplings (<3 cm DBH), the allometric equation (2) of Chaturvedi, Raghubanshi, and Singh (Citation2012b) was employed.
Belowground biomass was calculated from the AGB using a root-shoot ratio of 0.26 (Equation (3)) (Ravindranath & Ostwald, Citation2008).
Carbon of tropical dry forest is considered to be a fraction 44.53% of biomass, hence, 0.4453 was the conversion factor multiplied (Equation (4)) (Júnior et al., Citation2016).
Results
The total AGB of trees was greater in study site III (390.03 Mg/ha) than that of other two study sites (). Since belowground biomass and CS were computed based on AGB values, belowground biomass as well as CSs exhibited a similar trend. The total CS of trees ranged 44.51–218.84 Mg/ha. The AGB and CS of the adult tree and sapling population showed greater values in site III than that of other study sites. On the contrary, AGB and CS of juvenile tree population showed greater values in site I than in other study sites. Mean tree diameter was ranged 15.89–32.89 cm among the study sites. The CS of adult trees ranged 32.56–206.70 Mg/ha, juvenile tree population ranged 4.68–6.70 Mg/ha, and sapling population ranged 5.25–7.42 Mg/ha.
Table 1. Summary of quantitative assessment of biomass and carbon stocks of trees in the tropical dry forest of East Godavari region, Eastern Ghats.
Xylia xylocarpa accumulated greater biomass and CS in both sites I and II of adult tree population, followed by Pterocarpus marsupium, Terminalia alata, Albizia odoratissima, and Bridelia retusa in site I, and Grewia tiliifolia, Dillenia pentagyna, and Lannea coromandelica in site II ( and ), while Terminalia arjuna stored greater biomass and CS in site III followed by X. xylocarpa, Mangifera indica, and Pongamia pinnata. In both sapling and juvenile tree populations, X. xylocarpa was the top species that accumulated greater biomass and CS compared to other species in all three study sites. Top five species of respective study sites stored 61.69–73.04% of biomass and CS in adult stage, simillarly, in the juvenile and sapling populations, these accumulated 64.28–76.31% and 31.18–60.57% respectively. The species which contributed less than 1% of the AGB in the adult population are 30, 31, and 46 in sites I, II, and III, respectively. The juvenile population of 43 species in site I, 49 species in site II, and 39 species in site III has also shown less than 1% of aboveground biomass. A similar trend was exhibited in sapling population also (54 species in site I, 55 species in site II, and 54 species in site III).
Table 2. Species-wise contribution of aboveground biomass (top 25 species) in the tropical dry forest of East Godavari region, Eastern Ghats.
Table 3. Species-wise contribution of carbon stock (top 25 species) in the tropical dry forest of East Godavari region, Eastern Ghats.
Combretaceae was the highest contributor of biomass in adult tree population of site III compared to Leguminosae, which was the family that contributed the highest biomass in all developmental stages of trees in sites I and II, and juvenile and sapling stages of site III (). Eight families in site I, 7 families in site II, and 9 families in site III contributed less than 1% of biomass and CSs. Leguminosae, Combretaceae, Anacardiaceae, Malvaceae, Phyllanthaceae, and Dilleniaceae are the major tree biomass (adults + juveniles + saplings) accumulated families in sites I and II, whereas Combretaceae, Leguminosae, Anacardiaceae, Ebenaceae, and Myrtaceae are the major contributors in site III.
Table 4. Family-wise contribution of aboveground biomass in the tropical dry forest of East Godavari region, Eastern Ghats.
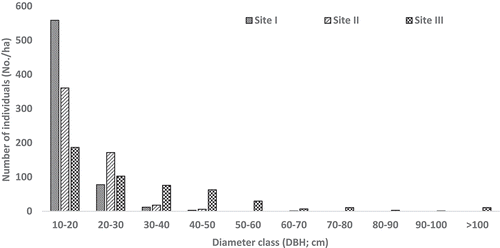
Diameter class-wise distribution of adult trees showed wide variation among the study sites (). In all the study sites, lower diameter class (≥10–<30 cm DBH) has a greater number of individuals, whereas in mid diameter class (30–60 cm DBH), higher number of individuals was recorded in site III (169 trees) followed by site II (24 trees) and site I (15 trees). However, larger diameter class (≥100 cm DBH) trees were obtained only in site III.
Figure 3. Diameter class-wise distribution of trees in the study sites in tropical dry forest of East Godavari region, Eastern Ghats.
Correlation and regression analysis indicated that adult tree basal area has shown a significant positive relationship with adult tree CS (), while juvenile basal area showed a negative relationship with saplings, adults and, total tree carbon. Similarly, adult tree density exhibited a weak negative relationship with adult tree carbon and total tree carbon, whereas it showed a positive relationship with juvenile CSs. Sapling density and juvenile density showed a significant positive relationship with saplings and juvenile carbon, respectively. Soil moisture showed a significant positive relationship with total tree CSs.
Table 5. Correlation (r value) between predictor variables with tree aboveground biomass (AGB) in the tropical dry forest of East Godavari region, Eastern Ghats.
Discussion
The AGB estimated in the present study ranged from 58.04 to 368.39 Mg/ha has exceeded the range (39–334 Mg/ha) given by Becknell et al. (Citation2012) who obtained the values while reviewing 40 published papers on seasonally dry tropical forests and 95–214 Mg/ha reported in Javadi hills of Eastern Ghats (Naveenkumar et al., Citation2017). Mean AGB in the present study (168.99 Mg/ha) is lower than the mean AGB of 260 African tropical forests (395.7 Mg/ha) estimated by Lewis et al. (Citation2013), in the forests of Borneo (445 Mg/ha, Slik et al., Citation2010), and Amazonian forests average of 289 Mg/ha (Malhi et al., Citation2006). The adult tree basal area showing a significant positive relationship with adult tree CS and productivity has been proven by many small-scale research and observations in natural ecosystems (Midgley et al., Citation2010). The mean aboveground CS was 75.26 Mg/ha in the present study (ranged 25.84–164.05 Mg/ha) that lies within the global range value (14–123 Mg C/ha) reported by Murphy and Lugo (Citation1986) in tropical deciduous forests and studies reported in the recent past (Becknell, Citation2012; Becknell & Powers, Citation2014; Gandhi & Sundarapandian, Citation2017).
Brown and Lugo (Citation1990) concluded that total amount of accumulated biomass in forest ecosystems may vary with variation in biophysical characteristics, microclimate, and level of anthropogenic disturbances. The present result pointed out that site I was facing relatively high disturbance over a period of time which influences the reduction in large diameter trees which in turn resulted in lower biomass than that of other study sites. The highest biomass in study site III (five and four-fold, respectively in sites I and II) could be attributed to numerous larger diameter trees in the study site which constituted significant biomass. The study site III is nearer to rivulet which could provide a conducive environment for the extensive growth of trees that resulted in the accumulation of greater biomass. The water availability due to rivulets influences the increase in the volume of trees and thereby accumulating greater AGB. Alvarez-davila et al. (Citation2017) found that water availability strongly influences forest structure and tree growth. Similarly, Becknell et al. (Citation2012) also stated that water availability is one of the main factors which regulates biomass accumulation. The larger diameter trees contribute majority of forest biomass and it was also confirmed by other researchers (Brown & Lugo, Citation1992; Brown et al., Citation1995; Brown, Citation1996; Chave et al., Citation2003; Culmsee, Leuschner, Moser, & Pitopang, Citation2010; Gandhi & Sundarapandian, Citation2017; Lung & Espira, Citation2015; Midgley & Niklas, Citation2004; Slik et al., Citation2010). The relative differences in anthropogenic pressures among the study sites could be the reason for low CSs in study sites I and II. Several studies enlightened that human disturbances could alter species composition and structure of forest ecosystem (Anbarashan & Parthasarathy, Citation2012; Htun, Mizoue, Kajisa, & Yosida, Citation2011; Sheil & Burslam, Citation2003; Srinivas & Sundarapandian, Citation2018) that resulted more stem density and lower basal area in site I than that of least disturbed study site (III) where less stem density and higher basal area were recorded. Even though the abundance of trees was more in sites I and II, their diameter and canopy sizes were low that creates canopy openings and light penetration to the ground level and thereby providing favorable conditions for tree regeneration and understory vegetation development.
The juvenile tree population CSs (4.68–6.70 Mg/ha) assessed in the present study were higher to the values (0.6–3.6 Mg/ha) reported by Chaturvedi et al. (Citation2012a) in tropical dry forests of Uttar Pradesh, India. The contribution of tree juvenile population (15.05% of CSs) to the total tree CSs was high in study site I while the least (2.16%) was observed in study site III. Human activates and cutting of trees might have resulted as coppicing which enhances sapling and juvenile tree populations in site I that inferences greater juvenile biomass contribution. However, tree sapling contribution to total CS was greater in study site III than other study sites. This may be due to water availability. Ceccon, Sanchéz, and Campo (Citation2004) and Marod, Kutintara, Tanaka, and Nakashizuka (Citation2004) stated that the early stages of tree growth may be regulated by sunlight availability, adequate soil nutrients, soil texture, and soil moisture content. The adult trees contributed significantly greater CS (73.2–94.5%) to total tree CS compared to tree juveniles and saplings contribution (5.5–26.8%). However, future carbon accumulation will depend on the present status of the tree sapling and juvenile populations. The significant variation observed in the biomass and CSs among the study sites could be attributed to variations in species composition, soil characteristics, level of anthropogenic pressures, etc.
X xylocarpa was a dominant contributor of biomass and CS in sites I and II, while T. arjuna was the highest contributor in site III. The present study revealed that T. arjuna and X. xylocarpa are the important adult tree species to endure and sink more carbon in the dry tropical forest in East Godavari region even under anthropogenic pressure. T. arjuna accumulated significant biomass carbon that could be due to favorable conditions, i.e., the rivulets are nearer to the study plots. Several studies also reported that the T. arjuna prefer water-rich areas for sustenance (Datta & Goyal, Citation1996; Johnsingh & Joshua, Citation1989; Kundu & Schmidt, Citation2015). The present study also has drawn the need for conservation of T. arjuna, X. xylocarpa, and other major contributors in order to fix significant CS in the tropical dry forest in the Eastern Ghats. Thus, the present study concludes that the tropical dry forests in East Godavari region stored a substantial amount of carbon as similar to the tropical dry forest of other parts of India as well as elsewhere. The CS of trees in the tropical dry forest could be attributed to soil moisture, the density of various stages of development of trees and anthropogenic pressure.
Conclusion
The dry tropical forest ecosystem is becoming particularly important because of facing high anthropogenic pressures which resulted in the loss of forest cover and biodiversity. Present work gave an emphasis on the tree biomass and CS contribution of trees at various stages of development, showing the necessity of further research work to understand the factors influencing biomass and CS contributions of other woody vegetation and nonwoody vegetation in the regional scale. Results from the present study indicate the utmost requirement of formal actions to curtail anthropogenic pressure and to take conservation measures in the tropical forest of East Godavari region, Andhra Pradesh for increase carbon sink in the climate change scenario.
Acknowledgments
The first author thanks UGC, Govt. of India for providing fellowship under UGC-BSR (RFSMS) scheme. We thank state forest department, Andhra Pradesh, for giving permission to execute fieldwork. We are also grateful to Dr. S. Karuppusamy, Department of Botany, The Madura College, Madurai, India for identification of plant species.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Achard, F., Eva, H. D., Mayaux, P., Stibig, H. J., & Belward, A. (2004). Improved estimates of net carbon emissions from land cover change in the tropics for the 1990s. Global Biogeochemical Cycles, 18(2), 1–11.
- Álvarez-Dávila, E., Cayuela, L., González-Caro, S., Aldana, A. M., Stevenson, P. R., Phillips, O., & Melo, O. (2017). Forest biomass density across large climate gradients in northern South America is related to water availability but not with temperature. PloS one, 12(3), 1–16, p.e0171072.
- Anbarashan, M., & Parthasarathy, N. (2012). Tree diversity and forest stand structure along disturbance gradients in Indian tropical dry evergreen forest. Ecotropica, 18(2), 119–136.
- Becknell, J. M. (2012). Carbon cycling in secondary tropical dry forest from species to 16 global scales. (Ph.D. Dissertation). University of Minnesota. Retrieved from the University of Minnesota Digital Conservancy, http://hdl.handle.net/11299/136056.
- Becknell, J. M., Kissing Kucek, L., & Powers, J. S. (2012). Aboveground biomass in mature and secondary seasonally dry tropical forests: A literature review and global synthesis. Forest Ecology and Management, 276, 88–95.
- Becknell, J. M., & Powers, J. S. (2014). Stand age and soils as drivers of plant functional traits and aboveground biomass in secondary tropical dry forest. Canadian Journal of Forest Research, 44(6), 604–613.
- Behera, S. K., & Misra, M. K. (2006). Aboveground tree biomass in a recovering tropical sal (Shorea robusta Gaertn. f.) forest of Eastern Ghats, India. Biomass and Bioenergy, 30(6), 509–521.
- Brown, I. F., Martinelli, L. A., Thomas, W. W., Moreira, M. Z., Ferreira, C. C., & Victoria, R. A. (1995). Uncertainty in the biomass of Amazonian forests: An example from Rondonia, Brazil. Forest Ecology and Management, 75(1–3), 175–189.
- Brown, S. (1996). Tropical forests and the global carbon cycle: Estimating state and change in biomass density. In M. J. Apps, & D. T. Price (Eds.), Forest ecosystems, forest management and the global carbon cycle (pp. 135–144). Berlin, Heidelberg: Springer.
- Brown, S., Gillespie, A. J., & Lugo, A. E. (1989). Biomass estimation methods for tropical forests with applications to forest inventory data. Forest Science, 35(4), 881–902.
- Brown, S., & Lugo, A. E. (1990). Tropical secondary forests. Journal of Tropical Ecology, 6(1), 1–32.
- Brown, S., & Lugo, A. E. (1992). Aboveground biomass estimates for tropical moist forests of the Brazilian Amazon. Interciencia. Caracas, 17(1), 8–18.
- Ceccon, E., Sanchéz, S., & Campo, J. (2004). Tree seedling dynamics in two abandoned tropical dry forests of differing successional status in Yucatán, Mexico: A field experiment with N and P fertilization. Plant Ecology, 170, 12–26.
- Chaturvedi, R. K., Raghubanshi, A. S., & Singh, J. S. (2012a). Effect of grazing and harvesting on diversity, recruitment and carbon accumulation of juvenile trees in tropical dry forests. Forest Ecology and Management, 284, 152–162.
- Chaturvedi, R. K., Raghubanshi, A. S., & Singh, J. S. (2012b). Biomass estimation of dry tropical woody species at juvenile stage. The Scientific World Journal, Article ID 790219, 1–5.
- Chave, J., Condit, R., Lao, S., Caspersen, J. P., Foster, R. B., & Hubbell, S. P. (2003). Spatial and temporal variation of biomass in a tropical forest: Results from a large census plot in Panama. Journal of Ecology, 91(2), 240–252.
- COP 21. (2015). Twenty-first meeting of the conference of the parties United Nations Framework Convention on Climate Change (UNFCCC). Paris, France. 30th November −11th December 2015. Retrived from https://unfccc.int/news/finale-cop21
- Culmsee, H., Leuschner, C., Moser, G., & Pitopang, R. (2010). Forest aboveground biomass along an elevational transect in Sulawesi, Indonesia, and the role of Fagaceae in tropical montane rain forests. Journal of Biogeography, 37(5), 960–974.
- Datta, A., & Goyal, S. P. (1996). Comparison of forest structure and use by the Indian Giant Squirrel (Ratufa indica) in two riverine forests of Central India. Biotropica, 28(3), 394–399.
- Djomo, A. N., Knohl, A., & Gravenhorst, G. (2011). Estimations of total ecosystem carbon pools distribution and carbon biomass current annual increment of a moist tropical forest. Forest Ecology and Management, 261(8), 1448–1459.
- Ebeling, J., & Yasué, M. (2008). Generating carbon finance through avoided deforestation and its potential to create climatic, conservation and human development benefits. Philosophical Transactions of the Royal Society B: Biological Sciences, 363(1498), 1917–1924.
- FAO. (1995). Forest Resources Assessment 1990. Global Synthesis. Rome: FAO.
- Gandhi, D. S., & Sundarapandian, S. (2017). Large-scale carbon stock assessment of woody vegetation in tropical dry deciduous forest of Sathanur reserve forest, Eastern Ghats, India. Environmental Monitoring and Assessment, 189(4), 187.
- Hertel, D., Moser, G., Culmsee, H., Erasmi, S., Horna, V., Schuldt, B., & Leuschner, C. (2009). Below-and above-ground biomass and net primary production in a paleotropical natural forest (Sulawesi, Indonesia) as compared to neotropical forests. Forest Ecology and Management, 258(9), 1904–1912.
- Htun, N. Z., Mizoue, N., Kajisa, T., & Yosida, S. (2011). Tree species composition and diversity at different levels of disturbance in Popa Mountain Park, Myanmar. Biotropica, 43(5), 597–603.
- Johnsingh, A. J. T., & Joshua, J. (1989). The threatened gallery forest of the river Tambiraparani, Mundanthurai Wildlife Sanctuary, south India. Biological Conservation, 47(4), 273–280.
- Júnior, P., Resende, L., Andrade, E. M. D., Palácio, H. A. D. Q., Raymer, P. C. L., Ribeiro Filho, J. C., & Pereira, F. J. S. (2016). Carbon stocks in a tropical dry forest in Brazil. Revista Ciência Agronômica, 47(1), 32–40.
- Kundu, M., & Schmidt, L. H. (Ed.) (2015). Terminalia Arjuna (Roxb. ex DC) Wight & Arn. Seed Leaflet, (166) Retrived from http://www.forskningsdatabasen.dk/en/catalog/2398337778.
- Lewis, S. L., Lopez-Gonzalez, G., Sonké, B., Affum-Baffoe, K., Baker, T. R., Ojo, L. O., … Ewango, C. E. (2009). Increasing carbon storage in intact African tropical forests. Nature, 457(7232), 1003.
- Lewis, S. L., Sonké, B., Sunderland, T., Begne, S. K., Lopez-Gonzalez, G., Van Der Heijden, G. M., … Bastin, J. F. (2013). Above-ground biomass and structure of 260 African tropical forests. Philosophical Transactions of the Royal Society B, 368(1625), 20120295.
- Lung, M., & Espira, A. (2015). The influence of stand variables and human use on biomass and carbon stocks of a transitional African forest: Implications for forest carbon projects. Forest Ecology and Management, 351, 36–46.
- Malhi, Y., Wood, D., Baker, T. R., Wright, J., Phillips, O. L., Cochrane, T., … Higuchi, N. (2006). The regional variation of aboveground live biomass in old‐growth Amazonian forests. Global Change Biology, 12(7), 1107–1138.
- Marod, D., Kutintara, U., Tanaka, H., & Nakashizuka, T. (2004). Effects of drought and fire on seedling survival and growth under contrasting light conditions in a seasonal tropical forest. Journal of Vegetation Science, 15, 691–700.
- Midgley, G. F., Bond, W. J., Kapos, V., Ravilious, C., Scharlemann, J. P., & Woodward, F. I. (2010). Terrestrial carbon stocks and biodiversity: Key knowledge gaps and some policy implications. Current Opinion in Environmental Sustainability, 2(4), 264–270.
- Midgley, J. J., & Niklas, K. J. (2004). Does disturbance prevent total basal area and biomass in indigenous forests from being at equilibrium with the local environment? Journal of Tropical Ecology, 20(5), 595–597.
- Mohanraj, R., Saravanan, J., & Dhanakumar, S. (2011). Carbon stock in Kolli forests, Eastern Ghats (India) with emphasis on aboveground biomass, litter, woody debris and soils. iForest-Biogeosciences and Forestry, 4(2), 61.
- Murphy, P. G., & Lugo, A. E. (1986). Ecology of tropical dry forest. Annual Review of Ecology and Systematics, 17(1), 67–88.
- Naveenkumar, J., Arunkumar, K. S., & Sundarapandian, S. M. (2017). Biomass and carbon stocks of a tropical dry forest of the Javadi Hills, Eastern Ghats, India. Carbon Management, 8(5–6), 351–361.
- Pan, Y., Birdsey, R. A., Fang, J., Houghton, R., Kauppi, P. E., Kurz, W. A., … Ciais, P. (2011). A large and persistent carbon sink in the world’s forests. Science, 333, 988–993.
- Pragasan, L. A. (2014). Carbon stock assessment in the vegetation of the Chitteri Reserve Forest of the Eastern Ghats in India based on non-destructive method using tree inventory data. Journal of Earth Science & Climatic Change,S11, 1–6.
- Pragasan, L. A. (2015). Assessment of aboveground biomass stock in the Pachaimalai forest of Eastern Ghats in India. Applied Ecology and Environmental Research, 13(1), 133–145.
- Pullaiah, T., Rao, D. M., Ramamurthy, K. S., Karuppusamy, S., & Rani, S. S. (2011). Floras of Eastern Ghats: Hill ranges of South East India (Vols. 1–4). Hyderabad, India: INTACH, Andhra Pradesh State Chapter publications.
- Ravindranath, N. H., & Ostwald, M. (2008). Methods for estimating above-ground biomass. In: Carbon inventory methods handbook for greenhouse gas inventory, carbon mitigation and roundwood production projects (pp. 113–147). Dordrecht: Springer Netherlands.
- Sahu, S. C., Sharma, J., & Ravindranath, N. H. (2015). Carbon stocks and fluxes for forests in Odisha (India). Tropical Ecology, 56(1), 77–85.
- Sahu, S. C., Suresh, H. S., & Ravindranath, N. H. (2016). Forest structure, composition and aboveground biomass of tree community in tropical dry forests of Eastern Ghats, India. Notulae Scientia Biologicae, 8(1), 125–133.
- Sheikh, M. A., Kumar, M., Bussman, R. W., & Todaria, N. P. (2011). Forest carbon stocks and fluxes in physiographic zones of India. Carbon Balance Manage, 6, 15.
- Sheil, D., & Burslem, D. F. R. P. (2003). Disturbing hypothesis in tropical forests. Trends in Ecology & Evolution, 18, 18–26.
- Slik, J. W. F., Aiba, S. I., Brearley, F. Q., Cannon, C. H., Forshed, O., Kitayama, K., … Poulsen, A. D. (2010). Environmental correlates of tree biomass, basal area, wood specific gravity and stem density gradients in Borneo’s tropical forests. Global Ecology and Biogeography, 19(1), 50–60.
- Srinivas, K., & Sundarapandian, S. (2018). Diversity, population structure and distribution of trees in tropical dry forests, East Godavari District, Eastern Ghats, India. International Journal of Ecology & Development, 33(2), 13–32.
- Sundarapandian, S. M., Dar, J. A., Gandhi, D. S., Srinivas, K., & Subashree, K. (2013). Estimation of biomass and carbon stocks in tropical dry forests in Sivagangai district, Tamil Nadu, India. International Journal of Environmental Science and Engineering Research, 4(3), 66–76.
- UNFAO. (2010). Managing forests for climate change. Food and Agriculture Organization of the United Nations, I1960E/1/11.10 Retrieved from www.fao.org/docrep/013/i1960e/i1960e00.pdf
- UNFAO. (2015). Durban declaration: 2050 vision for forests and forestry. Food and Agriculture Organization of the United Nations Rome. Retrieved from www.fao.org/fileadmin/user_upload/wfc2015/../Durban_Declaration_draft.pdf
- UNFCCC. (2014). Report of the conference of the parties on its nineteenth session. Warsaw, 11th–23rd November 2013. Addendum. FCCC/CP/2013/10/Add.1. UN Framework Convention on Climate Change, Bonn.
- Vieilledent, G., Vaudry, R., Andriamanohisoa, S. F., Rakotonarivo, O. S., Randrianasolo, H. Z., Razafindrabe, H. N., … Rasamoelina, M. (2012). A universal approach to estimate biomass and carbon stock in tropical forests using generic allometric models. Ecological Applications, 22(2), 572–583.
