Abstract
Research was conducted on breeding and metamorphosis of a species of the African clawed frog (Xenopus laevis). The experimental group consisted of 10 individuals each of albino and natural forms. Specimens were divided into pairs and numbered for better tracking. In experimental ponds, conditions simulating the mating season were created, which led to 100% reproduction of all the pairs. The aim of the work was to determine the effects of temperature on the gender of resulting metamorphic specimens, and how the length of metamorphosis affects their gender. Specimens were divided into two groups. The first group consisted of specimens bred at 19°C, and the second group of specimens were bred at 24°C. Mating covered the period from 5 May to 25 June 2016. Observations were completed on 31 October 2016. Five hundred and fourteen individual metamorphic specimens were bred during the experiment. The research shows that the length of metamorphosis has an effect on gender, in favour of females. The effect of temperature on gender was not confirmed. The experimental results can be used as a basis for similar research, since this topic has not been discussed previously.
Introduction
Propagation and metamorphosis research was carried out on an experimental group of the aquatic African clawed frog Xenopus laevis (Daudin, 1802) under artificial laboratory conditions. Geographically, African clawed frogs come from the African continent. Their biotope is stagnant or slowly flowing backwater. They have a stout body with a rather small head and smooth skin. The females can grow up to 12 cm, while the males are smaller in size (about 8 cm). The basic colouration is green to greyish with a lighter abdomen; an albinotic form with red eyes often occurs. Individuals with a yellow-orange tinge and those with black and white spots also occur very frequently (Evans et al. Citation2004).
Xenopus is an invaluable tool for the study of embryology and vertebrate development, basic cell and molecular biology, genomics, neurobiology and toxicology, as well as for modelling of human diseases (Hellsten et al. Citation2010). This is a model type of frog to use for research purposes, especially in the area of regenerative medicine (Krylov et al. Citation2007, Citation2010; Smith & Stoskopf Citation2007). The advantage of the genus Xenopus, unlike other amphibians, is its higher tolerance to environmental fluctuations, ability to feed on dead material, and relatively simple breeding in laboratory conditions. The females lay a large number of eggs, usually as many as 1000 in one lay; however, as many as 2000 have been recorded (Amaya et al. Citation1998).
The African clawed frog (Xenopus laevis) had been used in the past in hospitals as a pregnancy test because it responds very quickly to sexual hormones secreted during pregnancy (Wheeler & Brändli Citation2009; Olmstead et al. Citation2010). With the ability to withstand high mechanical resistance, embryos can also be used for microinjection and transplantation experiments (Hirsch et al. Citation2002). Thanks to the possibility of inducing ovulation at any time of the year, regardless of the life cycle of the frogs, this family has become an ideal model organism for the study of developmental processes (O’Rourke Citation2007). Further, they are used in research on Bisphenol A (BPA), which is used for the production of polycarbonates, epoxide and unsaturated polyester resins, the final products of which are used as covers for cans, tooth fillings and as an antioxidant in plastics (Fromme et al. Citation2002).
Materials and methods
Research on the reproduction and metamorphosis of experimental individuals was carried out under artificial laboratory conditions. Glass vessels with a volume of 20–35 L were used for rearing. Mating took place in the period from 5 May to 25 June 2016. The research was completed on 31 October 2016. There were 10 individuals of genus Xenopus laevis available. Two males at about 4 years of age came from the previous rearing, from the crossing of an albinotic female and a naturally coloured male. Both were green to greyish brown with a brighter stomach and lighter spots on the top of the body, with a body size of 8 cm. Before the experiment none of them were bred with the females. Another eight individuals (three females and five males) were purchased at the age of about 1 year, when they reached sexual maturity. These individuals had not been bred previously. Female colouring was natural; two females and three males were albinotic. For purposes of research, for better control of eggs laid and to avoid cannibalism on the eggs laid, the individuals were divided into pairs. Each pair had a separate aquarium sized 30 × 40 × 20 cm. For the purpose of better manipulation of the studied individuals, eggs and hatched larvae in the aquarium, no underlayer or filtration was used. Breeding couples were marked by numbers 1–5 ().
Table I. Evidence of pairs.
The tadpoles were reared in 10 groups divided according to individual breeding, which took on average 50 days, after the 62nd–63rd stage of development. After metamorphosis, they were divided into smaller groups by size to avoid cannibalism.
Mature individuals intended for reproduction were fed 2–3 times a week with pieces of fish and chicken meat, earthworms and insects (crickets, grasshoppers and cellar beetles). The tadpoles were administered infusoria from hay brine, spirula and yeast tablets, and later on frozen cyclopes and frozen brine shrimp (Artemia salina). The water was changed once a week and supplemented with chlorine-free stale water. Two control groups were created for mating. The water temperature in the first control group was 19°C, while it was 24°C in the second. Heating equipment for fish was used to heat the water. The water level throughout development was about 5 cm. The mating was stimulated by fresh water being exchanged daily in the aquariums. After mating and subsequent laying of the eggs, the African clawed frogs were displaced to a glass aquarium with water. Eggs were counted manually, and they were not manipulated until they hatched. In order to prevent mold formation in the aquarium, non-fertilised eggs were carefully removed.
The results obtained were recorded and evaluated daily. The following data were recorded: date of mating and hatching, feeding, number of tadpoles and their activity, water temperature, variations, sex ratio, growth rate and metamorphoses.
The results were processed by mathematical-statistical methods using the program SAS Deployment Manager 9.3.
The evaluation of genetic relationships between ZZ and ZW and sex steroids was not the subject of our research.
Results
In the course of the experiment, approximately 2145 eggs were laid, from which approximately 1728 larvae were hatched, of which 514 resulted in adult individuals. From the 10 matings, 10 groups of tadpoles were weaned, which, due to the identified cannibalism among different species in terms of size during adolescence, were divided into smaller groups.
After 50 hours of egg fertilisation, in the 35th to 36th stages of development, the eggs began to hatch. The larvae left their translucent cover and were fastened down on the glass of the aquarium. Already at this early stage it was possible to recognise their colour form. The albinotic form is white. The dark form is characterised by weak dark strips along the sides.
After 48 hours post hatching, the tadpole bodies were extended and their colour form was highlighted. Signs of the eyes could already be seen on the sides of the head, and the mouth opening was also visible. The yellow follicle was gradually lost; the tadpoles became more mobile and most of them were just below the top of the water in such a way that the mouth opening was touching the surface.
After 72 hours post hatching, the tadpoles began to move actively. Food was administered once a day, most frequently around lunch time.
At the beginning of the second week, the hind extremities appeared, and a week later front extremities appeared. We found that the first tadpoles metamorphosed after 5 weeks of intense growth, while for some individuals it took a longer time, despite all the tadpoles having had the same conditions for their development. Sexual maturity occurred in an average 30.11% of individuals. This is a natural phenomenon in nature, since not all individuals hatched are viable. Records of individual matings, data in individual categories and coloured forms arisen by crossing are given in .
Table II. Mating data, number of individuals in each category and their colour form.
The frogs were divided into six groups according to the body size to prevent cannibalism (19°C – 260 individuals and 24°C – 254 individuals; ).
Table III. Number of frogs in each group depending on the water temperature.
From the total number of individuals, 259 females were brought up (i.e. 50.39%) and 255 males (representing 49.61% of the total). The individual mean values found during the experiment are indicated in .
Table IV. Average values of monitored indicators.
During the experiment we found that the female sex metamorphosed faster than the male. Regardless of sex it may be stated that the length of metamorphosis ranges between 54 and 70 days; in females it is between 54 and 61 days while it is 58 to 70 days in males. In the evaluation of the influence of time and temperature on the rate of metamorphosis, extreme variations in six individuals were found. These included three males whose metamorphosis lasted over 70 days, and three females whose metamorphosis lasted less than 54 days. We found that an influence of temperature on sex during metamorphosis was not demonstrated ().
Figure 1. Correlation influence of time and temperature on the rate of metamorphosis and gender-converted individuals.
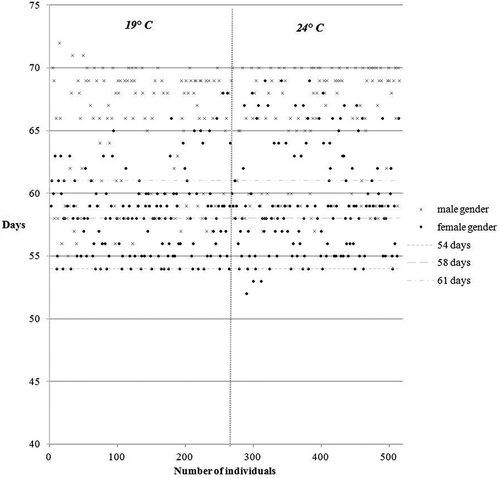
The results show that the influence of temperature and gender on the length of metamorphosis was statistically high ().
Table V. The influence of temperature and gender on the length of metamorphosis.
Discussion
Xenopus tropicalis (Gray, 1864) is an amenable model organism for genetic and developmental studies. Its diploid genome, containing 10 pairs of easily distinguishable chromosomes, has been sequenced and arranged in scaffolds (Klein et al. Citation2002). Xenopus tropicalis and its tetraploid relative Xenopus laevis (Daudin, 1802) constitute pivotal creatures from a gene duplication point of view (Pollet & Mazabraud Citation2006). Amphibians in general are a connecting link between lower vertebrates, such as fishes, and terrestrial tetrapods including humans. Despite their interesting taxonomic position, there is an almost complete lack of tools for the study of their chromosomal evolution (Smith & Voss Citation2006). The species X. laevis and X. (Silurana) tropicalis have a nonhomologous trigger for sex determination (Yoshimoto et al. Citation2008; Olmstead et al. Citation2010; Roco et al. Citation2015). These two species are members of different subgenera distinguished from each other by the number of chromosomes (x) carried by the gametes of their respective diploid ancestors, i.e. x = 10 for the subgenus Silurana and x = 9 for subgenus Xenopus (Evans et al. Citation2015). All extant species in the subgenus Xenopus are polyploid, but with disomic chromosomal inheritance, and tetraploids in this subgenus have 4x = 36 chromosomes. In X. laevis, a gene called DM-W is the master regulator of sex determination (Yoshimoto et al. Citation2008); this gene appeared in an ancestor of X. laevis after divergence from the ancestor of X. tropicalis, and is present in many close relatives of X. laevis (Bewick et al. Citation2011). In the subgenus Silurana, X. tropicalis has a complex trigger for sex determination that resides on Y, W and Z chromosomes. This system in X. tropicalis produces distorted sex ratios in some crosses. Thus, African clawed frogs use at least two systems for sex determination, and at least one of them evolved during the diversification of this group (Roco et al. Citation2015).
Xenopus is popular as a model for the study of vertebrate development, cell biology and immunology (Session et al. Citation2016). According to Cannatella (Citation2015) the African clawed frog Xenopus laevis has long been a model organism for a variety of studies at the mechanistic cellular and molecular levels. In recent years, its use has expanded as a model for integration across functional, phylogenetic and comparative levels of biology across multiple species: musculoskeletal anatomy, larval biology, gene duplication, behaviour, communication, functional morphology, etc.
Similarly, Vašeková (Citation2017) shows a significant influence of temperature on the length of metamorphosis and the sex of Xenopus laevis. Other authors observed other factors in their study, such as reproductive biomarkers, free radicals by electron spin resonance (Guerriero et al. Citation2017a), antioxidants using enzymatic assay (Guerriero et al. Citation2003), chromatography (Guerriero et al. Citation2004), and expression of steroid receptors (Guerriero et al. Citation2005, Citation2009, Citation2017b), in parallel to the approach already used.
Since the frogs are easy to raise and had other desirable properties such as large eggs, external development, easily manipulated embryos and transparent tadpoles, X. laevis gradually developed into one of the most productive model systems for vertebrate experimental embryology (Brown Citation2004).
However, X. laevis has a large paleotetraploid genome with an estimated size of 3.1 billion base pairs (Gbp) on 18 chromosomes and a generation time of 1–2 years. In contrast, the much smaller diploid western clawed frog, X. tropicalis, has a small genome, about 1.7 Gbp on 10 chromosomes, matures in only 4 months and requires less space than its larger cousin (Tymowska Citation1973). It is thus readily adopted as an alternative experimental subject for developmental and cell biology ().
Figure 2. Comparison of adults and tadpoles of Xenopus tropicalis and X. laevis. Adult body length is 5 and 10 cm respectively. A, tailbud; B, swimming tadpole; C, feeding tadpole. White scale bar = 1 mm (Hellsten et al. Citation2010).
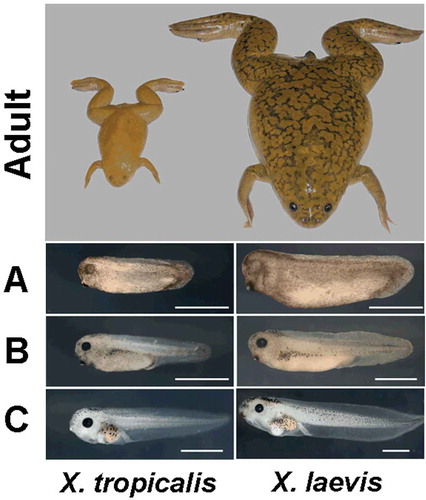
Nagano and Ode (Citation2014) reported that the larvae of aquatic African clawed frogs were hatched within 2–3 days, and after losing the yellow follicle, they began to feed on algae suspended in the water column. Further, they found that the speed at which larvae hatch depends on ambient temperature. Tinsley and McCoid (Citation1996) reported that the length of metamorphosis depends not only on temperature but also on the amount of food intake. Under optimal conditions, metamorphosis may be achieved within 2 months. The length of the conversion to a mature individual, depending on temperature and food, is approximately 6 to 8 weeks (Nagano & Ode Citation2014). Godfrey and Sanders (Citation2004) found that Xenopus laevis embryo development is also significantly affected by water hardness and the concentrations of chlorine and salts.
Since the African clawed frog (Xenopus laevis), which comes from South Africa, has created numerous invasive populations on several continents, it has become a convenient model organism for the study of environmental issues (Rödder et al. Citation2017). The African amphibian Xenopus laevis is generally considered to be an invasive species which constitutes a threat not only to local fauna but also to biodiversity the world over, as it has a strong invasive potential (Tinsley et al. Citation2015). Global climate change is affecting ecosystems and ecological communities, leading to changes in the geographical expansion or number of their populations (Measey et al. Citation2012; De Busschere et al. Citation2016; Ihlow et al. Citation2016).
Genetic sex-determining systems in vertebrates include two basic types of heterogamety, which are represented by the XX/XY and ZZ/ZW types. Both types occur among amphibian species. Recently, a W-linked gene, DM-W, was isolated as a paralog of DMRT1 in the African clawed frog Xenopus laevis, which has a female heterogametic ZZ/ZW-type sex-determining system (Yoshimoto & Ito Citation2011). In developmental biology Xenopus laevis is a widely used model organism, yet its genetics is far from clear. Almost all members of the Xenopus genus are of polyploidic origin and form a polyploid series of 2n, 4n, 8n, 12n starting with diploid X. tropicalis and tetraploid X. laevis (Kobel & Du Pasquier Citation1986; Mácha et al. Citation2003). According to Mácha et al. (Citation2003) X. laevis forms bivalents, revealing its tetraploid, strongly diploidised character, which is consistent with the high proportion of gene duplications in its genome. Breeding sex-reversed males with normal males produces only male progeny. Crosses of sex-reversed males and sex-reversed females result in all-female progeny. These results indicate Abraxas-type sex determination in X. laevis; in other words, the Xenopus male is homogametic (ZZ) and the Xenopus female is heterogametic (ZW).
However, the molecular mechanism of the ZZ/ZW-type system in vertebrates, including the clawed frog Xenopus laevis, is unknown. The sexual fate of metazoans is determined genetically or by environmental factors, such as temperature. In the former case, heterogametic sex chromosomes determine the male (XY♂) or female (ZW♀) fate in many species of vertebrates (Yoshimoto et al. Citation2008). Researchers have isolated an X. laevis female genome-specific DM-domain gene, DM-W, and obtained molecular evidence of a W chromosome in this species. Based on these results, they found that DM-W is a likely sex (ovary)-determining gene in X. laevis.
Note that an allotetraploid such as Xenopus laevis has two related subgenomes, but these subgenomes are each transmitted to progeny via conventional disomic inheritance. So immediately after allotetraploidisation, the new species is already genetically diploid. This is clearly the case for X. laevis (Session et al. Citation2016).
Conclusions
In our investigation of temperature influence on reproduction and the rate of metamorphosis the African clawed frog (Xenopus laevis) we found that during the monitored period, the number of eggs laid in individual pairs was about 2145 individuals, from which 172.8 individuals were hatched on an average. The number of converted individuals was 514.0 pieces. One hundred and twenty individualls were albinotically coloured, while 394 were naturally coloured. During research, an average of 30.11% of individuals survived. From the results it follows that under constant environmental conditions, the length of metamorphosis has a significant effect on the sex of the individuals. An effect of temperature on sex during metamorphosis was not demonstrated. The results obtained can be used for laboratory research, zoological gardens and for private breeding too. Since research on similar issues at home or abroad has not yet been realised so far, our aim was to contribute new knowledge to the issues in question. The influence of temperature and gender on the length of metamorphosis was statistically high.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Amaya E, Offield M, Grainger RM. 1998. Frog genetics: Xenopus tropicalis jumps into the future. Trends in Genetics 14:253–255.
- Bewick AJ, Anderson DW, Evans BJ. 2011. Evolution of the closely related, sex-related genes DM-W and DMRT1 in African clawed frogs (Xenopus). Evolution 65:698–712.
- Brown DD. 2004. A tribute to the Xenopus laevis oocyte and egg. Journal of Biological Chemistry 279:45291–45299.
- Cannatella D. 2015. Xenopus in space and time: Fossils, node calibrations, tip-dating, and paleobiogeography. Cytogenetic and Genome Research 145:283–301.
- De Busschere C, Courant J, Herrel A, Rebelo R, Rödder D, Measey GJ, Backeljau T. 2016. Unequal contribution of native South African phylogeographic lineages to the invasion of the African clawed frog, Xenopus laevis, in Europe. PeerJ 4:e1659.
- Evans BJ, Carter TF, Greenbaum E, Gvoždík V, Kelley DB, McLaughlin PJ, Pauwels OSG, Portik DM, Stanley EL, Tinsley RC, Tobias ML, Blackburn DC. 2015. Genetics, morphology, advertisement calls, and historical records distinguish six new polyploid species of African clawed frog (Xenopus, Pipidae) from west and central Africa. PLoS One 10:e0142823.
- Evans BJ, Kelley DB, Tinsley RC, Melnick DJ, Cannatella DC. 2004. A mitochondrial DNA phylogeny of African clawed frogs: Phylogeography and implications for polyploid evolution. Molecular Phylogenetics and Evolution 33:197–213.
- Fromme H, Küchler T, Otto T, Pilz K, Müller J, Wenzel A. 2002. Occurrence of phthalates and bisphenol A and Fin the environment. Water Research 36:1429–1438.
- Godfrey EW, Sanders GE. 2004. Effect of water hardness on oocyte quality and embryo development in the African clawed frog (Xenopus laevis). Comparative Medicine 52:170–175.
- Guerriero G, Brundo MV, Labar S, Bianchi AR, Trocchia S, Rabbito D, Palumbo G, Abdel-Gawad FK, De Maio A. 2017b. Frog (Pelophylax bergeri, Günther 1986) endocrine disruption assessment: Characterization and role of skin poly (ADP-ribose) polymerases. Environmental Science and Pollution Research International. DOI:10.1007/s11356-017-0395-2.
- Guerriero G, D’Errico G, Di Giaimo R, Rabbito D, Olanrewaju OS, Ciarcia G. 2017a. Reactive oxygen species and glutathione antioxidants in the testis of the soil biosentinel Podarcis sicula (Rafinesque 1810). Environmental Science and Pollution Research International. DOI:10.1007/s11356-017-0098-8.
- Guerriero G, Di Finizio A, Ciarcia G. 2003. Oxidative defenses in the sea bass, Dicentrarchus labrax. In: Dunn JF, Swartz HM, editors. Oxygen Transport to Tissue XXIV. Advences in Experimental Medicine and Biology. New York:Kluwer Academic/Plenum Plublisher. Vol. 530, pp. 681–688.
- Guerriero G, Ferro F, Russo GL, Ciarcia G. 2004. Vitamin E in early stages of sea bass (Dicentrarchus labrax) development. Comparative Biochemistry and Physiology Patr A Molecular & Integrative Physiology 138:435–439.
- Guerriero G, Prins GS, Birch L, Ciarcia G. 2005. Neurodistribution of androgen receptor immunoreactivity in the male frog, Rana esculenta. Annals of the New York Academy of Sciences 1040:332–336.
- Guerriero G, Roselli CE, Ciarcia G. 2009. The amphibian (Rana esculenta) brain progesterone receptor: Relationship to plasma steroids and vitellogenic cycle during the gonadal recovery phase. Annals of the New York Academy of Sciences 1163:407–409.
- Hellsten U, Harland RM, Gilchrist MJ, Hendrix D, Jurka J, Kapitonov V, Ovcharenko I, Putnam NH, Shu S, Taher L, Blitz IL, Blumberg B, Dichmann DS, Dubchak I, Amaya E, Detter JC, Fletcher R, Gerhard DS, Goodstein D, Graves T, Grigoriev IV, Grimwood J, Kawashima T, Lindquist E, Lucas SM, Mead PE, Mitros T, Ogino H, Ohta Y, Poliakov AV, Pollet N, Robert J, Salamov A, Sater AK, Schmutz J, Terry A, Vize PD, Warren WC, Wells D, Wills A, Wilson RK, Zimmerman LB, Zorn AM, Grainger R, Grammer T, Khokha MK, Richardson PM, Rokhsar DS. 2010. The genome of the western clawed frog Xenopus tropicalis. Science 5978:633–636.
- Hirsch N, Zimmerman LB, Grainger RM. 2002. Xenopus, the next generation: X. tropicalis genetics and genomics. Developmental Dynamics 225:422–433.
- Ihlow F, Courant J, Secondi J, Herrel A, Rebelo R, Measey GJ, Lillo F, De Villiers FA, Vogt S, De Busschere C, Backeljau T, Rödder D. 2016. Impacts of climate change on the global invasion potential of the African Clawed Frog Xenopus laevis. PLoS One 11:1–19.
- Klein SL, Strausberg RL, Wagner L, Pontius J, Clifton SW, Richardson P. 2002. Genetic and genomic tools for Xenopus research: The NIH Xenopus initiative. Developmental Dynamics 225:384–391.
- Kobel HR, Du Pasquier L. 1986. Genetics of polyploid Xenopus. Trends in Genetics 2:310–315.
- Krylov V, Kubíčková S, Rubes J, Macha J, Tlapáková T, Seifertova E, Sebkova N. 2010. Preparation of Xenopus tropicalis whole chromosome painting probes using laser microdissection and reconstruction of X. laevis tetraploid karyotype by Zoo-FISH. Chromosome Research 18:431–439.
- Krylov V, Tlapáková T, Macha J. 2007. Localization of the single copy gene Mdh2 on Xenopus tropicalis chromosomes by FISH-TSA. Cytogenetic and Genome Research 116:110–112.
- Mácha J, Tlapáková T, Krylov V, Kopský V. 2003. Xstir polymorphism and absence of sex linkage in Xenopus laevis ME2 Gene. Folia Biologica 49:115–117.
- Measey GJ, Rödder D, Green SL, Kobayashi R, Lillo F, Lobos G, Rebelo R, Thirion JM. 2012. Ongoing invasions of the African clawed frog, Xenopus laevis: A global review. Biological Invasions 14:2255–2270.
- Nagano Y, Ode KL. 2014. Temperature-independent energy expenditure in early development of the African clawed frog Xenopus laevis. Physical Biology 11:046008.
- O’Rourke DP. 2007. Amphibians used in research and teaching. Institute for Laboratory Animal Research Journal 48:183–187.
- Olmstead AW, Lindberg-Livingston A, Degitz SJ. 2010. Genotyping sex in the amphibian, Xenopus (Silurana) tropicalis, for endocrine disruptor bioassays. Aquatic Toxicology 98:60–66.
- Pollet N, Mazabraud A. 2006. Insights from Xenopus genomes. Genome Dynamics 2:138–153.
- Roco AS, Olmstead AW, Degitz SJ, Amano T, Zimmerman LB, Bullejos M. 2015. Coexistence of Y, W, and Z sex chromosomes in Xenopus tropicalis. Proceedings of the National Academy of Sciences 112:4752–4761.
- Rödder D, Secondi J, Courant J, Ihlow F, Lillo F, Measey GJ, Rebelo R, Herrel A, Backeljau T, De Busschere C, De Villiers FA. 2017. Global realized niche divergence in the African clawed frog Xenopus laevis. Ecology and Evolution 7:4044–4058.
- Session AM, Uno Y, Kwon T, Chapman JA, Toyoda A, Takahashi S, Fukui A, Hikosaka A, Suzuki A, Kondo M, van Heeringen SJ, Quigley I, Heinz S, Oqino H, Ochi H, Hellsten U, Lyons JB, Simakov O, Putnam N, Stites J, Kuroki Y, Tanaka T, Michiue T. 2016. Genome evolution in the allotetraploid frog Xenopus laevis. Nature 538:336–343.
- Smith JJ, Voss SR. 2006. Gene order data from a model amphibian (Ambystoma): New perspectives on vertebrate genome structure and evolution. BMC Genomics 29:219.
- Smith S, Stoskopf MK. 2007. The art of amphibian science. Institute for Laboratory Animal Research Journal 38:179–182.
- Tinsley RC, McCoid MJ. 1996. Feral populations of Xenopus outside Africa. In: Tinsley RC, Kobel HR, editors. The biology of Xenopus. Oxford: Oxford University Press. pp. 81–94.
- Tinsley RC, Stott LC, Viney ME, Mable BK, Tinsley MC. 2015. Extinction of an introduced warm-climate alien species, Xenopus laevis, by extreme weather events. Biological Invasions 17:3183–3195.
- Tymowska J. 1973. Karyotype analysis of Xenopus tropicalis Gray, Pipidae. Cytogenetic and Genome Research 12:297–304.
- Vašeková P 2017. Reproduction and metamorphosis of Xenopus laevis. Thesis. Nitra: Slovak University of Agriculture Press. p. 63.
- Wheeler GN, Brändli AW. 2009. Simple vertebrate models for chemical genetics and drug discovery screens: Lessons from zebrafish and Xenopus. Developmental Dynamics 238:1287–1308.
- Yoshimoto S, Ito M. 2011. A ZZ/ZW-type sex determination in Xenopus laevis. The FEBS Journal 7:1020–1026.
- Yoshimoto S, Okada E, Umemoto H, Tamura K, Uno Y, Nishida-Umehara C, Matsuda Y, Takamatsu N, Shiba T, Ito M. 2008. A W-linked DM-domain gene, DM-W, participates in primary ovary development in Xenopus laevis. Proceedings of the National Academy of Sciences 105:2469–2474.
