Abstract
Rocky tide pools are transition environments whose communities are affected by sudden temperature, salinity and nutrient fluctuations. Furthermore, these environments are exposed to multiple stressors and can be easily altered by human trampling. In particular, specific studies on rock pool heterobranchs communities are lacking for the Mediterranean Sea. In this study, the community of Heterobranchia (Mollusca: Gastropoda) living in an anthropized rock pools system in western Adriatic (Ancona, Italy) has been investigated and a first checklist of the sea slugs in this urbanized areas is provided. During the four months survey, a total of 452 specimens, belonging to 19 species and 12 families was recorded. Notable findings were the first record of Placida dendritica for the Conero Riviera, and the first records of Doto cervicenigra and Ercolania viridis for the western Adriatic Sea. Identification of trophic categories showed a diversified assemblage in terms of food sources mirroring a surprising species diversity. Moreover, we provide here the description of a peculiar behavior possibly used by sea slugs to cope with the stressful conditions within this semi-closed system.
Introduction
Rocky tide pools are closed or semi-closed dynamic systems widespread along the coastline worldwide. Their presence contributes to increase the spatial heterogeneity of the intertidal zone creating habitats that can host a very high variety of living organisms (Ranta Citation1982; Metaxas & Scheibling Citation1993; Griffiths et al. Citation2006). The exposition to sunlight, atmospheric agents and tidal currents induce a wide fluctuation of environmental variables (temperature, pH, salinity, and dissolved oxygen) leading to extreme stressful conditions for the species living in this habitat (Ganning Citation1970, Citation1971; Chan Citation2000; Nielsen Citation2001; Hulsmans et al. Citation2008). Nevertheless, rocky pools host a significant biodiversity providing refuge, feeding and nursery grounds for many species of intertidal invertebrates (Bussell et al. Citation2007; Vinagre et al. Citation2015) and fish (Bennett Citation1987; Pfister Citation1996; White et al. Citation2015).
Depending on the coastline geomorphology, rocky tide pools are marine habitats that can be easily accessible both by tourists and local people and this could represent an effective opportunity for educational activities as confirmed by the multiplicity of citizen science projects developed in this habitat, leading to an increase in public awareness on environmental issues (Fairchild et al. Citation2018; Lucrezi et al. Citation2019). Most frequently these activities result in a direct impact on marine organisms due to trampling (Keough & Quinn Citation1998; Pinn & Rodgers Citation2005), harvesting of bait and seafood (Keough & Quinn Citation2000), and playful interactions with people who touch and collect animals, often causing the death of the latter (Addison et al. Citation2008; Martens Citation2016). In addition, the growing coastal defense (e.g. seawalls, breakwaters, rip-raps) and beach nourishment pursued to protect the coastline from storms and erosion, alter the wave regime and sedimentation rate with negative implications on coastal biodiversity (Martin et al. Citation2005; Bulleri & Chapman Citation2010; Perkins et al. Citation2015; Afghan et al. Citation2020; Masucci et al. Citation2020; Sedano et al. Citation2021).
The northern and western Adriatic coast is mainly characterized by sandy and pebble beaches with the exception of few rocky promontories that interrupt this continuity (i.e. S. Bartolo and Conero Mounts) (UNEP/MAP-RAC/SPA Citation2015). The Conero Mount Regional Natural Park is located in the Marche region and includes 15 km of rocky coastline that represents an important substrate for hard-bottom benthos and a crucial stepping stone for larval dispersion in the western Adriatic (Di Camillo et al. Citation2010). Here, the presence of natural hard substrates facilitate the growth of habitat-forming species of macroalgae (e.g. Cystoseira sensu latu) (Rindi et al. Citation2019, Citation2020) and Mediterranean mussel beds (Mytilus galloprovincialis Lamarck, 1819) (Cerrano et al. Citation2014) that host a peculiar biodiversity of mollusks, crustaceans and fish considered rare in other areas of the Mediterranean Sea (Betti Citation2010, Citation2021; Riolo & Betti Citation2015). Moreover, the jagged morphology of the Conero Mount limestone coastline creates heterogeneous intertidal habitats, such as the presence of scattered rocky tide pools (Fruzzetti et al. Citation2011; Cerrano et al. Citation2014).
Members of subclass Heterobranchia (Mollusca: Gastropoda), commonly known as sea slugs, are present in a wide range of habitats in the Mediterranean Sea (e.g. Poizat Citation1984; Scipione et al. Citation1996; Doménech et al. Citation2006; Poursanidis & Koutsoubas Citation2015; Betti et al. Citation2017; Chiarore et al. Citation2019; Canessa et al. Citation2021; Gerovasileiou & Bianchi Citation2021; Toma et al. Citation2022), and studies about their presence in tide pools habitat are known from the literature (Nybakken Citation1974, Citation1978; Todd et al. Citation1998; Lambert Citation2013; Nimbs et al. Citation2015; Cyrne et al. Citation2018; Armstrong et al. Citation2019). Nevertheless, even if some studies on these molluscs included specimens occasionally sampled from the Mediterranean intertidal zone (Lipej et al. Citation2008; Furfaro et al. Citation2020), there are no specific studies about sea slugs communities in rocky tide pools for the Mediterranean basin.
In addition, contrary to other Mediterranean localities where studies on heterobranch biodiversity have been provided (Cervera et al. Citation2004; Crocetta et al. Citation2013, Citation2015; Betti et al. Citation2017; Furfaro et al. Citation2020; Parera et al. Citation2020), including Adriatic Sea localities (Turk Citation2000; Lipej et al. Citation2008, Citation2012; Rinaldi Citation2012; Zenetos et al. Citation2016; Prkić et al. Citation2018), the heterobranch fauna of Ancona was not investigated extensively in the past. Indeed, the only sea slugs accounts from the Conero Riviera area consist of the two popular books by Betti (Citation2011, Citation2021), accounting for a total of 72 species. Nevertheless, there are no quantitative assessments about heterobranch community composition of this area.
Comparison of historical data on population dynamics in the Pacific Ocean showed that sea slugs were associated to thermal anomalies and thus can be considered sentinel species for climate changes, highlighting the importance of collecting data on these communities (Schultz et al. Citation2011; Goddard et al. Citation2016).
Aim of the present study has been to assess the species and trophic diversity of Heterobranchia in the rocky tide pools of Ancona area, to provide a first baseline for the conservation of these neglected and vulnerable habitats.
Materials and methods
Study area
The study area is located in the northern part of the Conero Mount Regional Natural Park, along the waterfront of Passetto on the eastern coast of Ancona, Italy (43.618947 N, 13.531880 E; ). This stretch of rocky coast represents a historical place of entertainment for the local people and it is highly frequented by tourists during the summer season. The area is renowned for the presence of more than one hundred artificial caves built between the 18th and the half of the 19th centuries, historically used by fishermen as boat shelters and now converted to houses and meeting places for the citizens (Turchetti & Tarsetti Citation2007). The growing popularity of the site has led over the century to an increasing anthropization. The shoreline geomorphology has been heavily altered by beach nourishments, numerous illegal concrete docks used by local fishermen as private piers and the construction in the 1956 of a public elevator that connects the city center to the shore (; Comune di Ancona Citation2017). Nowadays, the natural rocky coast is restricted to the marginal sides of the bay, while the original pebble shoreline is now substituted by a promenade sidewalk interrupted by concrete docks.
Figure 1. Map of the study area. (a) The exact location in Ancona and Italy of the study area. (b) Aerial view of the rocky tide pools system: areas investigated where pools form are highlighted in yellow (northern crevice) and in red (southern crevice). (c) View of the rocky pools formed on the northern crevice. Image b copyrights to Apple, California.
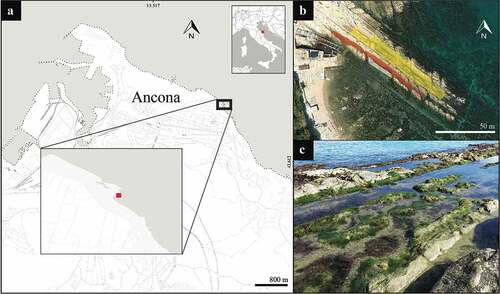
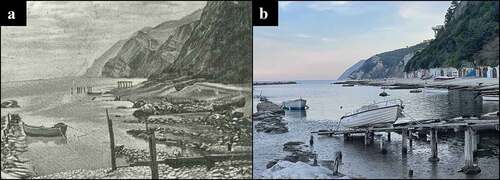
Figure 2. (a) View of the pristine coastline of the Passetto (1928), before the construction of the docks and fishermen caves. Picture modified from Turchetti and Tarsetti (Citation2007). (b) Nowadays view of the Passetto waterfront from the same angle, showing break walls, beach nourishments, caves and in the back the uppermost floor of the elevator.
The survey was conducted in a semi-closed rocky pool system, formed by the coastal limestones located at the westernmost end of the Passetto promenade. The area investigated is shown in (yellow and red squares). This system of pools has an overall surface extension (wet and rocky area) of about 750 m2 (). The rock formation follows the coastal slope and is excavated by two main crevices perpendicularly to the coastline. The southernmost crevice is narrower and deeper (maximum ~0.4 m deep at lowest tide; red area in ) while the northernmost is larger and shallower (maximum ~0.2 m deep at lowest tide; yellow area in ), and here majority of the pools are formed during low tide. Additional pools form also along the southernmost crevice proximally to the coastline, while its outermost part is well connected to the open sea. During the surveys, the minimum tide was −0.68 m (30/01/2018, 17:10, +1 GMT), while the maximum 0.64 m (11/03/2018, 17:10, +1 GMT), with a mean low water (MLW) of −0.14 ± 0.1 m (data retrieved from RMN – Rete Mareografica Nazionale, ISPRA, available at https://www.mareografico.it). This tide pool system, during the surveyed period, was characterized by a diversified macroalgal assemblage, rich in Dictyotales Bory, Corallinales P. C. Silva & H. W. Johansen, Ceramiales Nägeli and Ulvales Blackman & Tansley. Local intertidal fauna is mainly composed by cnidarians such as Exaiptasia diaphana (Rapp, 1829) and Obelia sp. Péron & Lesueur 1810, the bryozoan Bugula neritina (Linnaeus, 1758), and crustaceans, like Macropodia sp. Leach, 1814 [in Leach, 1813–1815], Pachygrapsus marmoratus (J. C. Fabricius, 1787), Perforatus perforatus (Bruguière, 1789), and Palaemon elegans Rathke, 1836. Among vertebrates, Blenniidae Rafinesque, 1820 as Aidablennius sphynx (Valenciennes, 1836), Lipophrys trigloides (Valenciennes, 1836), Parablennius incognitus (Bath, 1968), Salaria pavo (Risso, 1810), and Gobiidae Cuvier, 1816 like Gobius cobitis Pallas, 1814, are common sightings.
Field activities and data analysis
Monitoring of heterobranchs was carried out twice per month, with a minimum interval of 10 days, from January to April 2018 (8 surveys in total; see for replicates abbreviation used) with visual census and photographic methods. Considering that the rocky pool system was highly dynamic and characterized by sudden variations in the amount and volume of the pools, we decided to visually assess the whole area available during the daily minimum low tide. Each survey was carried out for a total of 60 minutes with the lowest tide after 30 minutes from the beginning in order to better cover the lowest tide period, to avoid the rising tide, and thus to facilitate specimen spotting. Specimens were photographed with a Canon G16 equipped with Inon S200 strobe for identification purposes at the lowest possible taxonomic level using the most up to date literature (i.e. Pruvot-Fol Citation1954; Schmekel & Portmann Citation1982; Lipej et al. Citation2008, Citation2012; Betti Citation2011; Trainito & Doneddu Citation2014; Zenetos et al. Citation2016; Prkić et al. Citation2018; Korshunova et al. Citation2019, Citation2020; Carmona Citation2020; Ghanimi et al. Citation2020). Diversity and abundance of species and trophic groups are reported. The assignment of trophic groups categories used here refers to Betti et al. (Citation2017).
Table I. List of Heterobranchs found in the rocky pool system at Passetto (Ancona, Italy). Number of specimens and species per each replicate and occurrence of upside-down behavior for each species are reported. Trophic groups abbreviations: SP = sponge-eaters; OM = omnivorous and opportunistic; OO = oophagous; BR = bryozoan-eaters; HE = herbivores; CN = cnidarian-eaters. Upside-down – observations of heterobranchs that shown the upside-down walking behavior during the surveys. The two replicates per month have been abbreviated as follows: J = January; F = February; M = March; A = April.
Results
During the 4 months of survey a total of 452 specimens of Heterobranchia have been found, belonging to three orders, 12 families and 19 species (, ). The most abundant and diversified family was Facelinidae Bergh, 1889 (Heterobranchia: Nudibranchia) with 244 specimens and 5 species: Facelina rubrovittata (A. Costa 1866), n = 236; Cratena peregrina (Gmelin, 1791), n = 2; Facelina dubia Pruvot-Fol, 1948, n = 2; Facelina vicina (Bergh, 1882), n = 2; Favorinus branchialis (Rathke, 1806), n = 2. The Family Plakobranchidae Gray, 1840 and Limapontiidae Gray, 1847 (Heterobranchia: Sacoglossa) were respectively the second and third most abundant families. Family Plakobranchidae was only represented by the species Elysia viridis (Montagu, 1804), n = 94, while Limapontiidae by Placida dendritica (Alder & Hancock 1843), n = 29, and Ercolania viridis (A. Costa 1866), n = 3.
Figure 3. Heterobranchs found during the tide pools surveys at Passetto. Order Pleurobranchida. Family Pleurobranchidae: (a) Berthella perforata. Order Nudibranchia. Suborder Doridina. Family Discodorididae: (b) Jorunna tomentosa. Family Polyceridae: (c) Polycera quadrilineata. Family Chromodorididae: (d) Felimare villafranca. Suborder Cladobranchia. Family Dotidae: (e) Doto cervicenigra; (f) Doto coronata. Family Janolidae: (g) Antiopella cristata. Family Eubranchidae: (h) Amphorina andra; (i) Amphorina linensis. Family Flabellinidae: (j) Edmundsella pedata. Family Trinchesiidae: (k) Trinchesia morrowae; (l) Trinchesia sp. Family Facelinidae: (m) Facelina dubia; (n) Facelina rubrovittata; (o) Facelina vicina; (p) Favorinus branchialis. Superorder Sacoglossa. Family Limapontiidae: (q)–(r) Ercolania viridis; (s) Placida dendritica. Family Plakobranchidae: (t) Elysia viridis. All scale bars around 1 cm.

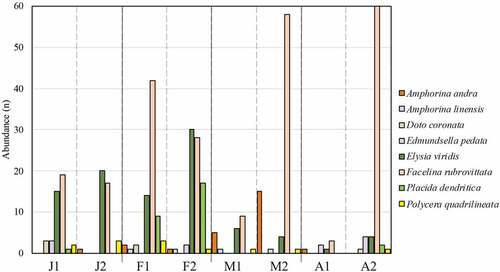
Comparison between replicates revealed a distribution pattern within the temporal interval surveyed (January to April) for some species while others were constantly or occasionally present. Abundances for the most representative species are reported (). Facelina rubrovittata was the most abundant species in all months and was present in every survey together with Elysia viridis (; ). The first showed high fluctuations between replicates in March and April, while Elysia viridis had an abundance peak in the second replicate of February (F2, n = 30). Polycera quadrilineata (O.F. Müller, 1776) was absent only in the first replicate of April and its highest abundance was recorded in January and February (J2 and F1, n = 3) (; ). The species P. dendritica, Amphorina andra Korshunova et al. 2020 and Edmundsella pedata (Montagu, 1816) were more abundant in F2 (n = 17), M2 (n = 15), and A2 (n = 4) respectively (; ). Favorinus branchialis, Felimare villafranca (Risso, 1818) and Jorunna tomentosa (Cuvier, 1804) were found only in one replicate (). Regarding species diversity per month, February was the most diversified month with 15 species in total, found respectively 12 in F1 and 10 in F2, followed by January and April with 11 species and March with eight species ().
Figure 4. Mean abundances (n) per replicate of the most represented taxa among the four-month survey. The two replicates per month have been abbreviated as follows: J = January; F = February; M = March; A = April.
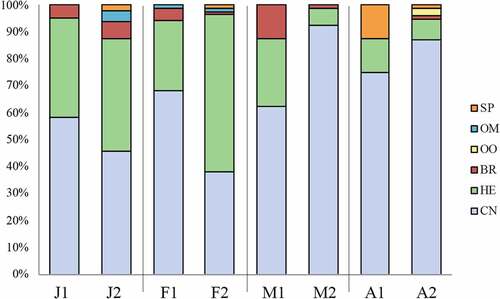
A total of six trophic groups were assigned: sponge-eaters (SP), omnivorous and opportunistic (OM), oophagous (OO), bryozoan-eaters (BR), herbivores (HE), and cnidarian eaters (CN) (; ). CN, HE and BR have been found in all the replicates, while SP, OO and OM appeared sporadically (; ). CN and HE showed the highest average percentage in every month, but HE presence decreased considerably in March and April while CN increased during these months (; ). BR were found constantly but in low abundance, ranging from 1.2% to 5.5% of the overall assemblage each month ().
Figure 5. Percentage of trophic groups found per each replicate. The two replicates per month have been abbreviated as follows: J = January; F = February; M = March; A = April. Abbreviations used for trophic groups: SP = sponge-eaters; OM = omnivorous and opportunistic; OO = oophagous; BR = bryozoan-eaters; HE = herbivores; CN = cnidarian-eaters.
Discussion
Owing to their interesting adaptive and aesthetic characters, heterobranchs have always been an attractive topic among scientists and naturalists, engaging also numerous passionate people, especially among SCUBA divers. Nevertheless, poor knowledge about their local diversity, ecology and life history at large is still wide for many sectors of the Mediterranean Sea. In the studied area, within a complex system of rocky pools, we found around the 26% of the heterobranch fauna reported for this trait of coast, considering previous assessment from the Conero area (Betti Citation2011, Citation2021).
Therefore, the Passetto rocky tide pools host a considerable heterobranch abundance and biodiversity during the surveyed period, suggesting that this littoral restricted habitat can sustain massive presence of heterobranchs in syntopy, with complex food-webs dynamics (Mendonça et al. Citation2018).
The macroalgal assemblage of the tide pools provides substrate and resources to a rich community (e.g. sponges, hydroids, bryozoans, ascidians) that result in a diversified food availability for heterobranchs. In this regard, the heterobranch community of the Passetto rocky pools was diversified also in terms of trophic groups. Hydrozoan-eaters were the most abundant and diversified (eight species) in all the replicates: F. rubrovittata, the most abundant species in our study, feeds on hydrozoans of the genus Eudendrium Ehrenberg, 1834, together with the other cladobranchian C. peregrina, Doto coronata (Gmelin, 1791) (Schmekel & Portmann Citation1982; Trainito & Doneddu Citation2014), and Trinchesia morrowae Korshunova et al. 2019 (possibly also on Sertularella spp. Gray, 1848; Cattaneo-Vietti et al. Citation1990). Other hydrozoan-eaters found were the Eubranchidae Odhner, 1934 A. andra and A. linensis (Garcia-Gomez, Cervera & Garcia, 1990), which possibly feed on Obelia spp. and Tubularia spp. Linnaeus, 1758 (McDonald & Nybakken Citation1997, Citation1999). Only recently the systematics of the genera Eubranchus Forbes, 1838 and Amphorina Quatrefages, 1844 have been updated by Korshunova et al. (Citation2020). Both A. andra and Eubranchus farrani (Alder & Hancock, 1844) show highly polymorphic color patterns but with specific diagnostic characters in the pigmentation and spot positioning. Specimens encountered showed exclusively patterns of A. andra (), coherently with previous findings in this area (see E. farrani sensu Betti Citation2011 and A. farrani sensu Betti, Citation2021). Moreover, both species are now known to be present in the Adriatic (Korshunova et al. Citation2020). In view of this, we identified the two species as Amphorina andra and Amphorina linensis. Among cladobranchian, we also add to the account of the species for the western Adriatic the Dotidae Gray, 1853 Doto cervicenigra Ortea & Bouchet, 1989, although only two specimens were found in the rocky pools out of the survey in the same period, and therefore not included in the results ().
Herbivores showed a high abundance of individuals but a lower number of species (n = 3). Elysia viridis is a generalist herbivore, feeding on different green and red algae (Schmekel & Portmann Citation1982), while P. dendritica and Ercolania viridis are known to feed on green macroalgae, respectively on Bryopsis sp. J. V. Lamouroux and Chaetomorpha sp. Kützing (Trinchese Citation1874; Schmekel & Portmann Citation1982; Micaroni et al. Citation2018). It is noteworthy that the two Limapontiidae P. dendritica and Ercolania viridis found during the surveys represent new records for Ancona and the Conero coasts, with Ercolania viridis being the first record from the western Adriatic (). The distribution of this species was recently updated with new records from the Ionian Sea (Salento Peninsula in Micaroni et al. Citation2018; Furfaro et al. Citation2020) and eastern Adriatic (Rovinji and Vranjic, Croatia in Prkić et al. Citation2018) but it was never recorded before at this latitude in western Adriatic.
During our surveys, sacoglossan specimens were attributed to P. dendritica in view of the white tipped auriculate rhinophores and cerata, the latter being not particularly pointed apically with the digestive gland diverticula branching through the cerata and showing lack of violet longitudinal bands along the foot (Alder & Hancock Citation1843; Costa Citation1866; Trinchese Citation1874, Citation1892; Pruvot-Fol Citation1954; Schmekel & Portmann Citation1982). However, P. dendritica is known to be highly polymorphic through its wide distribution range, from temperate to subtropical areas, showing a variety of color morphs according to its diet (Schmekel & Portmann Citation1982; Trowbridge Citation1992, Citation1997) and locality (Bleakney Citation1989; Rudman Citation2002). Following recent updates on the phylogeny of its congeneric Placida cremoniana (Trinchese Citation1892), which revealed cryptic diversity within this apparently widespread species (McCarthy et al. Citation2019; Durucan & Crocetta in Tsagarakis et al. Citation2021), it is likely that P. dendritica may also belong to a complex of species. Although in Betti (Citation2021) Placida cf. viridis is instead reported as an occasional sighting in the Passetto area, from our findings, specimens encountered () were closer to the typical P. dendritica described from Torquay (UK) by Alder and Hancock (Citation1843). Placida dendritica is not a common finding in this sector of the Mediterranean Sea, being more commonly found in the western Mediterranean (Rinaldi Citation2012; Trainito & Doneddu Citation2014). Although further molecular studies would be necessary to clarify the identification of this population, we record this species for the first time in Ancona to our knowledge.
It is also noteworthy that, during our surveys, several specimens of heterobranchs were found crawling upside-down the water surface of the tide pools (; ). We recorded this behavior for 11 species belonging to eight families and all the three orders found (Pleurobranchida, Nudibranchia, and Sacoglossa) for a total of 29 specimens (). Contrary to pleustonic heterobranchs (i.e. Glaucidae Gray, 1827) which are specialized in such lifestyle (Farmer Citation1970), benthic heterobranchs occasionally may use the surface tension of the water using mucous secretions to slide upside-down the water surface (Ponder et al. Citation2020). Reasons for this behavior are poorly understood, being seldomly described both in captivity (Colgan Citation1914; Alqudah et al. Citation2016) and in nature (Colgan Citation1914; Bertsch et al. Citation1972; Behrens Citation2018; Cyrne et al. Citation2018). In captivity, sea slugs have been seen crawling upside-down the surface soon after being moved to aquaria (Colgan Citation1914; Bertsch et al. Citation1972). Moreover, ventral-gilled nudibranchs as Phyllididae Rafinesque, 1814 have been seen displaying gills once on the surface, as in anoxic condition (Alqudah et al. Citation2016). Hence, it is possible that the heterobranchs of the Passetto tide pools system take advantage of this locomotion technique as a response to stressful conditions occurring in tide pools. Along with floating, also active and passive upside-down drifting through tidal currents have been noticed (). Locomotion tasks as food hunting in heterobranchs are driven by chemosensory feedbacks (Wyeth et al. Citation2006; Ponder et al. Citation2020), but is still not known if the upside-down floating locomotion may be driven by specific tasks and further studies on this peculiar behavior are needed.
Figure 6. Upside-down floating locomotion behavior assessed during the surveys. (a) Trinchesia morrowae; (b) Facelina rubrovittata; (c) Polycera quadrilineata; (d) Cratena peregrina; (e) Berthella perforata; (f) Facelina dubia drifting in the tidal current; (g) Elysia viridis. All scale bars around 1 cm.

It is acknowledged that majority of heterobranchs taxa are stenophagous (McDonald & Nybakken Citation1997, Citation1999), commonly undergoing seasonal fluctuations according to prey life cycles (Miller Citation1962; Todd Citation1981; Cattaneo Vietti & Balduzzi Citation1991; Sisson Citation2005; Canessa et al. Citation2021) with population blooms occurring locally on a year-basis for many species (Trowbridge Citation1997; Davis et al. Citation2017; Lombardo et al. Citation2020). For example, the occurrence of several specimens of Trinchesia sp. only in one replicate, as well as the massive presence of P. dendritica in February, are likely due to the sudden populations exploit which these species are subjected to. Nevertheless, reasons for the appearance and disappearance of massive amounts of heterobranchs specimens in such a short time frame in tide pools environments are not completely understood and several hypotheses have been proposed to explain such fluctuations (Cyrne et al. Citation2018). Among these, horizontal migrations from nearby areas to mate/spawn or fortuitous larval settlement followed by subsequent death by lack of food are supposed (Miller Citation1962; Nybakken Citation1974, Citation1978). As a matter of fact, heterobranchs are often seen reproducing in stressful conditions (Cyrne et al. Citation2018) and dying post spawning (Nybakken Citation1978; Doménech et al. Citation2002), although in our survey we did not observe mating behavior nor egg masses (together with a scarce presence of oophagous species), suggesting that the aggregations found were likely driven by food availability more than reproduction purposes. Nevertheless, the disappearance dynamics observed are not clear, but are potentially related to post-bloom food decrease or to environmental factors (e.g. temperature shifts, storms) (Cyrne et al. Citation2018). A more extended survey, covering also summer and fall months, could better clarify populations dynamics, prey–predator relations and possible anthropogenic stressors of heterobranch species here reported.
Rocky reefs, and thus also the tide pools, are listed among protected habitats in the EU Habitat Directive (92/43/EEC, code 1170), deserving further protection (UNEP/MAP-RAC/SPA Citation2015). These environments are still exposed to anthropogenic pressures that may affect their biota, such as human pressure through trampling (Pinn & Rodgers Citation2005) and construction of coastal defenses (Airoldi et al. Citation2005).
The western Adriatic Sea is affected by intense coastal erosion due to winter storms that led to the extensive construction of coastal defenses (Matteucci et al. Citation2010; Rosskopf et al. Citation2018). In particular, in the Marche region, more than 70% of the coast is protected by breakwaters (Lorenzoni et al. Citation2016), including a breakwater placed close to the study area in front of an artificial beach. Although these structures represent a suitable hard substrate for many species, the complex three-dimensionality of adjacent rocky pools and natural hard substrates support higher biodiversity than artificial ones (Bulleri & Chapman Citation2004; Airoldi et al. Citation2005; Moschella et al. Citation2005; Firth et al. Citation2013, Citation2014; Lai et al. Citation2018; Sedano et al. Citation2019, Citation2021). Moreover, placement of breakwaters modify the coastal current flows and sedimentation rates, thus altering benthic assemblages at intertidal and upper infralittoral level with cascade effects that can contribute to the general loss of biodiversity, not only at local scales (Airoldi et al. Citation2005; Afghan et al. Citation2020; Masucci et al. Citation2020).
We herein highlight the key role this semi-closed environment can play as a hotspot for local biodiversity, underlining that anthropic interventions such breakwaters placement and beach nourishment should consider the conservation of this important, albeit neglected, habitat in the Mediterranean Sea.
Authors contribution
A.C., A.R. and R.V. conceived the study, performed sampling activities and managed collected data. A.C. and R.V. wrote the original draft. A.C., A.R., R.V. and C.C reviewed and edited the manuscript. All the authors agreed to publish this version of the manuscript.
Disclosure statement
No potential conflict of interest was reported by the author(s)
References
- Addison PF, Koss RS, O’Hara TD. 2008. Recreational use of a rocky intertidal reef in Victoria: Implications for ecological research and management. Australasian Journal of Environmental Management 15(3):169–179. DOI:10.1080/14486563.2008.9725199.
- Afghan A, Cerrano C, Luzi G, Calcinai B, Puce S, Mantas TP et al. 2020. Main anthropogenic impacts on benthic macrofauna of sandy beaches: A review. Journal of Marine Science and Engineering 8(6):405. DOI: 10.3390/JMSE8060405.
- Airoldi L, Abbiati M, Beck MW, Hawkins SJ, Jonsson PR, Martin D, Moschella PS, Sundelöf A, Thompson RC, Åberg P. 2005. An ecological perspective on the deployment and design of low- crested and other hard coastal defence structures. Coastal Engineering 52(10–11):1073–1087. DOI:10.1016/j.coastaleng.2005.09.007.
- Alder J, Hancock A. 1843. Notice on a British species of Calliopaea d’Orbigny and on four new species of Eolis with observations on the development and structure of the nudibranchiate mollusca. Annals and Magazine of Natural History 12(77):233–236. DOI:10.1080/03745484309442517.
- Alqudah A, Saad S, Susanti D, Hadry NF, Khodzori MFA, Yusof MH, Rani MH. 2016. Observations on nudibranch behaviour patterns under laboratory conditions. Jurnal Teknologi 78(11):167–171. DOI:10.11113/.v78.6639.
- Armstrong EJ, Tanner RL, Stillman JH. 2019. High heat tolerance is negatively correlated with heat tolerance plasticity in nudibranch mollusks. Physiological and Biochemical Zoology 92(4):430–444. DOI:10.1086/704519.
- Behrens DW 2018. Comment on Dirona pellucida from Puget Sound by Mary Jo Adams. [Message in] Sea Slug Forum. Australian Museum, Sydney. Available: http://www.seaslugforum.net/find/18967. Accessed April 2022 06.
- Bennett BA. 1987. The rock-pool fish community of Koppie Alleen and an assessment of the importance of Cape rock-pools as nurseries for juvenile fish. African Zoology 22(1):25–32. DOI:10.1080/02541858.1987.11448016.
- Bertsch H, Gosliner TM, Wharton R, Williams G. 1972. Natural history and occurrence of opistobranch gastropods from the open coast of San Mateo County, California. The Veliger 14(3):302–314.
- Betti F. 2010. La fauna marina della riviera del Conero. Imola, Italy: EditriceLa Mandragora. pp. 180.
- Betti F. 2011. Il regno dei nudibranchi – Guida ai molluschi opistobranchi della Riviera del Conero. Imola, Italy: Editrice La Mandragora. pp. 200.
- Betti F, Bava S, Cattaneo-Vietti R. 2017. Composition and seasonality of a heterobranchassemblage in a sublittoral, unconsolidated, wave-disturbed community in the Mediterranean Sea. Journal of Molluscan Studies 83(3):325–332. DOI:10.1093/mollus/eyx019.
- Betti F. 2021. Il regno dei nudibranchi – Guida ai molluschi eterobranchi della Riviera del Conero. Imola, Italy: Editrice La Mandragora. pp. 192.
- Bleakney JS. 1989. Morphological variation in the radula of Placida dendritica (Alder & Hancock, 1843) (Opisthobranchia: Ascoglossa/Sacoglossa) from Atlantic and Pacific populations. The Veliger 32:171–181.
- Bulleri F, Chapman MG. 2004. Intertidal assemblages on artificial and natural habitats in marinas on the north-west coast of Italy. Marine Biology 145(2):381–391. DOI:10.1007/s00227-004-1316-x.
- Bulleri F, Chapman MG. 2010. The introduction of coastal infrastructure as a driver of change in marine environments. Journal of Applied Ecology 47(1):26–35. DOI:10.1111/j.1365-2664.2009.01751.x.
- Bussell JA, Lucas IA, Seed R. 2007. Patterns in the invertebrate assemblage associated with Corallina officinalis in tide pools. Journal of the Marine Biological Association of the United Kingdom 87(2):383–388. DOI:10.1017/S0025315407055385.
- Canessa M, Bavestrello G, Cattaneo-Vietti R, Furfaro G, Doneddu M, Navone A, Trainito E. 2021. Rocky substrate affects benthic heterobranch assemblages and prey/predator relationships. Estuarine, Coastal and Shelf Science 261:107568. DOI: 10.1016/j.ecss.2021.107568.
- Carmona L. 2020. Investigating the amphiatlantic status of Facelina bostoniensis (Couthouy, 1838) (Nudibranchia: Aeolidida). Journal of Molluscan Studies 86(1):64–71. DOI:10.1093/mollus/eyz034.
- Cattaneo Vietti R, Balduzzi A. 1991. Relationship between radular morphology and food in the Doridina (Mollusca: Nudibranchia). Malacologia 32:211–218.
- Cattaneo-Vietti R, Chemello R, Giannuzzi-Savelli R. 1990. Atlas of Mediterranean nudibranchs. Rome: La Conchiglia. pp. 264.
- Cerrano C, Pica D, Di Camillo G, Bastari A, Torsani F 2014. Caratterizzazione biocenotica e restituzione cartografia per l’individuazione di habitat e specie di interesse comunitario nelle Aree Protette delle Marche. Relazione Tecnica, Università Politecnica delle Marche, Italy, 53 pp.
- Cervera JL, Calado G, Gavaia C, Malaquias M, Templado J, Ballesteros M, Garcia-Gomez JC, Megina C. 2004. An annotated and updated checklist of the opisthobranchs (Mollusca: Gastropoda) from Spain and Portugal (including islands and archipelagos). Boletin Instituto Espanol de Oceanografia 20:1–122.
- Chan BKK. 2000. Diurnal physico-chemical variations in Hong Kong rock pools. Asian Marine Biology 17:43–54.
- Chiarore A, Bertocci I, Fioretti S, Meccariello A, Saccone G, Crocetta F, Patti FP. 2019. Syntopic Cystoseira taxa support different molluscan assemblages in the Gulf of Naples (southern Tyrrhenian Sea). Marine and Freshwater Research 70(11):1561–1575. DOI:10.1071/mf18455.
- Colgan N. 1914. The Opisthobranch fauna of the shores and shallow waters of County Dublin. The Irish Naturalist 23(8–9):161–204. http://www.jstor.org/stable/25524279.
- Comune di Ancona. 2017. Relazione tecnico-paesaggistica. Lavori di riqualificazione del passetto area compresa tra la pista di pattinaggio, i laghetti e le piscine comunali di Ancona. 2017/02
- Costa A. 1866. Saggio sui molluschi eolididei del Golfo di Napoli. Annuario del Museo Zoologico della Reale Università di Napoli 3:59–80.
- Crocetta F, Zibrowius H, Bitar G, Templado J, Oliverio M. 2013. Biogeographical homogeneity in the eastern Mediterranean Sea-I: The opisthobranchs (Mollusca: Gastropoda) from Lebanon. Mediterranean Marine Science 14(2):403–408. DOI:10.12681/mms.404.
- Crocetta F, Poursanidis D, Tringali LP. 2015. Biodiversity of sea slugs and shelled relatives (Mollusca: Gastropoda) of the Cretan Archipelago (Greece), with taxonomic remarks on selected species. Quaternary International 390:56–68. DOI: 10.1016/j.quaint.2015.02.061.
- Cyrne R, Rosa IC, Faleiro F, Dionísio G, Baptista M, Couto A, Pola M, Rosa R. 2018. Nudibranchs out of water: Long-term temporal variations in the abundance of two Dendrodoris species under emersion. Helgoland Marine Research 72(1):14. DOI:10.1186/s10152-018-0516-4.
- Davis TR, Harasti D, Kelaher B, Smith SDA. 2017. Spatial and temporal variation in subtidal molluscan diversity amongst temperate estuarine habitats. Marine Ecology 38(3):e12428. DOI:10.1111/maec.12428.
- Di Camillo CG, Bo M, Bartolucci I, Betti F, Calcinai B, Cerrano C, Coppari M, Martinelli M, Puce S, Bavestrello G. 2010. The benthic assemblage of Conero Promontory: A model for the study of seasonal cycles in the North Adriatic Sea. Biologia Marina Mediterranea 17:112–115.
- Doménech A, Avila C, Ballesteros M. 2002. Spatial and temporal variability of the opisthobranch molluscs of Port Lligat Bay, Catalonia, NE Spain. Journal of Molluscan Studies 68(1):29–37. DOI:10.1093/mollus/68.1.29.
- Doménech A, Avila C, Ballesteros M. 2006. Opisthobranch molluscs from the subtidal trawling grounds off Blanes (Girona, north-east Spain). Journal of the Marine Biological Association of the United Kingdom 86(2):383–389. DOI:10.1017/S0025315406013233.
- Fairchild TP, Fowler MS, Pahl S, Griffin JN. 2018. Multiple dimensions of biodiversity drive human interest in tide pool communities. Scientific Reports 8(1):1–11. DOI:10.1038/s41598-018-33155-x.
- Farmer WM. 1970. Swimming gastropods (opisthobranchia and prosobranchia). The Veliger 13(1):73–89.
- Firth LB, Thompson RC, White FJ, Schofield M, Skov MW, Hoggart SP, Jackson J, Knights AM, Hawkins SJ. 2013. The importance of water‐retaining features for biodiversity on artificial intertidal coastal defence structures. Diversity and Distributions 19(10):1275–1283. DOI:10.1111/ddi.12079.
- Firth LB, Schofield M, White FJ, Skov MW, Hawkins SJ. 2014. Biodiversity in intertidal rock pools: Informing engineering criteria for artificial habitat enhancement in the built environment. Marine Environmental Research 102:122–130. DOI: 10.1016/j.marenvres.2014.03.016.
- Fruzzetti VM, Segato D, Ruggeri P, Vita A, Sakellariadi E, Scarpelli G 2011. Fenomeni di instabilità della falesia del Monte Conero: Ruolo dell’assetto strutturale. Incontro Annuale dei Ricercatori di Geotecnica. IARG 2011 Turin, 4–6 July 2011.
- Furfaro G, Vitale F, Licchelli C, Mariottini P. 2020. Two seas for one great diversity: Checklist of the marine heterobranchia (Mollusca; Gastropoda) from the Salento Peninsula (South-East Italy). Diversity 12(5):171. DOI:10.3390/D12050171.
- Ganning B. 1970. Population dynamics and salinity tolerance of Hyadesia fusca (Lohman) (Acarina, Sarcoptiformes) from brackish water rockpools, with notes on the microenvironment inside Enteromorpha tubes. Oecologia 5(2):127–137. DOI:10.1007/BF00347626.
- Ganning B. 1971. On the ecology of Heterocypris salinus, H. incongruens and Cypridopsis aculeata (Crustacea: Ostracoda) from Baltic brackish-water rockpools. Marine Biology 8(4):271–279. DOI:10.1007/BF00348009.
- Gerovasileiou V, Bianchi CN. 2021. Mediterranean marine caves: A synthesis of current knowledge. Oceanography and Marine Biology 59. DOI: 10.1201/9781003138846-1.
- Ghanimi H, Schrödl M, Goddard JHR, Ballesteros M, Gosliner TM, Buske Y, Valdés A. 2020. Stargazing under the sea: Molecular and morphological data reveal a constellation of species in the Berthella stellata (Risso, 1826) species complex (Mollusca, Heterobranchia, Pleurobranchidae). Marine Biodiversity 50(1). DOI: 10.1007/s12526-019-01027-w.
- Goddard JHR, Treneman N, Pence WE, Mason DE, Dobry PM, Green B, Hoover C. 2016. Nudibranch range shifts associated with the 2014 warm anomaly in the northeast pacific. Bulletin of the Southern California Academy of Sciences 115(1):15–40. DOI:10.3160/soca-115-01-15-40.1.
- Griffiths SP, Davis AR, West RJ. 2006. Role of habitat complexity in structuring temperate rockpool ichthyofaunas. Marine Ecology Progress Series 313:227–239. DOI: 10.3354/meps313227.
- Hulsmans A, Vanschoenwinkel B, Pyke C, Riddoch BJ, Brendonck L. 2008. Quantifying the hydroregime of a temporary pool habitat: A modelling approach for ephemeral rock pools in SE Botswana. Ecosystems 11(1):89–100. DOI:10.1007/s10021-007-9110-3.
- Keough MJ, Quinn GP. 1998. Effects of periodic disturbances from trampling on rocky intertidal algal beds. Ecological Applications 8:141–161.
- Keough MJ, Quinn GP. 2000. Legislative vs. practical protection of an intertidal shoreline in southeastern Australia. Ecological Applications 10(3):871–881. DOI:10.1890/1051-0761(2000)010[0871:LVPPOA]2.0.CO;2.
- Korshunova T, Picton B, Furfaro G, Mariottini P, Pontes M, Prkić J, Fletcher K, Malmberg K, Lunding K, Martynov A. 2019. Multilevel fine-scale diversity challenges the ‘cryptic species’ concept. Scientific Reports 9(1):1–23. DOI:10.1038/s41598-019-42297-5.
- Korshunova T, Malmberg K, Prkić J, Petani A, Fletcher K, Lundin K, Martynov A. 2020. Fine-scale species delimitation: Speciation in process and periodic patterns in nudibranch diversity. ZooKeys 917:15. DOI: 10.3897/zookeys.917.47444.
- Lai S, Loke LH, Bouma TJ, Todd PA. 2018. Biodiversity surveys and stable isotope analyses reveal key differences in intertidal assemblages between tropical seawalls and rocky shores. Marine Ecology Progress Series 587:41–53. DOI: 10.3354/meps12409.
- Lambert WJ. 2013. Population biology of an intertidal dorid nudibranch (Onchidoris muricata) in the southern gulf of maine, U.SA.: Changes in phenology due to an invasive prey? American Malacological Bulletin 31(1):17–23. DOI:10.4003/006.031.0109.
- Lipej L, Dobrajc Ž, Mavrič B, Šamu S, Alajbegović S. 2008. Opisthobranch molluscs (Mollusca: Gastropoda) from Slovenian coastal waters (Northern Adriatic). Annales: Series Historia Naturalis 2(18):213–226.
- Lipej L, Mavrič B, Moškon S. 2012. New recordings of opisthobranch mollusks (Mollusca: Opisthobranchia) in the Slovenian portion of the Adriatic Sea. Annales: Series Historia Naturalis 2(22):133–136.
- Lombardo A, Marletta G, Leonardi G. 2020. Seasonality of Edmundsella pedata (Montagu, 1816) (Nudibranchia Flabellinidae) along the Ionian coasts of Sicily (Central Mediterranean Sea). Biodiversity Journal 11(2):631–638. DOI:10.31396/Biodiv.Jour.2020.11.2.631.638.
- Lorenzoni C, Postacchini M, Brocchini M, Mancinelli A. 2016. Experimental study of the short-term efficiency of different breakwater configurations on beach protection. Journal of Ocean Engineering and Marine Energy 2(2):195–210. DOI:10.1007/s40722-016-0051-9.
- Lucrezi S, Esfehani MH, Ferretti E, Cerrano C. 2019. The effects of stakeholder education and capacity building in marine protected areas: A case study from southern Mozambique. Marine Policy 108:103645. DOI: 10.1016/j.marpol.2019.103645.
- Martens L. 2016. From intergenerational transmission to intra-active ethical-generational becoming: Children, parents, crabs and rockpooling. Families, Relationships and Societies 5(3):447–462. DOI:10.1332/204674316X14758498374746.
- Martin D, Bertasi F, Colangelo MA, de Vries M, Frost M, Hawkins SJ, Macpherson E, Moschella PS, Satta MP, Thompson RC, Ceccherelli VU. 2005. Ecological impact of coastal defence structures on sediment and mobile fauna: Evaluating and forecasting consequences of unavoidable modifications of native habitats. Coastal Engineering 52(10–11):1027–1051. DOI:10.1016/j.coastaleng.2005.09.006.
- Masucci GD, Acierno A, Reimer JD. 2020. Eroding diversity away: Impacts of a tetrapod breakwater on a subtropical coral reef. Aquatic Conservation: Marine and Freshwater Ecosystems 30(2):290–302. DOI:10.1002/aqc.3249.
- Matteucci GA, Riccio SA, Rossini PA, Sisti EL, Bernucci ME, Pari PA, Benedettini MA, and Stanley CC. 2010. Shoreline evolution trend connected to progressive construction of segmented defense structures (Rimini, North Adriatic Sea, Italy). GeoActa Special Publication 3:135–141.
- McCarthy JB, Krug PJ, Valdés Á. 2019. Integrative systematics of Placida cremoniana (Trinchese, 1892) (Gastropoda, Heterobranchia, Sacoglossa) reveals multiple pseudocryptic species. Marine Biodiversity 49(1):357–371. DOI:10.1007/s12526-017-0812-2.
- McDonald GR, Nybakken JW. 1997. A worldwide review of the food of nudibranch mollusks I. Introduction and the suborder Arminacea. The Veliger 40(2):157–159.
- McDonald GR, Nybakken JW. 1999. A worldwide review of the food of nudibranch mollusks II. The suborder Dendronotacea. The Veliger 42(1):62–66.
- Mendonça V, Madeira C, Dias M, Vermandele F, Archambault P, Dissanayake A, Canning-Clode J, Flores AAV, Silva A, Vinagre C. 2018. What’s in a tide pool? Just as much food web network complexity as in large open ecosystems. PloS One 13(7):e0200066. DOI:10.1371/journal.pone.0200066.
- Metaxas A, Scheibling RE. 1993. Community structure and organization of tidepools. Marine Ecology Progress Series 98(1–2):187–198. DOI:10.3354/meps098187.
- Micaroni V, Strano F, Di Franco D, Crocetta F, Grech D, Piraino S, Boero F. 2018. Project “Biodiversity MARE Tricase”: A biodiversity inventory of the coastal area of Tricase (Ionian Sea, Italy)–Mollusca: Heterobranchia. European Zoological Journal 85(1):180–193. DOI:10.1080/24750263.2018.1462413.
- Miller MC. 1962. Annual cycles of some Manx nudibranchs, with a discussion of the problem of migration. Journal of Animal Ecology 31(3):545–569. DOI:10.2307/2053.
- Moschella PS, Abbiati M, Åberg P, Airoldi L, Anderson JM, Bacchiocchi F, Bullleri F, Dinesen GE, Frost M, Gacia E, Granhag L, Jonsson PR, Satta MP, Sundelöf A, Thompson RC, Hawkins SJ. 2005. Low-crested coastal defence structures as artificial habitats for marine life: Using ecological criteria in design. Coastal Engineering 52(10–11):1053–1071. DOI:10.1016/j.coastaleng.2005.09.014.
- Nielsen KJ. 2001. Bottom-up and top-down forces in tide pools: Test of a food chain model in an intertidal community. Ecological Monographs 71(2):187. DOI:10.2307/2657216.
- Nimbs MJ, Willan RC, Smith SD. 2015. Range extensions for heterobranch sea slugs (formerly opisthobranch) belonging to the families Diaphanidae, Plakobranchidae and Facelinidae on the eastern coast of Australia. Marine Biodiversity Records 8:e76. DOI: 10.1017/S1755267215000524.
- Nybakken J. 1974. A phenology of the smaller endronotacean, arminacean and aeolidacean nudibranchs at Asilomar State Beach over a twenty-seven month period. The Veliger 16(4):370–373.
- Nybakken J. 1978. Abundance, diversity and temporal variability in a California intertidal nudibranch assemblage. Marine Biology 45(2):129–146. DOI:10.1007/BF00390549.
- Parera A, Pontes M, Salvador X, Ballesteros MBV. 2020. Sea-slugs (Mollusca, Gastropoda, Heterobranchia): The other inhabitants of the city of Barcelona (Spain). Butlletí de la Institució Catalana d’Histōria Natural 84:75–100. http://hdl.handle.net/2445/169455.
- Perkins MJ, Ng TP, Dudgeon D, Bonebrake TC, Leung KM. 2015. Conserving intertidal habitats: What is the potential of ecological engineering to mitigate impacts of coastal structures? Estuarine, Coastal and Shelf Science 167:504–515. DOI: 10.1016/j.ecss.2015.10.033.
- Pfister CA. 1996. The role and importance of recruitment variability to a guild of tide pool fishes. Ecology 77(6):1928–1941. DOI:10.2307/2265796.
- Pinn EH, Rodgers M. 2005. The influence of visitors on intertidal biodiversity. Journal of the Marine Biological Association of the United Kingdom 85(2):263–268. DOI:10.1017/S0025315405011148h.
- Poizat C. 1984. Seasonal variations of Mediterranean interstitial opisthobranch assemblages. Hydrobiologia 118(1):83–94. DOI:10.1007/BF00031791.
- Ponder WF, Lindberg DR, Ponder JM. 2020. Biology and evolution of the mollusca. Vol. 2. Boca Raton, Florida: CRC Press. pp. 892.
- Poursanidis D, Koutsoubas D. 2015. A computerized database (CorMol) on the molluscan fauna from the Mediterranean reef ecosystems: Part I, the coralligenous formations. Quaternary International 390:29–43. DOI: 10.1016/j.quaint.2015.07.029.
- Prkić J, Petani A, Iglić D, Lanča L. 2018. Stražnjoškržnjaci Jadranskoga mora: Slikovni atlas I popis hrvatskih vrsta. Opisthobranchs of the Adriatic Sea: Photographic atlas and list of Croatian species. Bibinje, Croatia: Diving Club St Roko. pp. 463.
- Pruvot-Fol A. 1954. Mollusques opisthobranches. In: Faune de France 58. Paris, France: Lechevalier. pp. 460.
- Ranta E. 1982. Animal communities in rock pools. Annales Zoologici Fennici 19(4):337–347.
- Rinaldi A. 2012. Atlante della fauna e flora marina dell’Adriatico ord-occidentale. Imola, Italy: Editrice La Mandragora. pp. 642.
- Rindi F, Braga JC, Martin S, Peña V, Le Gall L, Caragnano A, Aguirre J. 2019. Coralline algae in a changing Mediterranean sea: How can we predict their future, if we do not know their present? Frontiers in Marine Science 6:723. DOI: 10.3389/fmars.2019.00723.
- Rindi F, Gavio B, Díaz-Tapia P, Di Camillo CG, Romagnoli T. 2020. Long-term changes in the benthic macroalgal flora of a coastal area affected by urban impacts (Conero Riviera, Mediterranean Sea). Biodiversity and Conservation 29(7):2275–2295. DOI:10.1007/s10531-020-01973-z.
- Riolo F, Betti F. 2015. First record of Europe’s smallest marine fish Lebetus guilleti (Gobiidae) in the Italian seas. Marine Biodiversity Records 8:e12. DOI: 10.1017/S1755267214001377.
- Rosskopf CM, Di Paola G, Atkinson DE, Rodríguez G, Walker IJ. 2018. Recent shoreline evolution and beach erosion along the central Adriatic coast of Italy: The case of Molise region. Journal of Coastal Conservation 22(5):879–895. DOI:10.1007/s11852-017-0550-4.
- Rudman WB 2002. Placida dendritica - Atlantic populations. Sea Slug Forum. Australian Museum, Sydney. Available: http://www.seaslugforum.net/find/placden3. Accessed April 2022 06.
- Schmekel L, Portmann A. 1982. Opisthobranchia des Mittelmeeres, Nudibranchia und Saccoglossa. Heidelberg, Germany: Springer-Verlag Berlin. pp. 410.
- Schultz ST, Goddard JHR, Gosliner TM, Mason DE, Pence WE, McDonald GR, Pearse VB, Pearse JS. 2011. Climate-index response profiling indicates larval transport is driving population fluctuations in nudibranch gastropods from the northeast Pacific Ocean. Limnology and Oceanography 56(2):749–763. DOI:10.4319/lo.2011.56.2.0749.
- Scipione M, Gambi M, Lorenti M, Russo G, and Zupo V 1996. Vagile fauna of the leaf stratum of Posidonia oceanica and Cymodocea nodosa in the Mediterranean Sea. in Seagrass biology: Proceedings of an International Workshop, Rottnest Island, Western Australia, 249–260.
- Sedano F, Florido M, Rallis I, Espinosa F, Gerovasileiou V. 2019. Comparing sessile benthos on shallow artificial versus natural hard substrates in the Eastern Mediterranean Sea. Mediterranean Marine Science 20(4):688–702. DOI:10.12681/mms.17897.
- Sedano F, Pavón A, Navarro-Barranco C, Guerra-García JM, Digenis M, Sempere-Valverde J, Espinosa F. 2021. Coastal armouring affects intertidal biodiversity across the Alboran Sea (western Mediterranean Sea). Marine Environmental Research 171:105475. DOI: 10.1016/j.marenvres.2021.105475.
- Sisson CG. 2005. Life history dynamics and biogeography of a nudibranch with contrasting developmental modes: A hypothesis for the evolution of larval types. Journal of Natural History 39(20):1719–1733. DOI:10.1080/00222930400024709.
- Todd CD. 1981. The ecology of Nudibranch Molluscs. Oceanography and Marine Biology, an Annual Review 19:141–234.
- Todd CD, Lambert WJ, Thorpe JP. 1998. The genetic structure of intertidal populations of two species of nudibranch molluscs with planktotrophic and pelagic lecithotrophic larval stages: Are pelagic larvae “for” dispersal? Journal of Experimental Marine Biology and Ecology 228(1):1–28. DOI:10.1016/S0022-0981(98)00005-7.
- Toma M, Betti F, Bavestrello G, Cattaneo-Vietti R, Canese S, Cau A, Andaloro F, Greco S, Bo M. 2022. Diversity and abundance of heterobranchs (Mollusca, Gastropoda) from the mesophotic and bathyal zone of the Mediterranean Sea. The European Zoological Journal 89(1):160–182. DOI:10.1080/24750263.2022.2033859.
- Trainito E, Doneddu M. 2014. Nudibranchi del Mediterraneo. Milano, Italy: Il castello. pp. 192.
- Trinchese S. 1874. Descrizioni di alcuni nuovi Eolididae del porto di Genova. Memorie della Reale Accademia delle Scienze dell’ Istituto di Bologna 3(4):197–203.
- Trinchese S. 1892. Nuove osservazioni sulla Placida viridis. Memorie dell’Accademia delle Scienze dell’Istituto di Bologna 3(5):539–547.
- Trowbridge CD. 1992. Phenology and demography of a marine specialist herbivore: Placida dendritica (Gastropoda: Opisthobranchia) on the central coast of Oregon. Marine Biology 114(3):443–452. DOI:10.1007/BF00350036.
- Trowbridge CD. 1997. Dietary induction of opisthobranch morphology: Placida dendritica (Alder & Hancock, 1843) on different green algal hosts. Journal of Molluscan Studies 63(1):29–38. DOI:10.1093/mollus/63.1.29.
- Tsagarakis K, Darmanin SA, Al Mabruk SAA, Auriemma R, Azzurro E, Badouvas N, Bakiu R, Bariche M, Battaglia P, Betti F, Borme D, Cacciamani R, Cali F, Corsini-Foka M, Crocetta F, Dalyan C, Deidun A, Digenis M, Domenichetti F, Dragičević B, Dulčić J, Durucan F, Guy-Haim T, Kesici NB, Lardi P, Manitaras Y, Michailidis N, Paraino S, Rizgalla J, Siapatis A, Soldo A, Stipa MG, Kurt TT, Tiralongo F, Tsiamis K, Vella A, Vella N, Zava B, Gerovasileiou V. 2021. New records of rare species in the Mediterranean Sea. Mediterranean Marine Science 22(3):627–652. DOI:10.12681/mms.26669.
- Turchetti M, Tarsetti M. 2007. Le grotte del Passetto. Storia ambientale e cultura materiale della marina di Ancona. Ancona, Italy: Affinità elettive. pp. 179.
- Turk T. 2000. The opistobranch mollusks (Cephalaspidea, Saccoglossa, Notaspidea, Anaspidea and Nudibranchia) of the Adriatic sea with special reference to the Slovenian coast. Annales: Series Historia Naturalis 2(21):161–172.
- UNEP/MAP-RAC/SPA. 2015. Adriatic Sea: Description of the ecology and identification of the areas that may deserve to be protected. In: Cerrano C, Cebrian D, Requena S, editors. Tunis: RAC/SPA, p. 92. DOI:10.13140/RG.2.2.14080.79368.
- Vinagre C, Dias M, Fonseca C, Pinto MT, Cabral H, Silva A. 2015. Use of rocky intertidal pools by shrimp species in a temperate area. Biologia 70(3):372–379. DOI:10.1515/biolog-2015-0046.
- White GE, Hose GC, Brown C. 2015. Influence of rock‐pool characteristics on the distribution and abundance of inter‐tidal fishes. Marine Ecology 36(4):1332–1344. DOI:10.1111/maec.12232.
- Wyeth RC, Woodward OM, Willows AOD. 2006. Orientation and navigation relative to water flow, prey, conspecifics, and predators by the Nudibranch Mollusc Tritonia diomedea. Biological Bulletin 210(2):97–108. DOI:10.2307/4134599.
- Zenetos A, Mačić V, Jaklin A, Lipej L, Poursanidis D, Cattaneo-Vietti R, Beqiraj S, Betti F, Poloniato D, Kashta L, Katsanevakis S, Crocetta F. 2016. Adriatic ‘opisthobranchs’ (Gastropoda, Heterobranchia): Shedding light on biodiversity issues. Marine Ecology 37(6):1239–1255. DOI:10.1111/maec.12306.
