Abstract
The European hare (Lepus europaeus) is cosmopolitan species, living in a variety of habitats and showing a diversified diet, that has been described mainly from agricultural meadows and crops, with little information available for extreme environments. Here, we describe, for the first time, the diet of the European hare from Mount Vesuvius, using DNA metabarcoding and high-throughput sequencing on DNA extracted from faecal pellets, a proxy for a population living in a volcanic environment. The DNA from pellets was first genetically assigned to European hare using high-resolution melting analysis. The diet of the hare on Vesuvius is mainly composed of herbaceous species belonging to Fabaceae (86.26% of total diet). The most frequent plant items ingested by the species are Galega officinalis and Lupinus angustifolius (67.10% of total diet), although these are detected only sporadically in the study area. Indeed, the spectrum of available plants also includes other easily accessible wild (i.e. Lolium sp., Bromus sp., Rumex sp.) and cultivated (i.e. Solanum lycopersicum, Cucumis melo, Pisum sativum) plant items, found only in traces in the diet of the hares. Our contribution adds information on the trophic ecology of the European hare, exploring its ability to live in an extreme environment. This could be useful to set a management strategy for conservation of the species, which is ecologically relevant on Vesuvius as prey for birds and mammals, as well as a vegetation modulator via selective grazing by endozoochory. Furthermore, our study represents the latest information on the diet of the hare living in an environment that no longer exists: an extensive fire destroyed about 80% of the woody area after our sampling. The post-fire regrowth is transforming the original environment and consequently the trophic availability for the European hare.
Key policy highlights
Conservation of biodiversity in a national park
Ecosystem service of the European hare
Management of a Lagomorpha game species
Introduction
The European hare (Lepus europaeus), also named the brown hare, is a cosmopolitan species, whose current distribution includes all regions from the northern Spain to northern portions of the Middle East to the Kazakhstan and Middle-Asian steppes, and areas of introduction as a game species in Argentina, Australia, Canada, New Zealand, southern Scandinavia, the United Kingdom, the United States and some islands, where the species has subsequently become established (Hacklander & Schai-Braun Citation2019).
The European populations have undergone a strong decline in many areas across the hare’s geographic range since the 1960s, mainly blamed on the agricultural intensification and mechanisation (Smith Citation2008; Chagnon et al. Citation2015) that led to dramatic landscape changes and the reduction of habitat diversity (Jennings et al. Citation2006; Reichlin et al. Citation2006; Pépin & Angibault Citation2007; Storkey et al. Citation2012). Furthermore, other factors, such as infective diseases (Tsokana et al. Citation2020) and the climate changes, could have magnified the effect of habitat losses (Edwards et al. Citation2000).
Currently, the conservation status of the European hare worldwide is Least Concern; however, population declines have resulted in its Red Listing as Near Threatened or Threatened (Hacklander & Schai-Braun Citation2019) in some countries, such as Norway, Germany, Austria and Switzerland. To face population decline, restocking operations with captive-bred individuals as well as the translocation of wild hares among countries have been performed, in some cases determining potential risk consequences for native populations (Caravaggi et al. Citation2015).
In Italy, the species was originally distributed throughout the north-central regions in the form of the native subspecies L. europaeus meridiei (Pierpaoli et al. Citation1999; Kasapidis et al. Citation2005; Stamatis et al. Citation2009), isolated during the last glaciation. However, since the beginning of the 20th century, the massive and protracted restocking with allochthonous individuals (mainly from east Europe) (Pierpaoli et al. Citation1999, Citation2003; Kasapidis et al. Citation2005; Fulgione et al. Citation2009) has heavily altered this distribution, causing the replacement of many local indigenous populations (Pierpaoli et al. Citation1999, Citation2003; Freschi et al. Citation2015, Citation2022) and leading to a spreading of the hare also into southern Italian regions. As a consequence, nowadays, the European hare is present across the whole Italian peninsula (except in Sicily and Sardinia), as a mix of native and exotic lineages (Angelici & Luiselli Citation2001; Kasapidis et al. Citation2005; Fulgione et al. Citation2009).
The European hare is one of the most managed mammals in Europe, mainly as game species. This raises problems related to its ability to hybridise and compete ecologically when in sympatry with other vulnerable hare species, such as the mountain hare (Lepus timidus) (Thulin Citation2003) and the Italian hare (Lepus corsicanus) (Angelici & Luiselli Citation2001; Mengoni et al. Citation2015; Pietri Citation2015; Buglione et al. Citation2020a). Furthermore, the European hare acts as a host of dangerous ticks, sandflies and other disease vectors (Ebani et al. Citation2016; Tsokana et al. Citation2020), spreading the related pathologies. This happens in the case of translocation of infected animals or when they serve as prey to the long-distance carnivores and omnivores travellers, coming into contact with humans and domestic animals (Bártová & Sedlak Citation2012). In terms of both conservation and management needs, it is necessary to know in depth the ecological requirements of the species in all relevant environmental contexts, to plan useful actions to meet these needs.
The European hare lives in a variety of habitats, from the arctic tundra and steppe to tropical savanna and desert, as well as in agricultural meadows, crops, and unimproved grassland; it avoids extended woodlands (Smith et al. Citation2005; Trocchi & Riga Citation2005; Smith Citation2008; Cardarelli et al. Citation2011; Sliwinski et al. Citation2019; Buglione et al. Citation2020a).
Accordingly, the species has a very diversified and variable diet, selecting mainly cultivated crops, but not disdaining weeds, wild grasses and forbs (Chapuis Citation1990). This flexible spectrum has only been partially described, and mainly for agricultural landscapes (Reichlin et al. Citation2006; Puig et al. Citation2007, Citation2017; Green et al. Citation2013; Schai-Braun et al. Citation2015; Castellaro et al. Citation2021); indeed, little information is available about the species’ adaptation to a volcanic environment, with soil partially covered by lava and stratified pyroclastic deposits, affecting vegetation and plant formations.
Thus, we characterised the diet of the European hare by DNA metabarcoding and high throughput sequencing (HTS) starting from excremental DNA (Reichlin et al. Citation2006; Buglione et al. Citation2018; Hacklander & Schai-Braun Citation2019), as a proxy of the food habits in extreme environmental conditions, in particular on Mount Vesuvius (South Italy). This Italian volcano represents an interesting study area because it is home to a population of introduced European hare that has no way of dispersing outside the volcanic cone. Indeed, a concentric enclosure of the crater, consisting of very compact urban area, completely isolates the prairies and forests growing on the volcanic soils, and the hares living there. Thus, our study could add a new puzzle piece to deepen the knowledge of the trophic ecology of a isolated volcanic population.
Furthermore, immediately after our data collection, there was an extensive fire that impacted about 80% of the woody area (according to the Vesuvius National Park data base). The post-fire spontaneous regrowth and planting carried out by the national park are transforming the original landscape and consequently the food availability for the European hare. Considering this, our study could represent the latest information on the diet of the hare population adapted to Vesuvian habitats in an environmental context that no longer exists.
Materials and methods
Study area
Vesuvius National Park (VNP) is a protected area that was established in 1995 (National Law no. 394/1991) in the Campania region (South Italy, 40° 49.260' N, 14° 25.560' E) covering 8482 ha.
It represents a volcanic system including Mount Somma (1132 m above sea level) and Mount Vesuvius (1277 m above sea level), the latter being a stratovolcano (De Natale et al. Citation2006) with a typical truncated cone shape, a crater with a diameter of 450 m and a depth of 300 m (as of 2022).
The area is characterised by a Mediterranean climate with hot and dry summers and mild winters, sometimes covered with snow at higher altitudes. The mean annual precipitation is 1100 mm, the average minimum temperature of the coldest month (January) is 5.7°C and the average maximum temperature of the warmest month (July) is 21.6°C (Ducci & Tranfaglia Citation2008).
The Vesuvian landscape is characterised by different land uses, such as volcanic geo-sites, mixed broadleaved and coniferous forests, shrubland, farmlands, and high-density urban areas. The plant population of the volcano is also characterised by a high number of species typical of the Mediterranean area, constituting more than 42% of the entire flora. Among them, 53.5% were from Eurimediterranean areas, 42.1% were from Stenomediterranean regions and only 4.2% are typical of the Mountain Mediterranean sites. The number of plant species from the European and Asian continents is not negligible, differently from the species of Nordic (Boreal) and Western European (Atlantic) regions which, as a whole, do not reach 10% of the entire flora. Other quite numerous plant species are cosmopolitan and exotic, both cultivated and adventitious, explained by the high incidence of a strong anthropic impact.
Most of the Somma–Vesuvius flora comprises annual species (Therophytes, exceeding 40% of the entire flora), perennial herbaceous plants (Hemicryptophytes, 28% of the entire flora), while shrubs and trees (Phanerophytes, 14%) are rather scarce (Ricciardi et al. Citation2000). In particular, Mount Somma hosts mesophilic and thermophilic forests with Alnus cordata, Arbutus unedo, Castanea sativa, Fraxinus ornus, Laurus nobilis, Quercus ilex and Quercus pubescens (Di Gennaro et al. Citation2002; Ricciardi et al. Citation2016).
On Mount Vesuvius, rocky lava flows are covered by the pioneer communities of endemic Vesuvius lichen (Stereocaulon vesuvianum) and typical spontaneous Mediterranean species such as Quercus ilex and Quercus pubescens, Cytisus scoparius and Spartium junceum, as well as non-native pine plantings, including stone pine (Pinus pinea), the most abundant planted species, and other Mediterranean coniferous species (Pinus pinaster, Pinus halepensis and Pinus nigra) (Di Gennaro et al. Citation2002; Ricciardi et al. Citation2016).
The volcano is surrounded by a vast urbanised matrix, with 13 municipalities and 351,018 residents (as of 2022; http://www.comuni-italiani.it/parco/vesuvio/) falling within VNP and five other municipalities that, considering spatial contiguity and homogeneity of characteristics, can be considered an integral part of the context. In this scenario, the agricultural environment covering the slopes of Vesuvius represents a buffer zone between natural and urban areas, making the national park an important safety zone for both wild animal and plant species.
Study plot
In detail, we have selected an area of 7.74 km2 ranging from about 500 to 1000 m above sea level, on the south-western side of the volcano, in order to avoid both soil covered exclusively with lava and pyroclastic deposits (up to 1000 m a.s.l), and intensive arable land due to its strong anthropisation (). The study area is characterised by lava layers that were deposited from 1798 to 1944 after repeated eruptions and solidification of flows of lava (Santacroce Citation1987). This ropy lava is covered by spontaneous Mediterranean vegetation with the prevalence of various species of broom (Genista tinctoria, Cytisus scoparius), shrubs, untilled farmland, mixed artificial pine forests and holm oak woods (Davoli et al. Citation2001). The undergrowth is typically rich in herbaceous and shrub species (Centranthus ruber, Festuca sp., Rosa canina, Rumex bucephalophorus, Ruscus aculeatus), and various species of wild orchids.
Figure 1. Study area. The blue inset shows the Campania region in South Italy. The red inset shows Mount Vesuvius (40° 49.260' N, 14° 25.560' E) with vegetation and land-use areas (Bagnaia et al. Citation2018). The yellow dashed line delimits the study plot. The satellite image was obtained from Maps version 2.1. 2012–2018, Apple Inc.
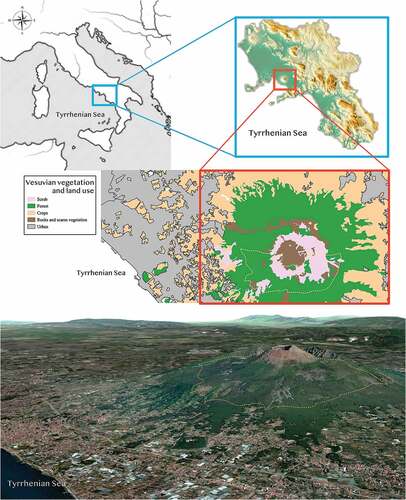
Although faecal pellets can be collected throughout whole National Park, we observed a higher likelihood of finding them in the study plot.
In addition to hare, some observations from rangers and technical documents report the presence of the wild rabbit (Oryctolagus cuniculus). The population of this lagomorph reached a peak of over 10,000 individuals in 2008 but is currently still considerably reduced (Accardo et al. Citation2008). Its distribution is focused mainly in plantations and arable lands at lower altitude of the southern slope, partially overlapping the European hare’s range (Accardo et al. Citation2008). However, further in-depth studies are needed to clarify this aspect and the potential ecological relationship between these two lagomorphs.
The predators of hare are represented by red fox (Vulpes vulpes), stone marten (Mortes foina) and diurnal raptors (e.g. Buteo buteo). Furthermore, as we learned from our preliminary study by analysing signs of predation and video trapping data, the presence of stray dogs and cats should not be overlooked – in particular, the predation pressure by felines on the young hare (unpublished data).
Collection of samples
The surveys were performed monthly in spring–summer 2016 (from March to July) in order to include the greatest diversity of plant items available for the hares.
Fresh faecal pellets (<1 day old) were collected by field operators walking along a transect of approximately 5 km. The discrimination of specimens was made by using visual searching and selecing on the basis of aspect patterns (e.g. morphology, moist shiny mucous coating, colour, sheen, etc.). To increase the chance of collecting only fresh material, the sampling site was inspected the evening before collection and any faecal pellet found was removed. Furthermore, in a sampling session we collected pellets 20 m apart from each other, to minimise the possibility of recording the same individual multiple times (Buglione et al. Citation2018, Citation2020b). The samples, handled with sterilised equipment, were preserved dried in sterile tubes with silica desiccant granule bags, transported at a controlled temperature to the laboratory and preserved at −20°C until processing.
All records were geo-referenced using a global positioning system.
Species discrimination
All experimental procedures were performed in a laboratory designed for molecular analyses on environmental samples, with different rooms and separate equipment for pre- and post-Polymerase chain reaction (PCR) analyses.
DNA extraction for species characterisation
For species determination, hare DNA was isolated from the outermost layer of each scat, sliced off with a scalpel. These surface slices were used to extract DNA using the QIAamp DNA Fast Stool Mini Kit (QIAGEN GmbH Valencia, CA, USA), that specifically includes InhibitEx Buffer specifically aimed at removing PCR inhibitors. Together with the samples, we systematically performed blank extractions without samples, to monitor for potential cross-contaminations. The quantity of total isolated DNA was measured using a Thermo-Scientific (Nanodrop 2000) Spectrometer (Nanodrop, Wilmington, DE, USA) while the DNA integrity was tested by 1% agarose gel electrophoresis.
High-resolution melting analysis
Taking into account the sympatry between the Italian hare and European hare in Campania (Pierpaoli et al. Citation1999; Fulgione et al. Citation2009; Buglione et al. Citation2020a) and the impossibility of discriminating the two species using faecal pellet morphology, we characterised the faecal DNAs with high-resolution melting (HRM) analysis (Farrar & Wittwer Citation2017), according to the protocol reported by Buglione et al. (Citation2020b).
Specifically, we performed HRM reactions on a Rotor-Gene Q 5-Plex (QIAGEN GmbH Valencia, CA, USA) with the Type-it HRM PCR Kit (QIAGEN GmbH Valencia, CA, USA), using 25–50 ng of DNA and 10 uM of each primer (Lep_F: 5’- TCAGCACCCAAAGCTGAAATTCTC-3’; Reverse_LE:5’-TGGGGAAGAGCTTTAATGCACGAT −3’; Reverse_LC: 5’- CGTCAATAGTGACAAGGTACTTGGA-3’ (Buglione et al. Citation2020b)). Cycling conditions were set up at an initial step for the HotStarTaq Plus DNA Polymerase activation for 5 min at 95°C, followed by 35 cycles of denaturation for 10 sec at 95°C, annealing for 30 sec at 62°C and extension for 10 sec at 72°C, activating fluorescence data acquisition on the green channel. The final melting step ramped from 65 to 80°C, with 0.1°C increments and 2 sec at each temperature. All runs included DNA from known Italian hare and European hare, as positive controls, and pure water as negative control. Two PCR replicates were performed for each sample.
Raw data were analysed using Rotor-Gene Q software v. 2.1.0 (QIAGEN GmbH Valencia, CA, USA), and the assignment of species was made by comparing the sample melting profile and the positive controls’ melting curves.
Diet characterisation
Isolation of DNA for diet analysis
The faecal pellets assigned to the European hare were processed for diet characterisation as reported by Buglione et al. (Citation2018). In brief, the innermost portion of the scats, with greater probability of containing the DNA of the ingested material, was used for the extraction of plant DNA by the hexade-cyltrimethylammonium bromide (CTAB) method (Doyle & Doyle Citation1987). Specifically, approximately 20 mg of material was homogenised in phosphate-buffered saline (PBS); after removing the soluble phase by high-speed centrifugation, isolated particles were suspended in CTAB buffer (2% CTAB, 100 mM TrisHCl pH 8.0, 20 mM Ethylen Diamino Tetracetyc Acid (EDTA), 1.4 M NaCl) with 0.2% β-mercaptoethanol and incubated at 70°C for 30 min. The DNA was isolated from lipids and proteins by mixing with chloroform/isoamyl alcohol (24:1) and centrifuging. Nucleic acids were precipitated with 70% isopropanol at −20°C overnight, then the supernatant was removed and the pellets were washed twice with 70% ethanol. Finally, the isolated DNA was dried and suspended in Tris-EDTA buffer. The contamination tests, integrity and purity of the isolated DNA were assessed as reported previously.
Sequencing on Illumina MiSeq platform
For diet characterisation, we used metabarcoding (Taberlet et al. Citation2007) and high-throughput sequencing (HTS) technology on faecal DNA. In particular, we followed the protocol reported by Buglione et al. (Citation2018). The PCRs were performed in 25 uL final volume reactions, with 50 ng of DNA as the template, 1 U of Taq Solis polymerase (BioDyne, Tartu, Estonia), 2.5 uL of 10X Buffer B (0.8 M Tris-HCl, 0.2 M (NH4)2 SO4, 0.2% w/v Tween-20), 2.5 uL of 25 mM MgCl2, 4 uL of 2.5 mM deoxyribonucleotide triphosphate (dNTP) mix and 2.5 uL of 25 uM of JK11 and JK14 primer mix (Yokota et al. Citation1989; Aceto et al. Citation1999; Frediani & Caputo Citation2005). The primers were modified with a specific adapter to the 5ʹ ends, aimed at recovering sequences from sample post-sequencing (Coissac Citation2012). Four independent PCR replicates were performed for each sample. To monitor the performance of the process and check for potential contamination, negative (pure water) controls were included in each amplification, as well as positive controls (a DNA mix containing 50 ng/uL from five plant families: Asteraceae, Fabaceae, Orchideaceae, Poaceae and Rosaceae). The PCR conditions were set up as an initial denaturation step for 3 min at 94°C, followed by 30 sec at 94°C, 45 sec at 52°C, and 45 sec at 72°C for 35 cycles, and, finally, an extension step for 7 min at 72°C.
After purification with Illustra GFX PCR DNA and Gel Band Purification Kit (GE Healthcare, Buckinghamshire, UK), PCR replicates were mixed in equimolar concentrations in all PCR samples. Finally, these were mixed to generate a unique pool representative of the European hare diet on the study area. The pool was tagged for a library with internal positive and negative controls to check for contamination, sequencing a sample containing a known microorganism. The library was tested using TapeStation (Agilent Technologies, Santa Clara, CA).
Library preparation and large-scale sequencing were performed at the Genomix4Life Srl (http://www.genomix4life.com/it/) on Illumina MiSeq platform (Illumina, Inc., San Diego, CA, USA) with a 2 × 300 bp paired-end run, following the Nextera DNA Sample Preparation protocol.
Processing of sequencing data
The quality control check of the short HTS raw reads was performed with the FastQC software v. 0.11.4 (http://www.bioinformatics.babraham.ac.uk). Subsequently, we trimmed and cropped the reads with a phred quality score of Q < 28 and a minimum length of 35 nucleotides, and clipped the primers and Illumina adapters using the Trimmomatic software v. 0.35 (Bolger et al. Citation2014). We removed the orphan reads and simultaneously processed for the analysis only paired reads, checking the filtering performance using, again, the FastQC program. The filtered reads were used for the assembly to create overlapping and contiguous sequences (contigs) with the SOAPdenovo2 127-mer (Luo et al. Citation2012) software. Then, we assigned each sequence to its taxonomic level by blasting against all nucleotide records at National Center for Biotechnology Information (NCBI) using the BLAST 2.3.0+ software (Camacho et al. Citation2009), selecting only the alignments with an E-value < 0.05, an identity >80.0%, and an alignment score >30%. We increased the accuracy of the automatic taxonomic assignation, filtering the blast results using a regional list of plant species (Ricciardi et al. Citation2000, Citation2016) (www.actaplantarum.org). When the same contig was assigned to more than two taxa, we selected the higher taxonomic level including all of them. We tagged as unclassified the sequences assigned only to a taxonomic level higher than family.
The mapping was performed using the bwa-0.7.12 (Li & Durbin Citation2009), samtools 1.3 (Li et al. Citation2009) and samstat 1.5.1 (Lassmann et al. Citation2011) software programs, aligning the reads against the reference contigs, previously assigned to the respective taxa during the blast analysis. Supplementary and secondary alignments were detected and removed with samtools 1.3 (see SAM Alignment/Map Format Specification at https://github.com/samtools/hts-specs). Finally, for each taxonomic assignment, we calculated the number of corresponding reads, retaining sequences with read counts > 2 for the barcoding identification (Mollot et al. Citation2014).
Results
We collected 50 faecal samples (10 pellets per month), of which 42 were genetically assigned to the European hare. For eight samples (one from April; one from May; two from June; four from July) species assignment was not possible because of DNA amplification failure, thus these were excluded for the subsequent analyses. For diet characterisation, we used DNA (N = 42) with a concentration of ≥ 50 ng/μL and both λ260/280 and λ260/230 > 1.60.
From 2,563,506 Illumina sequencing reads (35–301 bp), we obtained a total of 696,618 contigs, of which 621,215 found a match with blast records. At the end of the filtering, a total of 76,561 sequences had resulted from bioinformatic processing of data: 6.01% were identified to the family level, 23.90% were identified to the genus level and 70.08% were identified to the species level. No contamination was detected in the sequencing of the positive and negative controls.
The plants identified at these three taxonomic levels are included in 30 families. The Fabaceae, Polygonaceae and Poaceae (counts > 3500) were listed as the most representative, whereas the other families were represented in low percentages (<1%) ( and ).
Table I. Overview of the European hare diet on Vesuvius. Qualitative (family/taxon) and quantitative (read count; frequency of occurrence of each taxon in the corresponding family and in the total diet) analysis of the European hare diet. Family name reports the reads indeterminate within this taxonomic level. FO%, frequency of occurrence.
Figure 2. The European hare diet on Vesuvius. Read count (log10) for each plant taxon detected in the diet of the European hare. Dot size shows the frequency of occurrence (FO%) of each taxon in the corresponding plant family. * indicates items with FO% in the family <0.2.
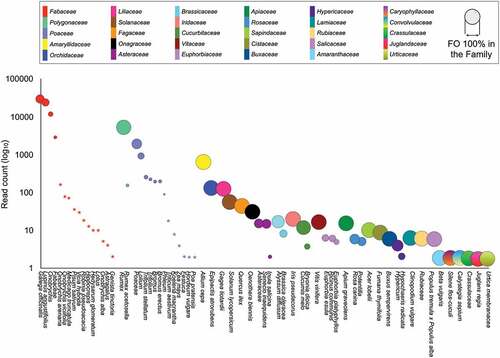
In particular, Fabaceae (86.26% of the total diet) showed major variability in terms of observed items (n = 15; excluding plant elements assigned only to family level), with Galega officinalis and Lupinus angustifolius accounting for more than half of the total diet (67.10%). Next, Polygonaceae (Rumex sp.) and Poaceae amount to 12.10% of the total diet. The remaining 1.64% of the total reads assigned accounted for 27 families, among which frequencies of occurrence of 0.82%, 0.18% and 0.16% were found for Amaryllidaceae, Orchidaceae and Liliaceae, respectively; meanwhile 0.46% included the other 24 families expressed at the lowest percentages (<0.07%).
Excluding the items identified only to the family level, the analysis showed a predominance of herbaceous species (93.81%) over arboreal ones (0.16%), of which the most abundant is Quercus ilex (). Also, the presence of cultivated species, such as Solanum lycopersicum (0.070%), Vitis vinifera (0.023%), Cucumis melo (0.018%), Brassica oleracea (0.010%), Zea mays (0.005%) and Hordeum vulgare (0.002%) can be highlighted, albeit in very low percentages.
Discussion
The European hare is an interesting herbivore to study considering its adaptability, with a broad-spectrum diet that changes in composition according to the diverse environments it colonises. Various studies have defined the variability of its diet in relation to different, even particularly extreme, environmental contexts (Puig et al. Citation2016, Citation2017; Naldi et al. Citation2020), but no one has ever taken into consideration the edibles of the species in a volcanic scenario.
Here, we analysed the diet of the European hare by using, for the first time on this species, a DNA metabarcoding approach, widely adopted for the characterisation of animals’ food intake (i.e. De Barba et al. Citation2014; Kartzinel et al. Citation2015; Goldberg et al. Citation2020; Buglione et al. Citation2020a, Citation2020c), allowing us to overcome many limitations related to the morphological method even if it is not without criticism (Holechek et al. Citation1982; Deagle et al. Citation2009; Soininen et al. Citation2009; Pompanon et al. Citation2012; Ingerson-Mahar Citation2014).
The diet of the hare on Vesuvius is mainly composed of herbaceous species, also commonly found along the roads, in uncultivated farmland and as pests in cultivated land, according to the dietary and environmental preferences reported in other studies (Reichlin et al. Citation2006; Karmiris et al. Citation2011; Schai-Braun et al. Citation2013, Citation2015; Buglione Citation2017). This result supports the interpretation of a food strategy that tends to avoid elements difficult to digest, such as woody parts, which contain higher proportions of less-digestible fibres compared with leaves (Dierenfeld et al. Citation2000). This could also provide the advantage of minimising the amount of ballast (Cheeke Citation1999), and hence also the weight handicap in the hare, which requires high running speeds to avoid predation (Hackländer et al. Citation2002).
The most frequent items ingested by the hare are goat’s rue (Galega officinalis) and blue (or narrow-leafed) lupin (Lupinus angustifolius). The former is usually present in Mediterranean and submediterranean areas of Italy below 1000 m a.s.l. in natural pasture and at the edge of cultivated fields (Lastrucci et al. Citation2010).
The vegetational and floristic characterisations available for the Vesuvian areas have not reported goat’s rue (Pasquale Citation1840; Licopoli Citation1873; Ricciardi et al. Citation1986, Citation2000, Citation2016), probably because such botanical studies devoted more attention to the native flora. Furthermore, low quantities of this species in historical times could explain its absence from the floristic-vegetational checklists. Indeed, intensive agriculture has been practised on the lower reaches of the slopes of Somma–Vesuvius since ancient times. However, these crops have undergone a progressive abandonment leading to a significant increase in vegetation with a low degree of naturalness, typical of ruderal areas and environments with a high degree of disturbance (Ricciardi et al. Citation2000).
The large amount of Galega officinalis in the diet could be traced back to a strategy used by females to stimulate milk production during lactation time, which occurred during our sampling period. In fact, a daily and controlled diet including goat’s rue is known to increase milk yield in animals, such as cows, sheep and rabbit (González‐Andrés et al. Citation2004; Penagos Tabares et al. Citation2014; Pałka et al. Citation2019; Darbyshire et al. Citation2022). However, it is not possible to know with certainty whether the presence of Galega officinalis was actually connected to the need for stimulating lactation in hare females because we did not determine the sex of the collected individuals and we pooled the samples for diet analysis. This opens the way for further investigations aimed at elucidating this aspect.
Furthermore, in some countries, goat’s rue has been included in lists of plants toxic to livestock (Parton & Bruere Citation2002), because of the potential poisoning effect due to the presence of some alkaloids, mainly galegine and derivatives (Patterson Citation1992; González‐Andrés et al. Citation2004). Thus, learning more about the systems used by the European hare to bypass such toxicity could be useful for optimising its use as forage for other animals (Peiretti & Gai Citation2006) or for human treatments, even if it is an exclusive adaptation developed by the European hare in this particular environmental context.
Blue lupin is a domestic legume crop species producing large seeds with up to 40% protein content. Different from wild lupins, in the domesticated forms, mainly growing at high altitudes as blue lupin on Vesuvius, seeds are less bitter and toxic because of minor quinolisidine alkaloid contents (below 0.02%) (Wink et al. Citation1995; Gresta et al. Citation2017). This sweet lupin is an attractive food source for various mammals, such rabbits and hares (Gresta et al. Citation2017). The lupin seeds, thanks to the lipid fraction, can be considered to fulfil an energy quota in animal nutrition (Hansen & Czochanska Citation1974; Musco et al. Citation2017). The hares prefer the parts of plants rich in fat, increasing energy assimilation, reducing foraging activity and producing milk with a high fat content (Hackländer et al. Citation2002; Smith et al. Citation2005).
The spectrum of available plants for hare on Vesuvius includes also other wild (i.e. Lolium sp., Bromus sp., Rumex sp.), cultivated (i.e. Solanum lycopersicum, Cucumis melo, Brassica oleracea, Allium cepa, Pisum sativum) or ornamental plant (i.e. Hypericum sp., Potentilla sp.) items (Pasquale Citation1840; Licopoli Citation1873; Ricciardi et al. Citation1986, Citation2000, Citation2016). The animals would have easy access to these resources, for example in plantations or farms, because they usually travel up to 1.8 km in search of suitable feeding areas, and may travel up to 15 km in one night while eating (Chapman & Flux Citation1990). However, these items are found only in trace quantities in the diet. All of this seems to suggests that although hares have the potential to take advantage of human-managed sites, they seem to persist in areas of volcanic environments, most frequently consuming plant species adapted to these soils, which sometimes are not the most abundant plants in the study area. To interpret these observations in the light of selective feeding behaviour, further investigations must be planned, also taking into account the movements of animals in volcanic areas, for example with video-trapping or radio-tracking.
The hare on Vesuvius plays a very important role, especially as prey for sedentary or migrating birds that use the Gran Cono as a resting place. Therefore, adequate management of the European hare is necessary, aiming at the maintenance of the species in a balanced relationship with the other components of the ecosystem. This can be achieved through the increasingly refined study of this population, to acquire basic information for understanding which elements of the environment to pay most attention to.
Last but not least, we must not overlook the potential role that the European hare could play in affecting the plant population dynamics and community structure through selective grazing and/or by the phenomenon of endozoochory (Chang et al. Citation2005; Green & Pickering Citation2013; Nomura & Tsuyuzaki Citation2015). In particular, this leads to a dispersal of seeds where the parental patches are not established, and pellets provide the gradual release of a long-term supply of seeds even in a fluctuating and disturbed ecosystem, such as on a volcano (Green & Pickering Citation2013). It would be interesting to investigate the possible role of the European hare on Vesuvius in the recolonisation process of the vegetation after the extensive fire affecting the area in 2017.
Author contributions
Conceptualisation: MB and DF; Methodology: MB and DF; Sample collection: MB, DF, GF and PC; Formal analysis: MB and DF; Investigation: MB and DF; Resources: DF and PC; Supervision: MB and DF; Writing – original draft: MB and DF; Writing – review & editing: MB, DF, GF and PC.
Disclosure statement
No potential conflict of interest was reported by the authors.
Data availability statement
Raw read data are available by request from the corresponding author.
Additional information
Funding
References
- Accardo Y, Caliendo M, de Filippo G, Foresta M, Fusco L, Rusch CE, Tomaselli E, Troisi S. 2008. Il Coniglio selvatico (Oryctolagus cuniculus) nel Parco Nazionale del Vesuvio. Dalla conoscenza alla gestione. Naples, Italy: Parco Nazionale del Vesuvio, Ottaviano. p. 64.
- Aceto S, Caputo P, Cozzolino S, Gaudio L, Moretti A. 1999. Phylogeny and evolution of orchis and allied genera based on ITS DNA variation: Morphological gaps and molecular continuity. Molecular Phylogenetics and Evolution 13(1):67–76. DOI: 10.1006/mpev.1999.0628.
- Angelici FM, Luiselli L. 2001. Distribution and status of the apennine Hare Lepus Corsicanus in continental Italy and Sicily. Oryx 35(3):245–249. DOI: 10.1046/j.1365-3008.2001.00182.x.
- Bagnaia R, Viglietti S, Laureti L, Giacanelli V, Ceralli D, Bianco PM, Loreto A, Luce E, Fusco L Carta della Natura della Regione Campania: Carta degli habitat alla scala 1:25.000, 2018.
- Bártová E, Sedlak K. 2012. Toxoplasmosis in animals in the czech republic - the last 10 years, and Djurkovi Djakovi O, editor. Toxoplasmosis - recent advances. Rijeka, Croatia: InTech. p. 200. DOI: 10.5772/50022.
- Bolger AM, Lohse M, Usadel B. 2014. Trimmomatic: A flexible trimmer for illumina sequence data. Bioinformatics 30(15):2114–2120. DOI: 10.1093/bioinformatics/btu170.
- Buglione M. 2017. Studio sulla nicchia ecologica di lepre italica (Lepus corsicanus). PhD thesis, Naples: University of Naples Federico II.
- Buglione M, Maselli V, Rippa D, de Filippo G, Trapanese M, Fulgione D. 2018. A pilot study on the application of DNA metabarcoding for non-invasive diet analysis in the Italian hare. Mammalian Biology 88:31–42. DOI: 10.1016/j.mambio.2017.10.010.
- Buglione M, Petrelli S, de Filippo G, Troiano C, Rivieccio E, Notomista T, Maselli V, Di Martino L, Carafa M, Gregorio R et al. 2020a. Contribution to the ecology of the Italian Hare (Lepus corsicanus). Scientific Reports 10(1):13071. DOI: 10.1038/s41598-020-70013-1.
- Buglione M, Petrelli S, Notomista T, de Filippo G, Gregorio R, Fulgione D. 2020b. Who is who? High Resolution melting analysis to discern between hare species using non-invasive sampling. Conservation Genetics Resources 12(4):727–732. DOI: 10.1007/s12686-020-01153-9.
- Buglione M, Petrelli S, Troiano C, Notomista T, Rivieccio E, Fulgione D. 2020c. The diet of otters (Lutra Lutra) on the agri river system, one of the most important presence sites in Italy: A molecular approach. PeerJ 8:e9606. DOI: 10.7717/peerj.9606.
- Camacho C, Coulouris G, Avagyan V, Ma N, Papadopoulos J, Bealer K, Madden TL. 2009. BLAST+: Architecture and Applications. BMC Bioinformatics 10(1):421. DOI: 10.1186/1471-2105-10-421.
- Caravaggi A, Montgomery WI, Reid N. 2015. Range expansion and comparative habitat use of insular, congeneric lagomorphs: Invasive European hares lepus Europaeus and endemic irish hares Lepus Timidus hibernicus. Biological Invasions 17(2):687–698. DOI: 10.1007/s10530-014-0759-1.
- Cardarelli E, Meriggi A, Brangi A, Vidus-Rosin A. 2011. Effects of arboriculture stands on european hare lepus europaeus spring habitat use in an agricultural area of Northern Italy. Acta Theriologica 56(3):229–238. DOI: 10.1007/s13364-010-0019-4.
- Castellaro G, Orellana CL, Escanilla JP. 2021. Summer diet of horses (Equus ferus caballus Linn.), Guanacos (Lama guanicoe Müller), and European Brown Hares (Lepus europaeus Pallas) in the high andean range of the coquimbo region, Chile. Animals 11(5):1313. DOI: 10.3390/ani11051313.
- Chagnon M, Kreutzweiser D, Mitchell EAD, Morrissey CA, Noome DA, Van der Sluijs JP. 2015. Risks of Large-scale use of systemic insecticides to ecosystem functioning and services. Environmental Science and Pollution Research 22(1):119–134. DOI: 10.1007/s11356-014-3277-x.
- Chang E, Zozaya E, Kuijper D, Bakker J. 2005. Seed dispersal by small herbivores and tidal water: Are they important filters in the assembly of salt-marsh communities? Functional Ecology 19(4):665–673. DOI: 10.1111/j.1365-2435.2005.01.
- Chapman J, Flux J. 1990. Rabbits, hares, and pikas: Status survey and conservation action plan. Gland, Switzerland: International Union for Conservation of Nature and Natural Resources.
- Chapuis J. 1990. Comparison of the diets of two sympatric lagomorphs, Lepus europaeus (Pallas) and Oryctolagus cuniculus (L.) in an Agroecosystem of the Ile-de-France. Z. Säugetierkd 55(3):176–185.
- Cheeke P. 1999. Applied animal nutrition: Feeds and feeding. 2nd ed. Englewood Cliffs, New Jersey: Prentice-Hall.
- Coissac E. 2012. OligoTag: A program for designing sets of tags for next-generation sequencing of multiplexed samples. In: Pompanon F, Bonin A, editors. Data production and analysis in population genomics. Methods in Molecular BiologyTM. Totowa, NJ: Humana Press. Vol. 888. pp. 13–31. DOI: 10.1007/978-1-61779-870-2_2.
- Darbyshire SJ, Francis A, Bromfield ESP, Mechanda S. 2022. The biology of canadian weeds: 158. Galega officinalis L. Canadian Journal of Plant Science 102(1):160–185. DOI: 10.1139/cjps-2020-0327.
- Davoli L, Fredi P, Russo F, Troccoli A. 2001. Natural and anthropogenic factors of flood hazards in the Somma-Vesuvius area (Italy)/Rôle des facteurs naturels et anthropiques sur les risques d’inondation autour du Vésuve-Somma (Italie). Morfo 7(3):195–207. DOI: 10.3406/morfo.2001.1102.
- Deagle BE, Kirkwood R, Jarman SN. 2009. Analysis of Australian Fur seal diet by pyrosequencing prey DNA in faeces. Molecular Ecology 18(9):2022–2038. DOI: 10.1111/j.1365-294X.2009.04158.x.
- De Barba M, Miquel C, Boyer F, Mercier C, Rioux D, Coissac E, Taberlet P. 2014. DNA metabarcoding multiplexing and validation of data accuracy for diet assessment: Application to omnivorous diet. Molecular Ecology Resources 14(2):306–323. DOI: 10.1111/1755-0998.12188.
- De Natale G, Troise C, Pingue F, Mastrolorenzo G, Pappalardo L. 2006. The Somma–vesuvius volcano (Southern Italy): Structure, dynamics and hazard evaluation. Earth-Science Reviews 74(1–2):73–111. DOI: 10.1016/j.earscirev.2005.08.001.
- Di Gennaro A, Aronne G, De Mascellis R, Vingiani S, Sarnataro M, Abalsamo P, Cona F, Vitelli L, Arpaia G. 2002. I Sistemi Di Terre Della Campania. Monografia e Carta 1:250.000. Con Legenda. Naples, Italy: University of Naples Federico II.
- Dierenfeld ES, Wildman REC, Romo S. 2000. Feed intake, diet utilization, and composition of Browses consumed by the Sumatran Rhino (Dicerorhinus Sumatrensis) in a North American Zoo. Zoo Biology 19(3):169–180. DOI: 10.1002/1098-2361(2000)19:3<169::AID-ZOO1>3.0.CO;2-D.
- Doyle J, Doyle J. 1987. A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochem Bulletin 19(1):11–15.
- Ducci D, Tranfaglia G. 2008. Effects of climate change on groundwater resources in campania (Southern Italy). Geological Society, London, Special Publications 288(1):25–38. DOI: 10.1144/SP288.3.
- Ebani VV, Poli A, Rocchigiani G, Bertelloni F, Nardoni S, Papini RA, Mancianti F. 2016. Serological survey on some pathogens in wild brown hares (Lepus europaeus) in central Italy. Asian Pacific Journal of Tropical Medicine 9(5):465–469. DOI: 10.1016/j.apjtm.2016.03.032.
- Edwards PJ, Fletcher MR, Berny P. 2000. Review of the factors affecting the decline of the european brown hare, Lepus europaeus (Pallas, 1778) and the use of wildlife incident data to evaluate the significance of paraquat. Agriculture, Ecosystems & Environment 79(2–3):95–103. DOI: 10.1016/S0167-8809(99)00153-X.
- Farrar JS, Wittwer CT. 2017. High-Resolution Melting curve analysis for molecular diagnostics. In: Patrinos GP, editor. Molecular diagnostics: London, UK: Academic Press. pp. 79–102. DOI: 10.1016/B978-0-12-802971-8.00006-7.
- Frediani M, Caputo P. 2005. Phylogenetic Relationships among annual and perennial species of the genus cicer as inferred from ITS sequences of nuclear ribosomal DNA. Biologia plantarum 49(1):47–52. DOI: 10.1007/s10535-005-7052-1.
- Freschi P, Fascetti S, Musto M, Cosentino C, Paolino R, Valentini V. 2015. Seasonal variation in food habits of the italian hare in a south apennine semi-natural landscape. Ethology Ecology & Evolution 1–15. DOI: 10.1080/03949370.2015.1022906.
- Freschi P, Fascetti S, Riga F, Rizzardini G, Fortebraccio M, Ragni M, Paolino R, Cosentino C. 2022. Diet selection by the Italian hare (Lepus corsicanus de Winton, 1898) in two protected coastal areas of latium. Animals 12(6):687. DOI: 10.3390/ani12060687.
- Fulgione D, Maselli V, Pavarese G, Rippa D, Rastogi RK. 2009. Landscape fragmentation and habitat suitability in endangered Italian hare (Lepus corsicanus) and European hare (Lepus europaeus) populations. European Journal of Wildlife Research 55(4):385–396. DOI: 10.1007/s10344-009-0256-5.
- Goldberg AR, Conway CJ, Tank DC, Andrews KR, Gour DS, Waits LP. 2020. Diet of a rare herbivore based on DNA metabarcoding of feces: Selection, seasonality, and survival. Ecology and Evolution 10(14):7627–7643. DOI: 10.1002/ece3.6488.
- González‐Andrés F, Redondo PA, Pescador R, Urbano B. 2004. Management of Galega officinalis L. and preliminary results on its potential for milk production improvement in sheep. New Zealand Journal of Agricultural Research 47(2):233–245. DOI: 10.1080/00288233.2004.9513591.
- Green K, Davis NE, Robinson WA, McAuliffe J, Good RB. 2013. Diet selection by European Hares (Lepus europaeus) in the alpine zone of the snowy mountains, Australia. European Journal of Wildlife Research 59(5):693–703. DOI: 10.1007/s10344-013-0723-x.
- Green K, Pickering C. 2013. Limited effect of hare grazing and short-term climatic variations on the most common alpine vegetation community in the snowy mountains, Australia. Plant Ecology & Diversity 6(3–4):511–522. DOI: 10.1080/17550874.2013.787466.
- Gresta F, Wink M, Prins U, Abberton M, Capraro J, Scarafoni A, Hill G. 2017. Lupins in European cropping systems. In: Murphy-Bokern D, Stoddard FL, Watson CA, editors. Legumes in cropping systems. Wallingford: CABI. pp. 88–108. DOI: 10.1079/9781780644981.0088.
- Hacklander K, Schai-Braun S. 2019. Lepus europaeus. The IUCN red list of threatened species 2019. DOI: 10.2305/IUCN.UK.2019-1.RLTS.T41280A45187424.en.
- Hackländer K, Tataruch F, Ruf T. 2002. The Effect of dietary fat content on lactation energetics in the European hare (Lepus europaeus). Physiological and Biochemical Zoology 75(1):19–28. DOI: 10.1086/324770.
- Hansen RP, Czochanska Z. 1974. Composition of the lipids of lupin seed (Lupinus angustifolius L. Var. “Uniwhite”). Journal of the Science of Food and Agriculture 25(4):409–415. DOI: 10.1002/jsfa.2740250409.
- Holechek J, Vavra M, Pieper R. 1982. Botanical composition determination of range herbivore diets: A review. Journal of Range Management 35(3):309–315. DOI: 10.2307/3898308.
- Ingerson-Mahar JM. 2014. Relating Diet and morphology of the head, mandibles and proventriculus in adult carabid beetles. Dissertation thesis. Graduate School-New Brunswick Rutgers, The State University of New Jersey. DOI: 10.7282/T3PG1Q6W.
- Jennings N, Smith RK, Hackländer K, Harris S, White PCL. 2006. Variation in Demography, condition and dietary quality of hares lepus europaeus from high-density and low-density populations. Wildlife Biology 12(2):179–189. DOI: 10.2981/0909-6396(2006)12[179:VIDCAD]2.0.CO;2.
- Karmiris I, Platis PD, Kazantzidis S, Papachristou TG. 2011. Diet selection by domestic and wild herbivore species in a coastal mediterranean wetland. Annales Zoologici Fennici 48(4):233–242. DOI: 10.5735/086.048.0404.
- Kartzinel TR, Chen PA, Coverdale TC, Erickson DL, Kress WJ, Kuzmina ML, Rubenstein DI, Wang W, Pringle RM. 2015. DNA metabarcoding illuminates dietary niche partitioning by African large herbivores. Proceedings of the National Academy of Sciences of the United States of America 112(26):8019–8024. DOI: 10.1073/pnas.1503283112.
- Kasapidis P, Suchentrunk F, Magoulas A, Kotoulas G. 2005. The shaping of mitochondrial DNA phylogeographic patterns of the brown hare (Lepus europaeus) under the combined influence of late pleistocene climatic fluctuations and anthropogenic translocations. Molecular Phylogenetics and Evolution 34(1):55–66. DOI: 10.1016/j.ympev.2004.09.007.
- Lassmann T, Hayashizaki Y, Daub CO. 2011. SAMStat: Monitoring biases in next generation sequencing data. Bioinformatics 27(1):130–131. DOI: 10.1093/bioinformatics/btq614.
- Lastrucci L, Landi M, Angiolini C. 2010. Vegetation analysis on wetlands in a tuscan agricultural landscape (Central Italy). Biologia 65(1):54–68. DOI: 10.2478/s11756-009-0213-5.
- Licopoli G. 1873. Storia naturale delle piante crittogame che crescono sulle lave vesuviane. Atti della Accademia Scuola Fisiche, Matematiche 3(2):1–58.
- Li H, Durbin R. 2009. Fast and accurate short read alignment with burrows-wheeler transform. Bioinformatics 25(14):1754–1760. DOI: 10.1093/bioinformatics/btp324.
- Li H, Handsaker B, Wysoker A, Fennell T, Ruan J, Homer N, Marth G, Abecasis G, Durbin R. 2009. 1000 genome project data processing subgroup. The sequence alignment/map format and SAMtools. Bioinformatics 25(16):2078–2079. DOI: 10.1093/bioinformatics/btp352.
- Luo R, Liu B, Xie Y, Li Z, Huang W, Yuan J, He G, Chen Y, Pan Q, Liu Y et al. 2012. SOAPdenovo2: An empirically improved memory-efficient short-read de novo assembler. GigaScience 1(1):18. DOI: 10.1186/2047-217X-1-18.
- Mengoni C, Mucci N, Randi E. 2015. Genetic diversity and no evidences of recent hybridization in the endemic Italian hare (Lepus Corsicanus). Conservation Genetics 16(2):477–489. DOI: 10.1007/s10592-014-0674-0.
- Mollot G, Duyck P-F, Lefeuvre P, Lescourret F, Martin J-F, Piry S, Canard E, Tixier P. 2014. Cover cropping alters the diet of arthropods in a banana plantation: A metabarcoding approach. PLoS One 9(4):e93740. DOI: 10.1371/journal.pone.0093740.
- Musco N, Cutrignelli MI, Calabrò S, Tudisco R, Infascelli F, Grazioli R, Lo Presti V, Gresta F, Chiofalo B. 2017. Comparison of nutritional and antinutritional traits among different species (Lupinus albus L, Lupinus luteusin L, Lupinus angustifolius L.) and varieties of lupin seeds. Journal of Animal Physiology and Animal Nutrition 101(6):1227–1241. DOI: 10.1111/jpn.12643.
- Naldi L, Greco I, Ferretti M, Zaccaroni M. 2020. Density estimates and habitat preferences of the European hare (Lepus europaeus) on mountainous areas in Italy. Mammal Study 45(2):123. DOI: 10.3106/ms2019-0057.
- Nomura N, Tsuyuzaki S. 2015. Hares promote seed dispersal and seedling establishment after volcanic eruptions. Acta Oecologica 63:22–27. DOI: 10.1016/j.actao.2015.02.003.
- Pałka S, Kmiecik M, Migdał Ł, Siudak Z. 2019. The effect of a diet containing fennel (Foeniculum Vulgare Mill.) and goat’s-rue (Galega officinalis L.) on litter size and milk yield in rabbits. Science Annalen Polonica Society Animal Products 15(4):73–78. DOI: 10.5604/01.3001.0013.6484.
- Parton K, Bruere A. 2002. Plant poisoning of livestock in New Zealand. New Zealand Veterinary Journal 50(sup3):22–27. DOI: 10.1080/00480169.2002.36262.
- Pasquale G. 1840. La Flora Del Vesuvio. Esercito Accademia Aspiranti Naturalisti 2(1):23–53.
- Patterson DT. 1992. Effect of temperature and photoperiod on growth and reproductive development of goatsrue. Journal of Range Management 45(5):449. DOI: 10.2307/4002901.
- Peiretti PG, Gai F. 2006. Chemical composition, nutritive value, fatty acid and amino acid contents of Galega officinalis L. during its growth stage and in regrowth. Animal Feed Science and Technology 130(3–4):257–267. DOI: 10.1016/j.anifeedsci.2006.01.007.
- Penagos Tabares F, Bedoya Jaramillo JV, Ruiz-Cortés ZT. 2014. Pharmacological overview of galactogogues. Veterinary Medicine International 2014:1–20. DOI: 10.1155/2014/602894.
- Pépin D, Angibault JM. 2007. Selection of resting sites by the european hare as related to habitat characteristics during agricultural changes. European Journal of Wildlife Research 53(3):183–189. DOI: 10.1007/s10344-007-0087-1.
- Pierpaoli M, Riga F, Trocchi V, Randi E. 1999. Species distinction and evolutionary relationships of the Italian hare (Lepus corsicanus) as described by mitochondrial DNA sequencing. Molecular Ecology 8(11):1805–1817. DOI: 10.1046/j.1365-294x.1999.00766.x.
- Pierpaoli M, Riga F, Trocchi V, Randi E. 2003. Hare populations in Europe: Intra and interspecific analysis of MtDNA variation. Comptes Rendus Biologies 326:80–84. DOI: 10.1016/S1631-0691(03)00042-8.
- Pietri C. 2015. Range and status of the Italian hare lepus corsicanus in corsica. Hystrix, the Italian Journal of Mammalogy 26(2). DOI: 10.4404/hystrix-26.2-11412.
- Pompanon F, Deagle BE, Symondson WOC, Brown DS, Jarman SN, Taberlet P. 2012. Who is eating what: Diet assessment using next generation sequencing: NGS diet analysis. Molecular Ecology 21(8):1931–1950. DOI: 10.1111/j.1365-294X.2011.05403.x.
- Puig S, Rosi MI, Videla F, Méndez E. 2016. Diet of brown hare (Lepus europaeus) and food availability in high andean mountains (Mendoza, Argentina). Mammalia 80(3):3. DOI: 10.1515/mammalia-2014-0142.
- Puig S, Rosi MI, Videla F, Mendez E. 2017. Flexibility in the food selection by the European hare (Lepus europaeus) along the altitudinal gradient of the southern andean precordillera (Argentina). Mammal Research 62(1):75–87. DOI: 10.1007/s13364-016-0288-7.
- Puig S, Videla F, Cona MI, Monge SA. 2007. Diet of the brown hare (Lepus Europaeus) and food availability in northern patagonia (Mendoza, Argentina). Mammalian Biology 72(4):240–250. DOI: 10.1016/j.mambio.2006.08.006.
- Reichlin T, Klansek E, Hackländer K. 2006. Diet selection by hares (Lepus europaeus) in arable land and its implications for habitat management. European Journal of Wildlife Research 52(2):109–118. DOI: 10.1007/s10344-005-0013-3.
- Ricciardi M, Aprile G, La Valv V, Caputo G. 1986. La flora del somma‑vesuvio. Bollettino della Società dei naturalisti in Napoli 95(1):3–121.
- Ricciardi M, Mazzoleni S, La Valva V. 2000. La flora e la vegetazione del Somma-Vesuvio. In: Ente Parco Nazionale del Vesuvio Naples (Italy), editor. Elementi di biodiversità del Parco Nazionale del Vesuvio. pp. 51–65.
- Ricciardi M, Motti R, Stinca A. 2016. Flora illustrata del Vesuvio: Storia, paesaggi, vegetazione. Doppiavoce: Napoli.
- Santacroce R. 1987. Somma-Vesuvius, CNR, Quaderni de la Ricerca Scientifica, 114, Progetto Finalizzato Geodinamica, Monografie Finali. Vol. 8. Roma, Italy: Consiglio Nazionale delle Ricerche. p. 251.
- Schai-Braun SC, Reichlin TS, Ruf T, Klansek E, Tataruch F, Arnold W, Hackländer K. 2015. The European hare (Lepus Europaeus): A picky herbivore searching for plant parts rich in fat. PLoS ONE 10(7):e0134278. DOI: 10.1371/journal.pone.0134278.
- Schai-Braun SC, Weber D, Hackländer K. 2013. Spring and autumn habitat preferences of active European hares (Lepus europaeus) in an agricultural area with low hare density. European Journal of Wildlife Research 59(3):387–397. DOI: 10.1007/s10344-012-0684-5.
- Sliwinski K, Ronnenberg K, Jung K, Strauß E, Siebert U. 2019. Habitat requirements of the European brown hare (Lepus europaeus Pallas 1778) in an intensively used agriculture region (Lower Saxony, Germany). BMC Ecology 19(1):31. DOI: 10.1186/s12898-019-0247-7.
- Smith AT. 2008. Conservation of endangered lagomorphs. In: Alves PC, Ferrand N, Hackländer K, editors. Lagomorph biology. Berlin Heidelberg: Springer Berlin Heidelberg. pp. 297–315. DOI: 10.1007/978-3-540-72446-9_20.
- Smith RK, Vaughan Jennings N, Harris S. 2005. A quantitative analysis of the abundance and demography of European Hares Lepus europaeus in relation to habitat type, intensity of agriculture and climate. Mammal Review 35(1):1–24. DOI: 10.1111/j.1365-2907.2005.00057.x.
- Soininen EM, Valentini A, Coissac E, Miquel C, Gielly L, Brochmann C, Brysting AK, Sønstebø JH, Ims RA, Yoccoz NG et al. 2009. Analysing diet of small herbivores: The efficiency of DNA barcoding coupled with high-throughput pyrosequencing for deciphering the composition of complex plant mixtures. Frontiers in Zoology 6(1):16. DOI: 10.1186/1742-9994-6-16.
- Stamatis C, Suchentrunk F, Moutou KA, Giacometti M, Haerer G, Djan M, Vapa L, Vukovic M, Tvrtković N, Sert H et al. 2009. Phylogeography of the brown hare (Lepus europaeus) in Europe: A legacy of South-Eastern mediterranean refugia? Journal of Biogeography 36(3):515–528. DOI: 10.1111/j.1365-2699.2008.02013.x.
- Storkey J, Meyer S, Still KS, Leuschner C. 2012. The impact of agricultural intensification and land-use change on the European Arable flora. Proceedings of the Royal Society B: Biological Sciences 279(1732):1421–1429. DOI: 10.1098/rspb.2011.1686.
- Taberlet P, Coissac E, Pompanon F, Gielly L, Miquel C, Valentini A, Vermat T, Corthier G, Brochmann C, Willerslev E. 2007. Power and limitations of the chloroplast TrnL (UAA) intron for plant DNA barcoding. Nucleic Acids Research 35(3):e14–e14. DOI: 10.1093/nar/gkl938.
- Thulin C-G. 2003. The distribution of mountain hares Lepus timidus in Europe: A challenge from brown hares L. europaeus?: distribution of European mountain hares. Mammal Review 33(1):29–42. DOI: 10.1046/j.1365-2907.2003.00008.x.
- Trocchi V, Riga F. 2005. I lagomorfi in Italia : Linee guida per la conservazione e gestione. Ozzano dell’Emilia: Istituto Nazionale per la fauna selvatica Alessandro Ghigi.
- Tsokana CN, Sokos C, Giannakopoulos A, Birtsas P, Valiakos G, Spyrou V, Athanasiou LV, Rodi Burriel A, Billinis C. 2020. European Brown hare (Lepus europaeus) as a source of emerging and re-emerging pathogens of public health importance: A review. Veterinary Medicine and Science 6(3):550–564. DOI: 10.1002/vms3.248.
- Wink M, Meißner C, Witte L. 1995. Patterns of quinolizidine alkaloids in 56 species of the genus lupinus. Phytochemistry 38(1):139–153. DOI: 10.1016/0031-9422(95)91890-D.
- Yokota Y, Kawata T, Iida Y, Kato A, Tanifuji S. 1989. Nucleotide sequences of the 5.8S RRNA gene and internal transcribed spacer regions in carrot and broad bean ribosomal DNA. Journal of Molecular Evolution 29(4):294–301. DOI: 10.1007/BF02103617.
