 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Phytoextraction involved the use of plants in rapid, efficient, less expensive, and environment friendly removal of toxic metals from contaminated soil. For this study, a pot experiment was conducted and plant species: Abelmoschus esculentus, Avena sativa, Guizotia abyssinica, and Glycine max were subjected to six copper concentrations i.e., 25, 50, 100, 150, 200, and 300 mg Cu kg−1 for the investigation of Cu phytotoxicity, tolerance, and accumulation for 12 weeks under green house. Soil without spike were taken as control. After 12 weeks of experiment, Cu toxicity on growth and chlorophyll contents were determined. Among four plant species, only A. sativa, C. tetragonoloba and S. indicum seeds were germinated at 300 mg Cu kg−1. The growth parameters were significantly (p < 0.05) reduced under high Cu stress (from 25 to 100 mg Cu kg−1) in G. abyssinica and G. max. The chlorophyll content found maximum at 25 mg Cu kg−1 in all plant species as compared to control. Significantly, high Cu accumulation was found in roots and shoots of A. sativa. The highest values of bioconcentration factor, bioaccumulation coefficient, translocation factor (all greater than 1), phytoremediation ratios, and accumulation with high tolerance suggested that A. sativa was a suitable plant for effective Cu phytoextraction.
Introduction
Soil pollution has become a serious and challenging environmental problem all over the world due to rapid industrialization and urbanization (Li, Citation2018). Various industries discharged their toxic heavy metals containing wastes directly into soil and water system that substantially enhanced the degradation process and significantly affected the ecosystem (Rashid, Manzoor, & Mukhtar, Citation2018; Wu, Zhang, Liu, & Chen, Citation2018). These unwanted chemicals cause severe health problems when exceed the permissible limits (Li, Li, & Wang, Citation2019).
Copper (Cu) is an abundant transition metal on the earth’s crust that exists in two different oxidation states either as monovalent (+1) or divalent (+2) (Rebecca, Citation2011). At low concentration, Cu is considered as an essential micronutrient for all living organisms (Mahmood & Islam, Citation2006; Muhammad et al., Citation2015; Wintz, Fox, & Vulpe, Citation2002) and plays several important roles in the number of metabolic activities, act as catalysts for various homogeneous and heterogeneous chemical reactions (Mildvan, Citation1970). The high concentration of Cu developed several deleterious effects in plants (Dresler, Hanaka, Bednarek, & Maksymiec, Citation2014) for instance, reduced seed germination, stunted root and shoot growth, low yield as well as formation of reactive oxygen species (ROS) (Azooz, Abou-Elhamd, & Al-Fredan, Citation2012; Stadtman & Oliver, Citation1991). Moreover, high Cu stress caused severe damage to metabolic pathways, disturbed the process of photosynthesis and biosynthesis of chlorophyll contents that in turn reduced the productivity of plants (Hegedus, Erdei, & Horvath, Citation2001). Several anthropogenic sources such as, mining and smelting operations, application of inorganic and organic fertilizers, liming treatments, inappropriate use of Cu-containing fungicides and pesticides, sewage sludge as well as wastewater irrigation system (Herawati, Suzuki, Hayashi, Rivai, & Koyoma, Citation2000; Mackie, Müller, & Kandeler, Citation2012; Muhammad et al., Citation2015; Rebecca, Citation2011) has released a large amount of Cu in natural environment and created soil contamination. Therefore, minimization of excess Cu from soil profile was needed.
Globally, management of soil pollution has become the greatest economic challenge, because after getting environmental acceptability, the success and practical implication of remediation techniques mainly depend upon cost (Padmavathiamma & Li, Citation2007). Various physical and chemical remediation methods have been reported in literature for the reclamation of metal-contaminated soil. The major drawbacks of conventional methods are the requirement of high cost and technical resources (Wuana & Okieimen, Citation2011). The conventional methods either physical or chemical were not successful in agricultural lands because of their adverse effects on soil properties. The technical requirement and costs of conventional techniques were also different from one another such as physical remediation required large amount of material resources and manpower whereas, chemical remediation like immobilization and soil washing required cost-effective methods (Khalid et al., Citation2017; Oh et al., Citation2013). Therefore, to overcome the limitations of aforementioned physicochemical methods, it is necessary to develop an efficient, environment compatible, and cost effective remediation technique for the removal of toxic contaminants from soil (Carolin, Kumar, Saravanan, Joshiba, & Naushad, Citation2017; Mani & Kumar, Citation2014). One of the novel approaches referred as phytoremediation particularly phytoextraction has been considered an alternative green solution to the problem of heavy metals contamination in soil and involved plants that efficiently accumulate toxic metals from surrounding areas and transport them from soil to above ground shoot (Rafati et al., Citation2011). The metal-enriched biomass after harvesting can be easily recycled, disposed, treated, and oxidized (Keller, Ludwig, Davoli, & Wochele, Citation2005; Rawat, Kumar, Mutanda, & Bux, Citation2011). Phytoextraction technique particularly suitable for those sites polluted with low to moderate levels of heavy metal contaminants (Malik & Biswas, Citation2012; Usman et al., Citation2012).
About 500 vascular plant species have been reported in literature, which are being used for the uptake of metals from heavily contaminated soil. The mechanism of metal uptake and translocation from soil to harvestable parts are different in plant species (Sharma, Singh, & Manchanda, Citation2014). For effective phytoextraction, the past studies have suggested that plants with high metal tolerance, accumulation potential, and translocation rate (i.e., from roots to shoots) may be preferred (Mehmood, Rashid, Mahmood, & Dawson, Citation2013). From the said information, the objectives of current research are:
To observe the phytotoxicity of Cu on growth performance and tolerance in A. esculentus, A. sativa, G. abyssinica and G. max;
To evaluate the phytoextraction potential; and
To estimate the accumulation potential of biofuel plant species under different Cu concentrations.
Materials and methods
Collection of seeds and process of sterilization
Seeds of tested plant species were procured, i.e., A. sativa, from the Institute of Fodder Research Program, G. max from the Institute of Oilseeds Research Program, National Agricultural Research Centre Islamabad, Pakistan, A. esculentus from Komal seed Production, Tandoallahyar, and G. abyssinica from Vine House Farm, UK. The seeds of selected plant species were surface sterilized with 0.1% HgCl2 solution for 10 min to keep them away from any microbial infection and then washed many times with tap water followed by distilled water (Pourakbar, Khayami, Khara, & Farbidina, Citation2007).
Soil samples collection and characterization
Soil samples were collected from the uncontaminated field of Jamshoro, Sindh, Pakistan with the help of hand shovel and brought to laboratory for further study. The collected soil samples were mixed well and air dried for 2 weeks. After that, soil samples were ground with pestle and mortar to pass through a size of 2 mm sieve and analyzed for physicochemical characteristics. Soil pH and electrical conductivity (EC) was measured with the help of pH-meter (InoLab-WTB GmbH; Weilheim, Germany) using glass electrode (Yoon, Cao, Zhou, & Ma, Citation2006) and EC meter (WTW – 330i) (Rachit, Verma, Meena, Yashveer, & Shreya, Citation2016) at 1:2 (w/v) ratio of soil to water suspension, respectively. Soil organic matter (OM) and organic carbon (OC) were measured according to Walkley and Black method (Fanrong et al., Citation2011). The physicochemical properties of soil are presented in .
Table 1. The physico-chemical properties of experimental soil
Preparation of copper (Cu) stock solution and soil spiking
The stock solution of Cu (1000 mg L−1) was prepared by dissolving 2.683 g of CuCl2 (cupric chloride) in 1000 mL (1L) deionized water. Soil in each pot (5 kg/pot) was artificially spiked with the increased concentration of 25, 50, 100, 150, 200, and 300 mg Cu kg−1 and kept for 2 weeks to attain equilibrium until soil was dried to attain a constant weight. Each of the above-mentioned dilutions together with control (without spiked) was performed in triplicate. The treatment details are given in .
Table 2. Cu treatment levels selected for pot experiment
Complete randomized experimental design
The experimental pots were arranged in complete randomized design (CRD) under greenhouse. Each pot contains 20 holes with equal distance, and in each hole, single seed was sown. After 7 days of sowing, seeds were germinated in each pot. At two-leaf stage, seedlings were thinned down to five in each experimental pot. A plastic plate was placed under the pot to collect the drain out liquid, which was returned back to pots at next watering. At the end of experiment (12 weeks), plants were harvested along with soil samples and investigated for Cu accumulation using atomic absorption spectrophotometer.
Quality assurance and instrumentation
All chemicals and reagents were of analytical grade with a certified purity of 99% procured from E. Merck (Germany). During sample analysis, high-quality glassware made up of Pyrex material were utilized having good resistance for acids. Working standards of respective metal were prepared by proper dilutions of standard stock solutions with double-distilled water.
Under optimum analytical conditions, the concentration of Cu in respective plant tissues and soil was determined by atomic absorption spectrophotometer (Perkin-Elmer, AAnalyst 800) equipped with a Cu hallow cathode lamp having current 5.0 mA and wavelength 327.4 nm. The standard calibration method was used for the calculation of results. Triplicate samples were run to insure the precision of quantitative results.
Germination percentage (%)
For seeds viability, the germination percentage was calculated as the total number of seeds germinated to the total numbers of seeds sown and are expressed in percentage (Talebi, Nabavi, & Sohani, Citation2014).
Growth parameters
The root and shoot lengths of plant species were measured using a centimeter scale and expressed in cm. With the help of analytical weight balance, fresh and dry weights of root and shoot were measured and expressed in gram. For dry weights, root and shoot were first air-dried and then oven-dried at 80 °C to attain constant weights.
Tolerance index (TI) of plants growth parameters
The tolerance index (TI) of plants toward metal stress was expressed as the ratio of plant growth parameters in Cu-contaminated soil in relation to that of plant growth parameters in control soil calculated as: (Wilkins, Citation1978)
Estimation of chlorophyll contents
Chlorophyll content was determined through UV-Visible spectrophotometer (Biochrom Libra S22) by Arnon (Citation1949). For chlorophyll extraction, 0.5 g of fresh leaves was ground with 80% acetone and then filtrated by means of Whatman™ filter paper No. 42. About 1 mL of suspension was diluted with an additional acetone (approximately 2 mL). The optical density (OD) was recorded using two wavelengths i.e., 663 and 645 nm against blank.
Copper (Cu) analysis in plants and soil samples
The Cu content in plants was determined by taking 0.5 g of ground plant sample in crucible and digested with a mixture of HNO3 and HClO4 (3:1, v/v), while for soil, the Cu content was determined by taking 1 g of soil sample and digested with aqua regia (3HNO3: 1HCl) and heated on a hot plate until the solution becomes cleared. The digested solution of plant and soil was filtered through Whatman™ filter paper No.42 and made up the volume up to mark 50 mL by adding deionized water. According to Monni, Salemma, and Millar (Citation2000), the concentration and accumulation of Cu in plant root and shoot were calculated as:
Phytoremediation efficiency
The phytoremediation efficiency was determined by following metal uptake indices:
Bioconcentration factor
The bioconcentration factor (BCF) was calculated as the ratio of Cu concentration in plant root to that of Cu concentration in soil:
Bioaccumulation coefficient
The bioaccumulation coefficient (BAC) was calculated as the ratio of Cu concentration in plant shoot to that of Cu concentration in soil:
Translocation factor
The translocation factor (TF) was calculated as the ratio of Cu concentration in plant shoot to that of Cu concentration in plant roots:
Phytoremediation ratio (%)
The phytoremediation ratio (PR) was used to measure the phytoextraction efficiency of plants (Awokunmi, Citation2016):
Statistical analysis
All data were statistically analyzed with PASW® Statistics 18 (SPSS Inc., Chicago, IL, USA). Analysis of variance (ANOVA) was performed for the comparison of treatment means. The significance difference among treatment means was determined by Duncan’s multiple range Post Hoc tests at a significance level of p < 0.05.
Results and discussion
Copper toxic effects on germination
Copper was considered as an essential micronutrient at low concentrations, and the maximum values for seed germination was observed at 25 mg Cu kg−1 in all tested plant species but gradual increase in copper concentration significantly (p < 0.05) reduced the germination percentage (). At 200 and 300 mg Cu kg−1, the percentage of seed germination of A. sativa was reduced about 55% in contrast to control, while in G. max no seeds were germinated at 200 and 300 mg Cu kg−1. On the other hand, no germination was recorded beyond 100 mg Cu kg−1 in G. abyssinica and at 200 mg Cu kg−1 in A. esculentus. Heavy metal phytotoxicity to seed germination in different plant species was significant to the metal tolerance and variability of resistance within the same and among different plant species. It is well documented in the literature that the seed is the only stage in the whole life cycle of plant that has well protected against the heavy metal stress. The germination of seeds was considered as the fundamental process that decided the impacts of heavy metal toxicity (Ansari et al., Citation2013). The seed coat acted like a barrier between the embryo and environment that protected the young embryo against the heavy metal toxicity. Heavy metal toxicity in terms of seed germination has associated with the interference of toxic metals with protease and amylase enzymes, which results in quick breakdown of stored food materials in seed and alteration of selective permeability properties of cell membrane (Singh, Nath, & Sharma, Citation2007). Past studies have suggested that the excess amount of Cu becomes responsible for the reduction of seed germination in most of the plants (Adhikari, Kundu, Biswas, Tarafdar, & Rao, Citation2012; Ahsan et al., Citation2007).
Table 3. Effects of Cu on germination and growth parameters of four plant species
Copper toxic effects on growth of plants
The high Cu concentration directly influenced the plant growth parameters. The growth parameters i.e., root length, shoot length, root fresh weight, shoot fresh weight, root dry weight, and shoot dry weight were all significantly (p < 0.05) increased at low Cu concentration (i.e., 25 mg Cu kg−1) over the control. At 300 mg Cu kg−1, growth parameters were adversely affected in all plant species indicated that Cu at higher levels reduced plants growth (). Among four plant species, A. esculentus showed better performance in terms of growth parameters. Besides seed germination, heavy metal stress was related to the process of growth inhibition that produced many morphological changes during development. Elongation of plant root and shoot demonstrated a notable sensitivity toward excess heavy metals in soil. The decrease in root length could be primarily due to the interference of heavy metals with the uptake of water and mineral nutrient. The decreased rate of water and mineral absorption was induced due to mineral deficiency in plants and reduced root cell division, cell elongation along with cell cycle that in turn reduced root length of plants (Muhammad et al., Citation2015). Toxicity of heavy metal reduced seedling height and affected root growth and development with the interference of plant metabolic activities (Barbosa et al., Citation2013). The increased transport rate of heavy metals toward shoot region directly affected sensitive parts of plants such as leaves and disturbed the process of photosynthesis as well as the cellular metabolism of shoot and thereby reduced plants’ height (Shaikh, Shaikh, Shaikh, & Shaikh, Citation2013). The hydrolytic enzyme activities was also affected as a result of heavy metal toxicity, and the food was not reached to the developing embryo that affected the seedlings length and ultimately led to the reduction of plants growth (Luo et al., Citation2010; Mukhopadhyay et al., Citation2013). The high plant biomass (i.e., fresh weight and dry weight) has the first pre-requisite for high plant yield. The biomass was mainly based on the growth performance of particular plant (Muhammad et al., Citation2015). Under severe metal toxicity, the plants displayed obvious symptoms of growth inhibition. The decrease in plant biomass was associated with disturbed metabolic activities, low photosynthetic reactions, and reduced uptake of essential mineral nutrients under heavy metal stress (Li et al., Citation2012).
Copper toxic effects on chlorophyll contents
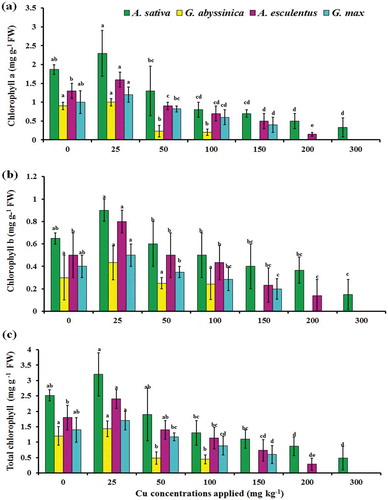
Chlorophyll contents (a, b, and total mg g−1 FW) were decreased significantly (p < 0.05) with steady raise of Cu concentration from 25 to 300 mg Cu kg−1 (). In all tested plant species, the maximum chlorophyll contents (a, b, and total mg g−1 FW) were observed at low Cu concentration (i.e., 25 mg Cu kg−1) as compared to control, while the lowest value for chlorophyll contents was found at 300 mg Cu kg−1. The heavy metal stress negatively affected the photosynthesis process and decreased the chlorophyll-a, chlorophyll-b, and total chlorophyll contents in all plant species at higher concentration. Heavy metal at toxic levels interfered with normal enzyme functions in plants and drastically affected the process of photosynthesis (Ali et al., Citation2015). The decreased rate of photosynthetic pigment was associated with metals that have the consequence of peroxidation of chloroplast membranes due to increased level of ROS generation (Srinivasan, Sahi, Paulo, & Venkatachalam, Citation2014). The production of ROS upon metal interference, directly and indirectly with photosynthesis process induced structural alteration to the pigment protein complexes by degradation and destabilization of proteins in antenna complex as well as complete distortion of thylakoid membranes that reduced the pigment contents and plant growth (Wodala, Eitel, Gyula, Ördög, & Horváth, Citation2012). The excess supply of toxic metals also impaired the uptake of essential photosynthetic pigment elements, such as potassium, calcium, magnesium, iron, and prevented the incorporation of divalent cations with heavy metals, therefore responsible for the reduction of chlorophyll pigments (Gopal & Rizvi, Citation2008). Literature has reported that the reduction of chlorophyll biosynthesis with the destruction of photosynthetic organization at thylakoid level has involved the hindrance of toxic metals with organized system of chlorophyll in plants (Kabata-Pendias & Pendias, Citation2001).
Figure 1. Effect of Cu stress on photosynthetic pigments chlorophyll-a (a) chlorophyll-b (b) and total chlorophyll (a + b) (c), of four plant species after 12 weeks of growth in soil contaminated with varying concentrations of applied Cu. Bars with the similar letters are statistically non-significant according to Duncan’s multiple range test (p < 0.05), Data are means (n = 3 ± SD), a in superscript represent significantly highest followed by later alphabets for lower means
Copper toxic effects on tolerance index
The growth parameters were significantly (p < 0.05) affected at higher Cu concentration in all tested plant species (). For Cu-treated soil, the tolerance indices (TIs) in terms of growth parameters i.e., root length, shoot length, root fresh weight, shoot fresh weight, root dry weight, and shoot dry weight were significantly (p < 0.05) higher in A. sativa than A. esculentus, G. abyssinica and G. max from 25 to 300 mg Cu kg−1. Metal tolerance was considered as basic prerequisite for metal accumulation and consequently for phytoremediation. Literature has reported that plant species utilized for phytoremediation must have high tolerance and metal accumulation capacity in their harvested biomass (Monni et al., Citation2000). Plants have various mechanisms for heavy metal tolerance for instance, exclusion and inclusion (Raskin & Ensley, Citation2000), cell wall binding, active transport of ions into the vacuole, chelation through the induction of metal-binding peptides, and the formation of metal complexes (Memon & Schroder, Citation2009). Growth inhibition represented the common response to heavy metal stress and therefore considered as an important factor among the major agricultural indices used for toxic metal stress tolerance (Tong, Kneer, & Zhu, Citation2004). Plant tolerance to heavy metal stress was estimated on their root and/or shoot development and growth inhibition as affected by the heavy metal toxicity (Srinivasan et al., Citation2014). Literature has reported that the plants with TI values <1 experienced stress because of metal contamination along with a net reduction of plant biomass. By contrast, the plant species with TI > 1 developed tolerance along with a net increase of biomass (hyper-accumulator). On the other hand, the plant species with TI values equals to 1 was unaffected by metal toxicity and indicated no difference in relation to control treatment (Audet & Charest, Citation2007). Therefore, plants’ tolerance to substantial metal toxicity was evaluated in accordance to their roots or shoots development limited by the toxic metal in soil.
Table 4. Effect of Cu stress on the tolerance indices (TIs) of four plant species
Copper distribution in different plant tissues
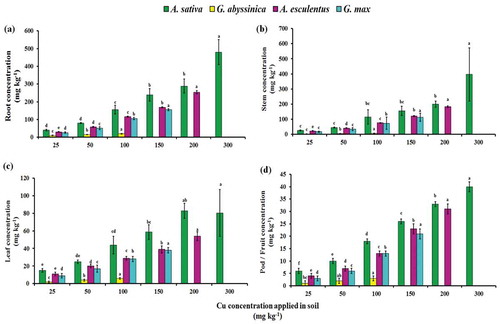
The Cu concentration in different parts of four plant species is presented in . The concentration of Cu in all plant parts i.e., root, stem leaves, and pod/fruit were increased with increased Cu levels in soil. In A. esculentus, A. sativa, G. abyssinica, and G. max, the maximum Cu concentration were in the order of root followed by stem, leaf, and pod/fruit at 300 mg Cu kg−1 treated soil. Studies demonstrated that phytoremediation efficiency depended on the take-up of metals, as well as their distribution and translocation to different plant tissues. The extent of metal tolerance in plants are reliant on metal bioavailability, plant species, and metabolic systems (Rohan, Mayank, João, & Paul, Citation2013). The metal concentrations was also varied among different plant species and influenced by the concentration and availability of heavy metal in soil as well as different condition of soil (Seregin & Ivanov, Citation2001).
Figure 2. Cu concentrations in root (a), stem (b), leaf (c), and pod/fruit (d) of four plant species after 12 weeks of growth in soil contaminated with varying concentrations of applied Cu. Bars with the similar letters are statistically non-significant according to Duncan’s multiple range test (p < 0.05), Data are means (n = 3 ± SD), a in superscript represent significantly highest followed by later alphabets for lower means
Copper accumulation in roots and shoots
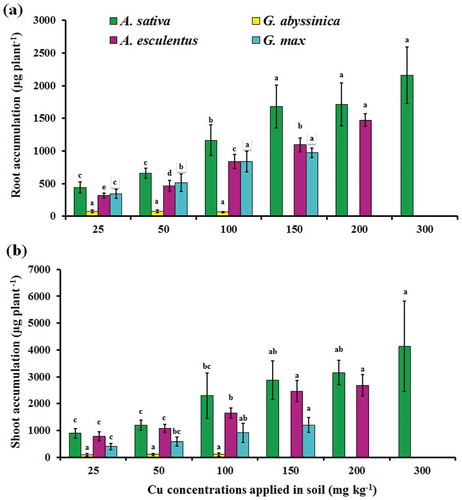
For all plant species, the highest Cu accumulation was found in shoots than roots per plant because of high shoot biomass (). The results showed that A. sativa accumulated the greatest amount of Cu in both root and shoot from 25 to 300 mg Cu kg−1 treatment whereas, minimum was accumulated in G. abyssinica. Overall, the results suggested that A. sativa was the effective candidate to extract Cu from contaminated soil than other tested plant species especially at higher Cu concentration i.e., 300 mg Cu kg−1. Beside concentrations, the accumulation of heavy metals in the above ground biomass (shoot) has an important factor that determined the plant capability for appropriate phytoremediation (Hanen et al., Citation2010) and affected plant remediation efficiency. Previous study has reported that the plant species suitable for successive phytoremediation have high metal tolerance and accumulation capacity in harvestable parts (Salt, Smith, & Raskin, Citation1998). The accumulation potential of plants depends on two main factors, i.e., metal concentration in soil and plants biomass for accurate metal quantity measurements (Vymazal, Citation2016). Past study reported that increased treatment level leads to enhanced metal accumulation in plants (Sun, Zhou, Wang, & Liu, Citation2009). Previous study showed that the uptake of metals, partition, and translocation to different plant parts as well as the degree of tolerance depends on the metal concentration and availability, the plant species, and metabolism. The plant biomass was also considered for the evaluation of metal accumulation potential (Yue-bing, Qixing, Jing, Wei-tao, & Rui, Citation2009).
Figure 3. Accumulation of Cu in root (a) and shoot (b) of four plant species after 12 weeks of growth in soil contaminated with varying concentrations of applied Cu. Bars with the similar letters are statistically non-significant according to Duncan’s multiple range test (p < 0.05), Data are means (n = 3 ± SD), a in superscript represent significantly highest followed by later alphabets for lower means
Phytoextraction efficiency
For all Cu treatments, A. sativa, A. esculentus, and G. max had BCF, BAC, and TF values > 1 among the tested plant species, which suggested that the studied plant species have high capacity to transport Cu from roots to shoots and suitable for phytoextraction (). On the other hand, G. abyssinica has BCF, BAC, and TF values <1 and signified that G. abyssinica has not categorized exclusively as Cu phytoextractor or phytostabilizer. BCF has an excellent indicator of metal accumulation capacity because it was taken into account that the plants ability to extract heavy metals from substrate and compared the plant species for phytoremediation potentials (McGrath & Zhao, Citation2003; Odjegba & Fasidi, Citation2007). The BAC has another useful parameter for evaluating the ratio of heavy metal in shoot to that in soil (Yoon et al., Citation2006) whereas, TF helped to evaluate the ratio of heavy metals in plant shoot to that in plant root (Cui, Zhou, & Chao, Citation2007; Li, Luo, & Su, Citation2007). For phytoextraction purpose, the plant species with all factors, the BCF, BAC, and TF values >1 was supposed to be a good phytoextractor and suitable for phytoextraction of metal-contaminated soil, while plant species with BCF and TF values <1 was probably not suitable for phytoextraction purpose (Fitz & Wenzel, Citation2002). Following the criteria, the plant species with BCF values >1 and TF values <1 was suitable for phytostabilziation (Mendez & Maier, Citation2008).
Table 5. Bioconcentration factor (BCF), bioaccumulation coefficient (BAC) and translocation factor (TF) for Cu in four plant species
Phytoremediation ratio (%)
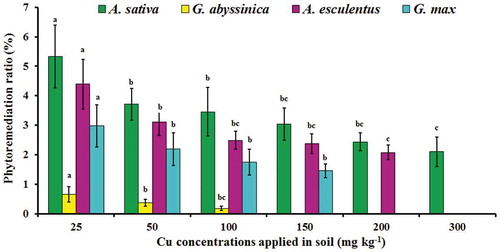
The PRs of four plant species, grown under different Cu-treated soil are reported in . The highest PR value for Cu was found in A. sativa, (5.33%) followed by A. esculentus (4.39%), G. max (2.97%) and G. abyssinica (0.66%) at 25 mg Cu kg−1. However, the PR (%) value was decreased by 2.10% for A. sativa at 300 mg Cu kg−1. In A. esculentus the PR (%) value was decreased by 2.08% at 200 mg Cu kg−1, while in G. max, decreased by 1.45% at 150 mg Cu kg−1. On the other hand, in G. abyssinica the PR (%) value was decreased by 0.17% at 100 mg Cu kg−1. The plants’ efficiency for metal removal and reclamation of soil polluted with metals depended on plants’ biomass production and the metal distribution among plant tissues. Previous study suggested that the plants used for phytoremediation must have fast growth rate, developed large biomass as well as easy to cultivate and harvest (Ciura, Poniedziałek, Sękara, & Jędrszczyk, Citation2005).
Figure 4. Phytoremediation ratio (%) of four plant species after 12 weeks of growth in soil contaminated with varying concentrations of applied Cu. Bars with the similar letters are statistically non-significant according to Duncan’s multiple range test (p < 0.05), Data are means (n = 3 ± SD), a in superscript represent significantly highest followed by later alphabets for lower means
Conclusion
The present study has provided an efficient, cost effective, environmental friendly, and solar-driven method using natural potential of plants in metals removal from contaminated soil. The results from the remediation of Cu-polluted soil have concluded that the germination, growth, and chlorophyll contents were significantly (p < 0.05) higher in A. sativa whereas, the minimum growth performance was observed in G. abyssinica at higher Cu concentration. Among four plant species, significant highest Cu accumulation was found in the roots and shoots of A. sativa. Overall, the results together with the fact suggested that A. sativa was a species that grow well in metal stress condition with short cycle, high tolerance, and accumulation potential. Moreover, the high values of BCF, BAC, TF, and PR recommended that A. sativa was a suitable plant for effective Cu phytoextraction and remediated the Cu-contaminated soil in quick and successive flushes than other tested plant species. The harvested plant biomass was biodegradable that could be easily disposed-off and utilized either as alternative source of biofuel energy or as raw material for large-scale composting or phytomining process. Furthermore, people of affected areas also took advantage from the natural safe method by toxic metals removal from contaminated agricultural soil up to acceptable safe limits that irrigated with untreated water.
Acknowledgments
The authors are grateful to Institute of Plant Sciences, University of Sindh, Jamshoro, Pakistan for providing facilities to carry out this research work.
Disclosure statement
The authors reported no potential conflict of interest.
References
- Adhikari, T., Kundu, S., Biswas, A. K., Tarafdar, J. C., & Rao, A. S. (2012). Effect of copper oxide nano particle on seed germination of selected crops. Journal of Agricultural Science and Technology, A, 2(6A), 815.
- Ahsan, N., Lee, D. G., Lee, S. H., Kang, K. Y., Lee, J. J., Kim, P. J., & Lee, B. H. (2007). Excess copper induced physiological and proteomic changes in germinating rice seeds. Chemosphere, 67, 1182–1193.
- Ali, S., Shahbaz, M., Shahzad, A. N., Fatima, A., Khan, H. A., Anees, M., & Haider, M. S. (2015). Impact of copper toxicity on stone-head cabbage (Brassica oleracea var. capitata) in hydroponics. PeerJ, 3, e1119.
- Ansari, M. K. A., Oztetik, E., Ahmad, A., Umar, S., Iqbal, M., & Owens, G. (2013). Identification of the phytoremediation potential of Indian mustard genotypes for copper, evaluated from a hydroponic experiment. Clean: Soil Air Water, 41, 789–796.
- Arnon, D. I. (1949). Copper enzymes in isolated chloroplasts: Polyphenol oxidase in beta vulgaris. Plant Physiology, 24, 1–15.
- Audet, P., & Charest, C. (2007). Heavy metal phytoremediation from a meta-analytical perspective. Environmental Pollution, 147, 231–237.
- Awokunmi, E. E. (2016). The potential of abelmoschus esculentus in EDTA-assisted phytoextraction of heavy metals from soil of Bashiri Dumpsite, Ado Ekiti, Nigeria. International Journal of Environmental Protection, 6, 9–14.
- Azooz, M. M., Abou-Elhamd, M. F., & Al-Fredan, M. A. (2012). Biphasic effect of copper on growth, proline, lipid peroxidation and antioxidant enzyme activities of wheat (Triticum aestivum’cv. Hasaawi) at early growing stage. Australian Journal of Crop Science, 6, 688–694.
- Barbosa, R. H., Tabaldi, L. A., Miyazaki, F. R., Pilecco, M., Kassab, S. O., & Bigaton, D. (2013). Foliar copper uptake by maize plants: Effects on growth and yield. Ciencia Rural, 43, 1561–1568.
- Carolin, C. F., Kumar, P. S., Saravanan, A., Joshiba, G. J., & Naushad, M. (2017). Efficient techniques for the removal of toxic heavy metals from aquatic environment: A review. Journal of Environmental Chemical Engineering, 5(3), 2782–2799.
- Ciura, J., Poniedziałek, M., Sękara, A., & Jędrszczyk, E. (2005). The possibility of using crops as metal phytoremediants. Polish Journal of Environmental Studies, 14, 17–22.
- Cui, S., Zhou, Q., & Chao, L. (2007). Potential hyperaccumulation of Pb, Zn, Cu and Cd in endurant plants distributed in an old smeltery, northeast China. Environmental Geology, 51, 1043–1048.
- Dresler, S., Hanaka, A., Bednarek, W., & Maksymiec, W. (2014). Accumulation of low-molecular-weight organic acids in roots and leaf segments of Zea mays plants treated with cadmium and copper. Acta Physiologiae Plantarum, 36, 1565–1575.
- Fanrong, Z., Shafaqat, A., Haitao, Z., Younan, O., Boyin, Q., Feibo, W., & Guoping, Z. (2011). The influence of pH and organic matter content in paddy soil on heavy metal availability and their uptake by rice plants. Environmental Pollution, 159, 84–91.
- Fitz, W. J., & Wenzel, W. W. (2002). Arsenic transformation in the soil rhizosphere plant system, fundamentals and potential application of phytoremediation. Journal of Biotechnology, 99, 259–278.
- Gopal, R., & Rizvi, A. H. (2008). Excess lead alters growth, metabolism and translocation of certain nutrients in radish. Chemosphere, 70(9), 1539–1544.
- Hanen, Z., Tahar, G., Abelbasset, L., Rawdha, B., Rim, G., Majda, M., … Chedly, A. (2010). Comparative study of Pb-phytoextraction potential in Sesuvium portulacastrum and Brassica juncea: Tolerance and accumulation. Journal of Hazardous Materials, 183, 609–615.
- Hegedus, A., Erdei, S., & Horvath, G. (2001). Comparative studies of H2O2 detoxifying enzymes in green and greening barley seedings under cadmium stress. Plant Science, 160, 1085–1093.
- Herawati, N., Suzuki, S., Hayashi, K., Rivai, I. F., & Koyoma, H. (2000). Cadmium, copper and zinc levels in rice and soil of Japan, Indonesia and China by soil type. Bulletin of Environmental Contamination and Toxicology, 64, 33–39.
- Kabata-Pendias, A., & Pendias, H. (2001). Trace elements in soils and plants (3rd ed.). Boca Raton: CRC Press.
- Keller, C., Ludwig, C., Davoli, F., & Wochele, J. (2005). Thermal treatment of metal-enriched biomass produced from heavy metal phytoextraction. Environmental Science & Technology, 39(9), 3359–3367.
- Khalid, S., Shahid, M., Niazi, N. K., Murtaza, B., Bibi, I., & Dumat, C. (2017). A comparison of technologies for remediation of heavy metal contaminated soils. Journal of Geochemical Exploration, 182, 247–268.
- Li, F. (2018). Heavy metal in urban soil: Health risk assessment and management. Heavy Metals, 337.
- Li, L., Li, X., & Wang, B. (2019). Public health challenges in China. In Introduction to public health in China (pp. 63–68). Singapore: Springer.
- Li, M. S., Luo, Y. P., & Su, Z. Y. (2007). Heavy metal concentrations in soils and plant accumulation in a restored manganese mine land in Guangxi, South China. Environmental Pollution, 147, 168–175.
- Li, X., Yang, Y., Zhang, J., Jia, L., Li, Q., Zhang, T., … Ma, S. (2012). Zinc induced phytotoxicity mechanism involved in root growth of Triticum aestivum L. Ecotoxicology and Environmental Safety, 86, 198–203.
- Luo, Z. B., He, X. J., Chen, L., Tang, L., Gao, S., & Chen, F. (2010). Effects of zinc on growth and antioxidant responses in Jatropha curcas seedlings. International Journal of Agriculture & Biology, 12, 119–124.
- Mackie, K. A., Müller, T., & Kandeler, E. (2012). Remediation of copper in vineyards - a mini review. Environmental Pollution, 167, 16–26.
- Mahmood, T., & Islam, K. R. (2006). Response of rice seedlings to copper toxicity and acidity. Journal of Plant Nutrition, 29, 943–957.
- Malik, N., & Biswas, A. K. (2012). Role of higher plants in remediation of metal contaminated sites. SciRev Chemical Communications, 2(2), 141–146.
- Mani, D., & Kumar, C. (2014). Biotechnological advances in bioremediation of heavy metals contaminated ecosystems: An overview with special reference to phytoremediation. International Journal of Environmental Science and Technology, 11(3), 843–872.
- McGrath, S. P., & Zhao, F. J. (2003). Phytoextraction of metals and metalloids from contaminated soils. Current Opinion in Biotechnology, 14, 561–572.
- Mehmood, F., Rashid, A., Mahmood, T., & Dawson, L. (2013). Effect of DTPA on Cd solubility in soil accumulation and subsequent toxicity to lettuce. Chemosphere, 90, 1805–1810.
- Memon, A. R., & Schroder, P. (2009). Implications of metal accumulation mechanisms to phytoremediation. Environmental Science and Pollution Research, 16, 162–175.
- Mendez, M. O., & Maier, R. M. (2008). Phytostabilization of mine tailings in arid and semiarid environments - an emerging remediation technology. Environment Health Perspective, 116, 278–283.
- Mildvan, A. S. (1970). Metal in enzymes catalysis. In D. D. Boyer (Ed.), The enzymes (Vol. 11, pp. 445–536). London: Academic Press.
- Monni, S., Salemma, M., & Millar, N. (2000). The tolerance of Empetrum nigrum to copper and nickel. Environmental Pollution, 109, 221–229.
- Muhammad, A., Shafaqat, A., Muhammad, R., Muhammad, I., Farhat, A., Mujahid, F., … Saima, A. B. (2015). The effect of excess copper on growth and physiology of important food crops: A review. Environmental Science and Pollution Research, 22(11), 8148–8162.
- Mukhopadhyay, M., Das, A., Subba, P., Bantawa, P., Sarkar, B., Ghosh, P. D., & Mondal, T. K. (2013). Structural, physiological and biochemical profiling of tea plants (Camellia sinensis (L.) O. Kuntze) under zinc stress. Biologia Plantarum, 57, 474–480.
- Odjegba, V., & Fasidi, I. (2007). Phytoremediation of heavy metals by Eichhornia crassipes. The Environmentalist, 27(3), 349–355.
- Oh, K., Li, T., Cheng, H. Y., Xie, Y., Yonemochi, S., Yan, L., & Shinichi, Y. (2013). Development of profitable phytoremediation of contaminated soils with biofuel crops. Journal of Environmental Protection, 4, 58–64.
- Padmavathiamma, P. K., & Li, L. Y. (2007). Phytoremediation technology: Hyper accumulation metals in plants. Water, Air, and Soil Pollution, 184(1–4), 105–126.
- Pourakbar, L., Khayami, M., Khara, J., & Farbidina, T. (2007). Physiological effects of copper on some biochemical parameters in Zea mays L. seedlings. Pakistan Journal of Biological Sciences, 10, 4092–4096.
- Rachit, K., Verma, K. S., Meena, T., Yashveer, V., & Shreya, H. (2016). Phytoextraction and bioconcentration of heavy metals by Spinacia oleracea grown in paper mill effluent irrigated soil. Nature Environment and Pollution Technology, 15, 817–824.
- Rafati, M., Khorasani, N., Moattar, F., Shirvany, A., Moraghebi, F., & Hosseinzadeh, S. (2011). Phytoremediation potential of Populus alba and Morus alba for cadmium, chromuim and nickel absorption from polluted soil. International Journal of Environmental Research, 5, 961–970.
- Rashid, H., Manzoor, M. M., & Mukhtar, S. (2018). Urbanization and its effects on water resources: An exploratory analysis. Asian Journal of Water, Environment and Pollution, 15(1), 67–74.
- Raskin, I., & Ensley, B. D. (2000). Phytoremediation of toxic metals: Using plants to clean up the environment. New York: Wiley.
- Rawat, I., Kumar, R. R., Mutanda, T., & Bux, F. (2011). Dual role of microalgae: Phycoremediation of domestic wastewater and biomass production for sustainable biofuels production. Applied Energy, 88(10), 3411–3424.
- Rebecca, R.C. (2011). Copper: Inorganic and coordination chemistry. Encyclopedia of Inorganic and Bioinorganic Chemistry, John Wiley and Sons, Ltd. USA.
- Rohan, D., Mayank, V., João, P., & Paul, M. S. (2013). Spatial distribution of heavy metals in soil and flora associated with the glass industry in North Central India: Implications for phytoremediation. Soil and Sediment Contamination: an International Journal, 22, 1–20.
- Salt, D. E., Smith, R. D., & Raskin, I. (1998). Phytoremediation. Annual Review of Plant Physiology, 49, 643–668.
- Seregin, T. V., & Ivanov, V. B. (2001). Physiological aspects of toxin action of cadmium and lead on high plants. Plant Physiology, 48, 606–630.
- Shaikh, I. R., Shaikh, P. R., Shaikh, R. A., & Shaikh, A. A. (2013). Phytotoxic effects of heavy metals (Cr, Cd, Mn and Zn) on wheat (Triticum aestivum L.) seed germination and seedlings growth in black cotton soil of Nanded, India. Research Journal of Chemical Sciences, 3(6), 14–23.
- Sharma, S., Singh, B., & Manchanda, V. K. (2014). Phytoremediation: Role of terrestrial plants and aquatic macrophytes in the remediation of radionuclides and heavy metal contaminated soil and water. Environmental Science and Pollution Research, 22, 946–962.
- Singh, D., Nath, K., & Sharma, Y. K. (2007). Response of wheat seed germination and seedling growth under copper stress. Journal of Environmental Biology, 28, 409–414.
- Srinivasan, M., Sahi, S. V., Paulo, J. C. F., & Venkatachalam, P. (2014). Lead heavy metal toxicity induced changes on growth and antioxidative enzymes level in water hyacinths [Eichhornia crassipes (Mart.)]. Botanical Studies, 55(1), 54.
- Stadtman, E. R., & Oliver, C. N. (1991). Metal catalyzed oxidation of proteins. Physiological consequences. Journal of Biological Chemistry, 266(4), 2005–2008.
- Sun, Y. B., Zhou, Q. X., Wang, L., & Liu, W. T. (2009). The influence of different growth stages and dosage of EDTA on Cd uptake and accumulation in Cd hyperaccumulator (Solanium nigrum L.). Bulletin of Environmental Contamination and Toxicology, 82, 348–353.
- Talebi, S., Nabavi, K. S. M., & Sohani, D. A. L. (2014). The study effects of heavy metals on germination characteristics and proline content of Triticale (Triticoseale wittmack). International Journal of Farming & Allied Sciences, 3, 1080–1087.
- Tong, Y. P., Kneer, R., & Zhu, Y. G. (2004). Vacuolar compartmentalization: A second generation approach to engineering plants for phytoremediation. Trends in Plant Science, 9, 7–9.
- Usman, A. R., Lee, S. S., Awad, Y. M., Lim, K. J., Yang, J. E., & Ok, Y. S. (2012). Soil pollution assessment and identification of hyperaccumulating plants in chromated copper arsenate (CCA) contaminated sites, Korea. Chemosphere, 87(8), 872–878.
- Vymazal, J. (2016). Concentration is not enough to evaluate accumulation of heavy metals and nutrients in plants. Science of the Total Environment, 544, 495–498.
- Wilkins, D. A. (1978). The measurement of tolerance to edaphic factors by means of root growth. New Phytologist, 80, 623–633.
- Wintz, H., Fox, T., & Vulpe, C. (2002). Responses of plants to iron, zinc and copper deficiencies. Biochemical Society Transactions, 30, 766–768.
- Wodala, B., Eitel, G., Gyula, T. N., Ördög, A., & Horváth, F. (2012). Monitoring moderate Cu and Cd toxicity by chlorophyll fluorescence and P700 absorbance in pea leaves. Photosynthetica, 50, 380–386.
- Wu, Q., Zhang, X., Liu, C., & Chen, Z. (2018). The de-industrialization, re-suburbanization and health risks of brownfield land reuse: Case study of a toxic soil event in Changzhou, China. Land Use Policy, 74, 187–194.
- Wuana, R. A., & Okieimen, F. E. (2011). Heavy metals in contaminated soils: A review of sources, chemistry, risks and best available strategies for remediation. Isrn Ecology, 2011, 1–20.
- Yoon, J., Cao, X., Zhou, Q., & Ma, L. Q. (2006). Accumulation of Pb, Cu and Zn in native plants growing on a contaminated Florida site. Science of the Total Environment, 368, 456–464.
- Yue-bing, S., Qixing, Z., Jing, A., Wei-tao, L., & Rui, L. (2009). Chelator enhanced phytoextraction of heavy metals from contaminated soil irrigated by industrial waste water with the hyperaccumulator plant (Sedum alfredii Hence). Geoderma, 150, 105–112.
