Abstract
The majority of jumping spiders are visual hunters that capture a wide range of prey. While they are known to use specific predatory techniques against different prey, their prey identification mechanisms are poorly understood. A generalist jumping spider, Yllenus arenarius, employs different predatory techniques to capture prey with two different escape potentials. The aim of the present study was to identify the characteristics used by the spider to classify prey into one of these categories. Freshly-emerged spiderlings were used in the experiments to analyse pre-programmed visual predatory preferences. The spiders were presented with: a) their natural prey with different escape potentials (flies, thrips, caterpillars) and b) video images constructed from different combinations of features of their natural prey. The images varied with regard to five characteristics: body length (short vs long images), the presence or absence of details (0 or 4 details, including head spot, antennae, legs, wings), the local motion of legs (moving vs still), the type of global motion (crawling vs non-crawling) and the direction of global motion (horizontal motion vs vertical small scale jumps). Prey-specific behaviours indicated which characteristics were used by the spider to ascertain the prey’s escape potentials. Our findings indicate that during visual prey categorization, Y. arenarius can rely solely on general prey characteristics, such as body proportions and the type of prey motion, while ignoring other stimuli, such as the presence of details and the local motion of legs. This mechanism of prey identification, based on these two easily-recognizable prey characteristics, enables fairly quick and precise categorization of a wide range of prey according to their escape potentials. The study shows how generalist jumping spiders can categorize their diverse prey into a limited number of groups and discusses the presence of the mechanism in other jumping spiders and other animals.
Introduction
It is believed that animals are able to recognise objects by perceiving their physical characteristics and by assigning certain semantic features to perceived objects. This process enables them to classify the objects to categories, such as predators, prey, mates, others and distinguish between them. The study of visual object recognition has a long tradition going back to the early twentieth century and the competing theoretical frameworks of Gestalt Psychology, originating in the work of Max Wertheimer, and the structuralism of Wilhelm Wundt (Wagemans et al. Citation2012). The discussion has since evolved to include two concepts: global and local processing. The former postulates that all the elements of an object must be perceived to recognize it, while the latter assuming that only certain key elements need to be identified (Kimchi Citation1992; Förster & Higgins Citation2005). These hypotheses can be tested by establishing the cues used in visual identification. This has been widely performed in higher vertebrates, such as birds and mammals, as well as humans (Kourtzi & Connor Citation2011; Soto & Wasserman Citation2014), but computationally-limited animals, such as invertebrates and lower vertebrates, have been highly understudied (Enns Citation2004). Nevertheless, visual object recognition has been studied in toads and frogs (Ingle Citation1983; Ewert Citation2004), mantids (Kral & Prete Citation2004), cuttlefish (Darmaillacq et al. Citation2004) and jumping spiders (Forster Citation1985; Bednarski et al. Citation2012; Jackson & Nelson Citation2012; Nelson & Jackson Citation2012a,Citationb; Dolev & Nelson Citation2014; Citation2016).
In toads and praying mantids, most studies have examined the characteristics used to distinguish prey from non-prey. It was found that the key features appear to be the movement and the shape of the object. Toads primarily rely on the motion of an object, its size and its length-to-width ratio in relation to the direction of movement (Wachowitz & Ewert Citation1996; Ewert Citation2004), while praying mantids look for the size of a compact stimulus, the length of the leading edge of an elongated stimulus, its contrast with the background, location of the stimulus in the visual field and the speed of the stimulus (Prete Citation1992a; Citationb, Citation1993; Prete & Mahaffey Citation1993; Prete & McLean Citation1996, Citation1999; Kral & Prete Citation2004).
The experiments with toads and mantids provided an insight into the characteristics used during prey identification by generalist predators. These findings have been extended by other studies on jumping spiders that have shown how highly specialized predators, such as Portia spp. and Evarcha culicivora, identify their prey. Portia, a jumping spider that specializes in capturing other jumping spiders, uses its principal eyes as a major cue characteristic to distinguish between its preferred prey and other objects (Harland & Jackson Citation2000, Citation2002). In addition, E. culicivora uses female mosquito-specific cues to feed on blood-filled Anopheles mosquitoes; these clues include its characteristic resting posture, the blood-fed abdomen, and the thorax, head and the antennae of the mosquito (Jackson et al. Citation2005; Nelson & Jackson Citation2006, Citation2012b). Interestingly, not only can an abstract stick figure be used to fool the spider into recognising an Anopheles mosquito, but also a figure composed of disarranged and disconnected elements (Dolev & Nelson Citation2014).
Highly-specialized jumping spiders, such as Portia and Evarcha culicivora, were tested with stationary objects, which corresponds to the scenes these spiders often encounter in natural circumstances. Such identification and categorization of stationary objects based on configurational cues, a highly-demanding visual task initially attributed only to highly-specialized spiders, has recently been found to also occur in nonspecialized euryphagous salticids, such as Salticus scenicus; this common jumping spider was found to be able to detect, recognize and appropriately respond to static predators (other jumping spiders) using salticid principal eyes as a major cue (Rößler et al. Citation2022).
In addition, motion patterns have also been found to play a part in object identification, in invertebrates: Menemerus semilimbatus was reported to be able to distinguish biological motion from nonbiological motion (De Agrò et al. Citation2021). It has been suggested that motion pattern may be used by a number of jumping spiders (Edwards & Jackson Citation1993, Citation1994; Bartos Citation2008; Bartos & Szczepko Citation2012), and this has been supported by experiments based on animations of modified prey with different motion patterns. As such, prey locomotion type has been added to the list of possible cues used in object recognition by jumping spiders (Nelson & Card Citation2016, Shamble et al. Citation2017).
These elegant studies have revealed that jumping spiders can be extremely discerning predators (Jackson & Pollard Citation1996; Harland & Jackson Citation2004; Nelson & Jackson Citation2012a); however, both Portia and E. culicivora, being highly-specialized oligophagous predators, are rather atypical of the majority of Salticidae, the most diverse spider family with over 6400 species and 662 genera (World Spider Catalog Citation2022), which seems to consist mainly of euryphagous species. Most jumping spiders capture a diverse range of prey, often using prey-specific techniques. Evidence suggests that these spiders seek a wide range of prey, categorizing them into different groups based on prey-specific characteristics. Moreover, it has been reported that some prey capable of efficient escape, such as flies, leafhoppers and grasshoppers, are captured in a similar manner by various salticids (Forster Citation1977, Citation1982; Freed Citation1984; Jackson Citation1988a; Richman & Jackson Citation1992; Edwards & Jackson Citation1993, Citation1994; Li et al. Citation1996; Bear & Hasson Citation1997, Citation1999; Bartos Citation2002; Nelson et al. Citation2005, Citation2007), suggesting that at least some generalist jumping spiders use common characteristics during prey identification. However, the wide morphological diversity demonstrated by the prey, such as the presence of wings and antennae, the appearance of legs, and their sizes, shapes and colours, beg the question of how they can be recognised by the spiders.
The study has three key aims. It examines whether a generalist visual predator capturing a wide range of diverse prey can 1) identify an object as prey based on simplified visual information (body outline with certain proportions of length to width devoid of any details (head spot, antennae, legs, wings) and natural forward movement; 2) use simplified information to discriminate between prey with high or low escape potentials (short vs long body, crawling vs non-crawling motion); 3) use prey details enhanced by the motion of legs in discrimination and categorization. In addition, the study aims to confirm whether virtual prey images are accepted by the spiders as equivalents of the natural prey; this was tested using highly-simplified images based on natural prey.
Jumping spiders are highly visual animals that depend on their exceptional eyesight during predation (Nelson & Jackson Citation2011), as well as communication with conspecifics (Forster Citation1982; Uhl & Elias Citation2011), locomotion and other activities (Jakob et al. Citation2011). Their modular visual system consists of eight simple eyes spaced around the carapace, which provide a combined visual field of about 360°. Six pairs of small “secondary eyes” positioned along the sides of the carapace have wide visual fields, but relatively low resolution (Land & Nilsson Citation2001; Zurek et al. Citation2010), while a pair of large forward-facing “principal eyes” has very high spatial resolution and enables these spiders to discern even very small image details (Williams & McIntyre Citation1980; Harland et al. Citation2012), perceive colours (Blest et al. Citation1981; Peaslee & Wilson Citation1989) and estimate distance (Nagata et al. Citation2012). In contrast to the secondary eyes, the principal eyes possess a very narrow horizontal visual field of about 2–5°, which can be enlarged to about 60° when the eye tubes move (Land Citation1969). In this modular visual system, the secondary eyes function primarily, but not exclusively, as movement detectors. They also initiate optomotor responses to an object that has entered the visual field, which allow the spider to face the object and engage in further visual inspection with the high-resolution principal eyes (Zurek & Nelson Citation2012a,Citationb). The secondary eyes also mediate responses to looming objects (Spano et al. Citation2012), thus possibly playing a major role in initiating escape.
To examine how a euryphagous predator categorizes prey based solely on visual signals, the study used Yllenus arenarius Menge 1868, a euryphagous jumping spider with a natural diet consisting of over 50 species of spiders and insects ranging from flies, wasps, ants and caterpillars, to thrips, aphids and antlion larvae (Bartos Citation2004, Citation2011) (). During prey capture, the spider uses a conditional predatory strategy with two easily-distinguishable prey-specific predatory techniques (Bartos Citation2002): one is used when capturing prey with high escape potentials, such as flies, orthopterans or wasps, and the other when capturing prey with low escape potentials, such as thrips or caterpillars. Both predatory techniques are observed during capture by freshly-hatched spiderlings, which suggests that these techniques, and the prey identification mechanisms, are preprogramed (Bartos Citation2008, Citation2013; Bartos & Szczepko Citation2012). Y. arenarius is also known to use a preprogramed identification mechanism when targeting predatory strikes, which has been revealed in a study (Bartos & Minias Citation2016) exploiting a similar experimental setup to that used herein. The authors manipulated the number of head-indicating details (ranging from prey with four details, including a head spot, antennae, legs and wings, to prey lacking any details), the position of these details in relation to motion direction (in the leading part of the body versus the trailing part), the local motion of legs and the presence of horizontal motion. They found that the spiders identify a preferred target on the prey’s body using the direction of the prey’s motion and the complex arrangement of head-indicating details.
Figure 1. Yllenus arenarius with its natural prey: A) fly, B) orthopteran, C) wasp, D) butterfly caterpillar.

The present study uses images that differed according to five characteristics (): a) body length (short vs long), b) the presence of horizontal motion (images proceeding forwards vs those staying in the same place but performing only slight vertical jerks to attract the spider’s attention), c) the type of global motion (crawling like a caterpillar vs moving without caterpillar-like crawls), d) the complexity of the body (0 vs 4 details, viz. head spot, legs, wings and antennae), e) the local motion of the legs (legs moving vs legs still).
Table I. The characteristics of images used in the tests: body length (short vs long); the presence of horizontal motion (images proceeding forward vs not proceeding); the type of global motion (crawling like a caterpillar vs moving without caterpillar-like crawls); the number of body details (0 vs 4 details, including head spot, legs, wings and antennae); the local motion of legs (legs moving vs legs still).
Methods
General methods
The methods used in this study are similar to those described by Bartos and Minias (Citation2016). In this study, the same model spider and similar prey characteristics were used to analyse the cues used in targeting predatory strikes and test the efficacy of a “false head”, an antipredator adaptation used by some animals to deflect initial predatory strikes.
The spiders tested in this study were newly-hatched Yllenus arenarius (mean body length of 1.71 ± 0.11 [SD] mm) collected in Central Poland (Kwilno, Zgierz County). In order to collect the freshly-emerged spiderlings, dune patches were inspected daily, starting about two weeks before their expected time of hatching (Bartos Citation2005). The spiderlings were separately transported and caged in the lab. The spiders were not fed before the tests. Each spiderling was used only once and after two days, it was released in the field. Tests were carried out between 0900 h and 1600 h (laboratory photoperiod 12 L:12D, lights on at 0700 h).
The predatory behaviours of test spiders (prey noticing, stalking, frontal approach and pouncing) were recorded during all experiments. Prey type was indicated by the use of stalk and frontal approach, previously recorded as specific for capturing prey with high (stalk) or low (frontal approach) escape potentials (Edwards & Jackson Citation1993, Citation1994; Bartos Citation2007). Stalk is a slow choppy gait combined with visual fixation on the prey. Frontal approach on the other hand, is characterized by a quick walk or run towards the prey. The path of this movement is arc-shaped and it is accompanied by, at least temporary, principal eye-fixation on the prey, which results in sideways movement by the spider in the final part of approach. The spider stops several millimetres in front of the prey on its predicted path, turns to the prey and waits for the prey to approach. Finally the spider pounces on the prey. All behaviours used in this study as indicators of decisions by the spider (noticing, frontal approach, stalking and pouncing) are reliably identifiable and have already been used in other studies with various salticids (Forster Citation1977; Nelson & Jackson Citation2012b; Dolev & Nelson Citation2014) including Y. arenarius (Bartos Citation2008, Citation2011).
The study consisted of two experiments: Experiment 1, which uses natural prey (flies, thrips, caterpillars), and Experiment 2, using video images of virtual prey possessing different combinations of the features demonstrated by natural prey used in Experiment 1.
Experiment 1 – with natural prey
This experiment used three species of natural prey with different escape potentials and different appearances: Drosophila melanogaster (flies), Chirothrips manicatus (thrips) and the larvae of Pyralis farinalis (butterfly caterpillars). The aim of this experiment was to record the predatory behaviour demonstrated against natural prey; the recordings would be used for comparison with the behaviour expressed by the spiders approaching the virtual prey used in Experiment 2. All the insects used in Experiment 1 are readily captured by juvenile Y. arenarius in the field and in the laboratory (Bartos Citation2004, Citation2011). The flies and the caterpillars were obtained from laboratory culture. Thrips were collected in the field on the day of the experiment or the previous day. All prey used in the tests were within the size range of ±20% of the spider’s body length. Each prey was chosen randomly for each test.
The test arena for natural prey experiments was a white cardboard cylinder (height: 150 mm; diameter: 200 mm) with a 10 mm thick layer of sand on the bottom. An incandescent light bulb (100 W) and a CCD camera with a macro lens were placed about 0.5 m above the centre of the arena. The camera was connected to a PC clone computer with a video card (Matrox Marvel G450 eTV).
The test started when a spider was placed in the arena. After one minute, a prey animal was dropped at a distance of about 80 mm from the spider. In order to record when the spider noticed the prey it was placed 30°–40° to the left or to the right from the visual axis of the spider’s AM eyes (position chosen at random). Before the tests, each prey was kept for 15 min in a refrigerator (5°C) to decrease its mobility and the risk of escape from the arena. Each prey was left with the spider for five minutes or until the prey was attacked. The test was aborted when the spider or prey climbed the walls of the arena or when the prey escaped.
Between tests, the surface layer of the sand was removed to dispose of any draglines and chemical signals from a previously-used prey or a spider. The arena was refilled with new sand and evened with a wooden tile to the original level. Each individual was used in one test for only one type of prey. During the test, the spider was recorded and observed on the monitor and was unable to see the experimenter.
Experiment 2 – with virtual prey
The stimuli consisted of videos of continuously-moving images created using Macromedia Flash 8 in greyscale (). The images differed with respect to the five characteristics given above: body length (short images with a length-to-height ratio of 3/1 vs long images with a ratio of 10/1); the presence or absence of details (0 or 4 details, including head spot, antennae, legs, wings); the local motion of legs (moving vs still); the type of global motion (crawling vs non-crawling) and the direction of global motion (horizontal motion vs vertical small scale jerks).
The global motion (horizontal and vertical) of the whole image and the local motion of the details were modelled on those demonstrated by the natural prey used in Experiment 1. All stimuli consisted of simplified images based on the general characteristics of flies (D. melanogaster), thrips (C. manicatus) and caterpillars (P. farinalis). Short images were based on body proportions of flies (D. melanogaster), while long images were based on body proportions of thrips (C. manicatus) and caterpillars (P. farinalis). The length on the screen of the short image was 1.17 mm, and of the long image was 3.89 mm. Both image lengths are within the preferred prey size range of juvenile Y. arenarius (Bartos Citation2011).
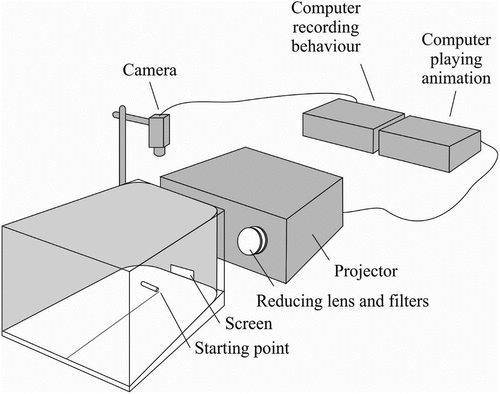
The videos were displayed by a projector on a screen mounted into a wall of the cardboard arena (). The arena itself was a white cardboard cuboid with an isosceles trapezium as the bottom (height: 100 mm, trapezium legs: 250 mm, wide base: 200 mm, narrow base: 100 mm). The images were projected onto an unmarked fine = grained matt glass screen (height: 30 mm, length: 30 mm) built in the narrowest wall of the arena. The top half of the screen was masked to focus the attention of the spider on the bottom half with a displayed image. The arena was filled with dune sand, which is the natural substrate for Y. arenarius, successfully used in earlier studies with freshly-hatched juvenile spiderlings (Bartos Citation2008; Bartos & Szczepko Citation2012).
The movies were rendered by a SHARP XR-10X-L data projector mounted about 100 mm behind the screen (1400 x 1050 pixels). The brightness of the image was reduced with neutral-density filters mounted between the screen and the projector. To display life-size images in high resolution, which makes them more realistic for visually-efficient jumping spiders, the images were displayed large and their size was subsequently reduced with a lens mounted between a projector and the screen. A similar approach was used in other studies with jumping spiders (Harland & Jackson Citation2002; Nelson & Jackson Citation2006, Citation2012b; Dolev & Nelson Citation2014). The videos were played on a continuous loop using Macromedia Flash Player 8. The images in the videos moved from one side of the screen to the other, then disappearing off the screen for 5 s, which resembled disappearing behind an obstacle. Following this, they re-entered the screen from the side they disappeared and moved in the opposite direction.
At the beginning of the test, a spider was placed in the centre of the arena, at a distance of 25 mm from the screen: this equals about 15 body lengths of juvenile Y. arenarius. Before the test, the spider was kept in an opaque tube (length: 80 mm, inner diameter: 8 mm) sealed on one side with a plug (). The tube was positioned parallel to the screen to prevent the spider from seeing the screen after the plug’s removal. This allowed the moment when the spider faced the screen and noticed the image to be recorded. If the spider did not leave the tube within 10 minutes after plug removal, or jumped out of the tube during or after plug removal, or if the spider left the arena without noticing the image on the screen, the trial was excluded from the analyses. The playback projection started before the plug was removed, as the timing of playback initiation may influence the spider’s decision (Clark & Uetz Citation1993). During the test, the spider was recorded and observed on a monitor and was unable to see the experimenter.
Testing was carried out between 0900 and 1600 hours. In order to remove any draglines and chemical cues from previously-used spiders, the sand was brushed after each test and a 5 mm thick surface of sand was removed. The arena was refilled with new sand and evened with a wooden tile to the original level. After each test, the screen was wiped with a piece of cotton dipped in 95% ethanol and the screen was allowed to dry between trials. No individual spider was used in more than one test of any one image type.
Behavioural analyses
Video recordings were analysed frame-by-frame using VLC media player 3.0.11 Vetinari (http://www.videolan.org/). During the analyses it was recorded whether the spider engaged in prey noticing, frontal approach, stalking or pouncing.
Statistical analyses
The effects of body proportions and motion types, as well as the presence of details and the local motion of legs on the frequency of stalk and frontal approach were tested using logistic regression with binomial error. Nonsignificant (P ≥ 0.05) variables were removed by backward removal. The significance of particular effects was assessed with the Wald statistic (W). The strength of the overall association between the predictors in the model was estimated using Nagelkerke’s R2 (Nagelkerke Citation1991). Logistic regression coefficients (β) and standard errors (β, SE) were used to assess the character and strength of significant relationships. Effect sizes are quoted as odds ratios (OR) with 95% confidence intervals (CI). The logistic regression models contained all horizontally-moving prey (all images but VSN0, VLN0 shown in ). The responses of the spider to these two images were analysed separately due to the fact that VSN0 and VLN0 could not be described by all the variables included in the models, such as the type of motion and the local motion of legs. The responses of the spiders to natural prey (flies, thrips and caterpillars) and their analogous virtual images were tested using the G test. All values are presented as means ± SD. All analyses were performed using STATISTICA 10.0 software (Statsoft, Tulsa, OK, U.S.A.).
Results
The spiders demonstrated similar behaviours when approaching natural prey and the corresponding virtual images. The frequency of stalking did not differ significantly between spiders approaching flies (20 out of 28 spiders; 71%) and those approaching SN4 (nine out of 20; 45%), the virtual fly (G1 = 1.71, P = 0.191). In addition, the frequency of frontal approach did not differ between caterpillars (13 out of 25 spiders; 52%) and their analogous LC4 images (10 out of 2; 40%) (G1 = 0.36, P = 0.547), or between thrips (11 out of 26 spiders; 42%) and their corresponding LN4 images (5 out of 16; 31%) (G1 = 0.26, P = 0.610).
Non-specific behaviours for natural prey types were uncommon. Stalk was observed in 2 of 25 spiders approaching caterpillars (8%) and in 3 of 26 spiders approaching thrips (12%), while frontal approach was observed in 1 of 28 spiders approaching flies (4%).
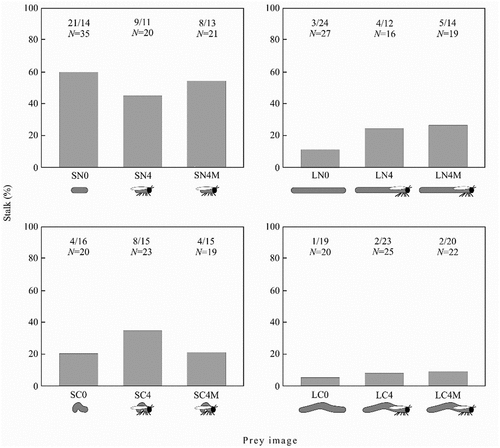
In Experiment 2, prey body length (β = 1.44 ± 0.32, W = 20.19, P < 0.001) and motion type (β = 1.07 ± 0.31, W = 11.88, P < 0.001) significantly predicted whether the images would be stalked (). The short-bodied images demonstrated four-times greater odds of being stalked (CI: 2.2–7.9) than the long-bodied images. In addition, the non-crawling images were found to have three-times greater odds of being stalked (CI: 1.6–5.3) than the crawling images. The number of details (β = −0.03 ± 0.09, W = 0.09, P < 0.767) and the local motion of legs (β = 0.22 ± 0.32, W = 0.47, P < 0.493) were found to be nonsignificant variables and were excluded from the model. The reduced model fitted the data (χ12 = 36.61, P < 0.001, Nagelkerke R2 = 0.13).
Figure 3. The frequency of stalk in the experiments with horizontally-moving prey. The number of spiders performing stalk, the number of spiders that did not perform stalk and total number of spiders are given above each bar.
Body length (β = −1.27 ± 0.31, W = 17.13, P < 0.001) and motion type (β = −0.81 ± 0.30, W = 7.26, P < 0.007) significantly predicted whether spiders performed a frontal approach against the prey images (). The odds of a frontal approach were almost 3.5 times greater for long-bodied images (CI: 1.9–6.5) than for short-bodied images, and 2.2 times greater for long-bodied images (CI: 1.2–4.0) than short-bodied images. The number of details (β = −0.14 ± 0.09, W = 0.03, P < 0.872) and the local motion of legs (β = −0.55 ± 0.33, W = 2.72, P < 0.098) were found to be nonsignificant and were excluded from the model. The reduced model fitted the data (χ12 = 27.06, P < 0.001, Nagelkerke R2 = 0.14).
Figure 4. The frequency of frontal approach in the experiments with horizontally-moving prey. The number of spiders performing frontal approach, the number of spiders that did not perform frontal approach and total number of spiders are given above each bar.
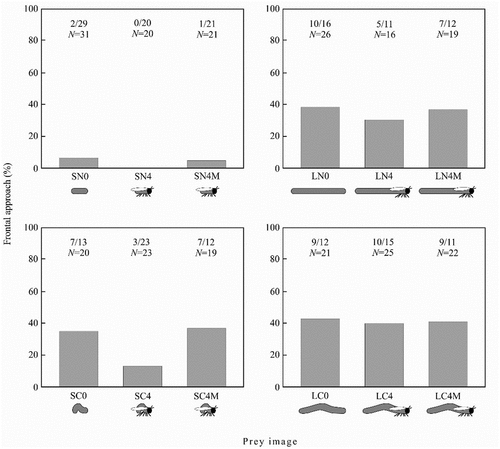
The spiders that attacked VSN0 images (N = 9) and VLN0 images (N = 8) did not perform stalk or frontal approach in any of the predatory attempts. Instead they directly walked towards the images and onto the image presented on the screen.
Discussion
The main finding of this study is that Y. arenarius can rely solely on general prey characteristics during visual prey categorization, more specifically body proportions and the type of prey motion, and can ignore other details. Such a simplified mechanism of prey identification based on only two characteristics allows the spider, and possibly other generalist jumping spiders, to categorize a wide range of prey into a limited number of groups, and subsequently capture them with the use of prey-specific predatory techniques. For Y. arenarius and other such predators, precise and quick prey identification is crucial to ensure predatory success. The characteristics used by Y. arenarius, namely short vs long body and crawling vs non-crawling motion, seem to fulfil these requirements. These characteristics are easily visible, unambiguous and can be used against a potentially large number of prey. In addition, this approach seems to result in only a limited number of costly misinterpretations. For example, insect larvae incapable of efficient escape, such as caterpillars and maggots, adopt a crawling motion, while non-crawling insect larvae, such as campodeiforms, and some winged insect imagines, such as thrips and staphylinids, demonstrate elongated bodies. In contrast, a short body and (or) non-crawling motion are typical for the majority of arthropods with high escape potentials, such as flies, leafhoppers, grasshoppers and wasps. While a number of short-bodied and non-crawling arthropods are incapable of efficient escape, such as wingless aphids, aradids or lygaeids (Bartos Citation2004), any resulting misclassification of prey with regard to escape potentials seems to result in fairly low costs, related mainly to prolonged approach time rather than escape; these animals are nevertheless still found in the natural diet of Y. arenarius.
Our findings also indicate that some prey identification mechanisms employed by generalist jumping spiders differ from those used by specialized jumping spiders. It seems likely that at least some generalist jumping spiders do not rely on diverse and complex prey-specific details to make correct predatory decisions, as Y. arenarius did not appear to use the wings, legs, antennae or head spot for prey identification and categorization. This observation corresponds with the lack of reaction to interior spatial detail observed in the experiments with Phidippus audax, another euryphagous jumping spider, showing no preference between moving images of a cricket and a rectangle of the same size and colour (Bednarski et al. Citation2012). These observations are particularly interesting when compared with previous reports that such details play a key role in visual prey identification by specialized jumping spiders like Portia (Harland & Jackson Citation2000, Citation2002) and E. culicivora (Jackson et al. Citation2005; Nelson & Jackson Citation2006, Citation2012b).
The observation that prey details may not have any strong influence on the resulting categorisation begs the question of whether they were relevant or realistic enough to be used in prey categorization, or if they were detected at all. The possibility of remaining undetected seems unlikely, as the resolution of jumping spider eyes enables adults (Nelson Citation2010; Harland et al. Citation2012; Nelson & Jackson Citation2012b) and juveniles (Goté et al. Citation2019) to detect very small objects, and generally speaking, jumping spiders strongly rely on such signals in communication (Clark & Uetz Citation1993; Nelson & Jackson Citation2007; Elias et al. Citation2012) and predation (Jackson & Pollard Citation1996; Nelson et al. Citation2005; Nelson & Jackson Citation2011). More importantly, however, such details were found to be detected and exploited by naïve Y. arenarius in targeting predatory strikes (Bartos & Minias Citation2016).
While it appears likely that the details were perceived in the present experiment, their realism was relatively low, as they were drawn as generalizations of certain structures of the natural prey. This possible low realism might have been the reason for the lack of the effect observed for the leg movement and the vertical motion in the detail-less images. Previous experiments with Hypoblemum albovittatum (Dolev & Nelson Citation2016) found that low levels of realism in the images presented to jumping spiders may affect their identification and predatory decisions.
The prey identification mechanism observed in Y. arenarius is also likely to be widespread among other jumping spiders. This assumption is supported by the fact that both stalk and frontal approach exist within the routine predatory repertoires of many jumping spiders (Forster Citation1977, Citation1982; Freed Citation1984; Jackson Citation1988a; Richman & Jackson Citation1992; Edwards & Jackson Citation1993, Citation1994; Li et al. Citation1996; Bear & Hasson Citation1997, Citation1999; Bartos Citation2002; Nelson et al. Citation2005, Citation2007). Therefore, it is possible that similar prey characteristics employed by Y. arenarius are also used by other jumping spiders when capturing similar prey. In fact, the observations of jumping spider predatory behaviour support this hypothesis. Stalk was observed in a number of jumping spiders capturing short-bodied, non-crawling prey; this group includes various members of both the salticoid genera: Aelurillus, Euryattus, Evarcha, Hypoblemum, Jacksonoides, Plexippus, Tauala and Trite (Forster Citation1982; Jackson Citation1988a,Citationb; Bear & Hasson Citation1997; Li et al. Citation1999; Nelson et al. Citation2005; Dolev & Nelson Citation2016) and spartaeinae genera: Portia, Brettus, Cocalus and Cyrba (Jackson & Hallas Citation1986; Jackson Citation1990; Harland & Jackson Citation2000, Citation2001). Other than Y. arenarius (Bartos Citation2007, Citation2008), only Phidippus has demonstrated frontal approach (Edwards & Jackson Citation1993, Citation1994) and only when capturing caterpillars (long-bodied, crawling prey); this is generally due to the fact that insect larvae have rarely been used in prey-capture studies.
Our findings indicate the presence of a pre-programmed mechanism of prey categorization that enables inexperienced spiderlings to identify different prey groups based solely on the body proportions and the mode of prey motion. The mechanism enables the spider to identify groups of prey animals based on simple, universal and easily visible cues, thus increasing predatory success, as well as decreasing the risk of injury or becoming a meal of a would-be prey. The mechanism may play a significant role throughout its lifetime, as two-year-old and three-year-old spiders use similar prey-specific predatory behaviour against similar prey categories (Bartos & Szczepko Citation2012). This, however, seems to be especially important for the freshly-emerged spiderlings possessing no prior experience with prey, because it simply enables them to use a prey-specific predatory technique from the first predatory attempt. Predatory behaviour may, however, change during the life cycle, as it has been shown for Phidippus regius (Edwards & Jackson Citation1994) and Y. arenarius (Bartos & Szczepko Citation2012). Adult E. culicivora even lose an innate predatory strategy, which is specifically used by juveniles against Anopheles mosquitoes, the preferred prey of all age groups (Nelson et al. Citation2005). Although these changes do not seem to be accompanied by any alterations in the identification and categorization mechanisms used by E. culicivora, this does not seem to be the case for E. culicivora, a predatory specialist the feeds throughout its life on blood-fed Anopheles mosquitos. Similarly Y. arenarius continues to use similar prey-specific predatory tactics against different prey throughout its life cycle (Bartos & Szczepko Citation2012); the fact that this long-lived salticid (Bartos Citation2004) changes its diet during its lifespan (Bartos Citation2011) attests to the versatility of this mechanism: spiders from three different cohorts coexisting simultaneously in the field (freshly hatched spiderlings, one-year-old and two-year-olds) capture different prey taxa categorised on the basis of similar cues.
Shape and mode of motion are used by many visual predators for distinguishing potential prey from non-prey objects (Wachowitz & Ewert Citation1996; Ewert Citation2004; Kral & Prete Citation2004; Cronin Citation2005). This seems obvious, as these cues characterize the majority of non-sedentary invertebrate prey, all moving along their long axis, crawling or non-crawling. Studies on insects and amphibians visually distinguishing their prey from non-prey (Ewert Citation2004; Kral & Prete Citation2004; Cronin Citation2005) suggest that the methods used by Y. arenarius may also be shared by different taxa from distant groups, such as mantises (Prete Citation1992a; Citationb, Citation1993; Prete & Mahaffey Citation1993; Prete & McLean Citation1996, Citation1999) and toads (Wachowitz & Ewert Citation1996; Ewert Citation2004).
It has been proposed that mantises categorise their prey based on the concept of a perceptual envelope, which encompasses all of the various combinations of certain original key parameters characteristic for prey items (Kral & Prete Citation2004). This concept was also used as a starting point by Dolev and Nelson (Citation2016) to describe the perceptual categories of generalist and specialist jumping spiders. They propose that the evolutionary spectrum of jumping spider predatory specialisations includes extreme generalists such as P. audax, which possess some very crude neural representations of prey characteristics (Bednarski et al. Citation2012) and possibly capture a very wide spectrum of prey, and salticid generalists with narrower perceptual envelopes, such as Y. arenarius, which capture various prey but with prey-specific predatory techniques (Bartos Citation2002, Citation2007, Citation2008, Citation2012, Citation2013, Citation2016). In addition, the spectrum encompasses extreme specialists, such as E. culicivora, which focus on a specific type of prey (Nelson & Jackson Citation2006, Citation2012b; Dolev & Nelson Citation2014, Citation2016). Our present findings revealing the perceptual mechanisms of Y. arenarius used in the categorization of different prey groups appear to be in line with Dolev and Nelson (Citation2016).
Geolocation information
Poland, Kwilno, Zgierz County, coordinates 51ʹ59°N, 19ʹ30°E.
Acknowledgements
I would like to thank Jerzy Krysiak for his help designing the displaying module of the experimental set-up and Bartłomiej Dana, Michel Mabiki and Wioleta Gruszka for technical assistance. I would like to thank two Anonymous Referees for their valuable comments and suggestions on this manuscript. This work was supported in part by the Polish Ministry of Scientific Research and Information Technology under grant SCSR 6P04F07215, and by the University of Lodz. The Article Publishing Charge has been covered by the University of Lodz.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Bartos M. 2002. Distance of approach to prey is adjusted to the prey’s ability to escape in Yllenus arenarius Menge (Araneae, Salticidae). In: Toft S, Scharff N, editors. European arachnology. Aarhus, Denmark: Aarhus University Press. pp. 33–38.
- Bartos M. 2004. The prey of Yllenus arenarius (Araneae, Salticidae). Bulletin of the British Arachnological Society 13:83–85.
- Bartos M. 2005. The life history of Yllenus arenarius (Araneae, Salticidae): Evidence for sympatric populations isolated by the year of maturation. Journal of Arachnology 36:300–305. DOI:10.1636/CSt107-134.1.
- Bartos M. 2007. Hunting prey with different escape potentials: Alternative predatory tactics in a dune-dwelling salticid. Journal of Arachnology 33:214–221. DOI:10.1636/04-73.1.
- Bartos M. 2008. Alternative predatory tactics in a juvenile jumping spider. Journal of Arachnology 36:300–305.
- Bartos M. 2011. Partial dietary separation between coexisting cohorts of Yllenus arenarius (Araneae: Salticidae). Journal of Arachnology 39:230–235. DOI:10.1636/CP10-63.1.
- Bartos M. 2013. Optical cues used in prey identification by a juvenile jumping spider, Yllenus arenarius (Araneae, Salticidae). Dr. Hab. thesis, University of Łódź, Łódź University Press, Łódź, Poland.
- Bartos M, Minias P. 2016. Visual cues used in directing predatory strikes by the jumping spider Yllenus arenarius (Araneae, Salticidae). Animal Behaviour 120:51–59. DOI:10.1016/j.anbehav.2016.07.021.
- Bartos M, Szczepko K. 2012. Development of prey-specific predatory behavior in a jumping spider (Araneae: Salticidae). Journal of Arachnology 40:228–233. DOI:10.1636/Hi11-66.1.
- Bear A, Hasson O. 1997. The predatory response of a stalking spider, Plexippus paykulli, to camouflage and prey type. Animal Behaviour 54:993–998. DOI:10.1006/anbe.1997.0525.
- Bednarski JV, Taylor P, Jakob EM. 2012. Optical cues used in predation by jumping spiders, Phidippus audax (Araneae, Salticidae). Animal Behaviour 84:1221–1227. DOI:10.1016/j.anbehav.2012.08.032.
- Blest AD, Hardie RC, McIntyre P, Williams DS. 1981. The spectral sensitivities of identified receptors and the function of retinal tiering in the principal eyes of a jumping spider. Journal of Comparative Physiology A 145:227–239. DOI:10.1007/BF00605035.
- Clark DL, Uetz GW. 1993. Signal efficacy and the evolution of male dimorphism in the jumping spider, Maevia inclemens. Proceedings of the National Academy of Sciences of the United States of America, 90 (24): 11954–11957. DOI:10.1073/pnas.90.24.11954.
- Cronin TW. 2005. The visual ecology of predator-prey interactions. In: Barbosa P, Castellanos I, editors. Ecology of predator-prey interactions. Oxford: Oxford University Press. pp. 105–138.
- Darmaillacq AS, Chichery R, Poirier R, Dickel L. 2004. Effect of early feeding experience on subsequent prey preference by cuttlefish, Sepia officinalis. Developmental Psychobiology 45:239–244. DOI:10.1002/dev.20034.
- De Agrò M, Rößler DC, Kim K, and Shamble PS. 2021. Perception of biological motion by jumping spiders. PLoS biology 19(7):e3001172. DOI:10.1371/journal.pbio.3001172.
- Dolev Y, Nelson XJ. 2014. Innate pattern recognition and categorization in a jumping spider. PLoS One 9(6):e97819. DOI:10.1371/journal.pone.0097819.
- Dolev Y, Nelson XJ. 2016. Biological relevance affects object recognition in jumping spiders. New Zealand Journal of Zoology 43:42–53. DOI:10.1080/03014223.2015.1070183.
- Edwards GB, Jackson RR. 1993. Use of prey-specific predatory behaviour by North American jumping spiders (Araneae, Salticidae) of the genus Phidippus. Journal of Zoology 229:709–716. DOI:10.1111/j.1469-7998.1993.tb02666.x.
- Edwards GB, Jackson RR. 1994. The role of experience in the development of predatory behaviour in Phidippus regius, a jumping spider (Araneae, Salticidae) from Florida. New Zealand Journal of Zoology 21:269–277. DOI:10.1080/03014223.1994.9517994.
- Elias DO, Maddison WP, Peckmezian C, Girard MB, Mason AC. 2012. Orchestrating the score: Complex multimodal courtship in the Habronattus coecatus group of Habronattus jumping spiders (Araneae: Salticidae). Biological Journal of the Linnean Society 105(3):522–547. DOI:10.1111/j.1095-8312.2011.01817.x.
- Enns JT. 2004. The thinking eye, the seeing brain: Explorations in visual cognition. New York: W. W. Norton & Company.
- Ewert J-P. 2004. Motion perception shapes the visual world of amphibians. In: Prete FR, editor. Complex worlds from simpler nervous systems. Cambridge: MIT Press. pp. 117–160.
- Forster LM. 1977. A qualitative analysis of hunting behaviour in jumping spiders (Araneae: Salticidae). New Zealand Journal of Zoology 4:51–62. DOI:10.1080/03014223.1977.9517936.
- Forster L. 1982. Visual communication in jumping spiders (Salticidae). In: Witt PN, Rovner JS, editors. Spider communication: Mechanisms and ecological significance. Princeton: Princeton University Press. pp. 161–212.
- Forster L. 1985. Target discrimination in jumping spiders (Araneae: Salticidae). In: Barth FG, editor. Neurobiology of Arachnids. Berlin: Springer-Verlag. pp. 249–274.
- Förster J, Higgins ET. 2005. How global versus local perception fits regulatory focus. Psychological Science 16:631–636. DOI:10.1111/j.1467-9280.2005.01586.x.
- Freed AN. 1984. Foraging behaviour in the jumping spider Phidippus audax: Bases for selectivity. Journal of Zoology 203:49–61. DOI:10.1111/j.1469-7998.1984.tb06043.x.
- Goté JT, Butler PM, Zurek DB, Buschbeck EK, Morehouse NI. 2019. Growing tiny eyes: How juvenile jumping spiders retain high visual performance in the face of size limitations and developmental constraints. Vision Research 160:24–36. DOI:10.1016/j.visres.2019.04.006.
- Harland DP, Jackson RR. 2000. Cues by which Portia fimbriata, an araneophagic jumping spider, distinguishes jumping-spider prey from other prey. Journal of Experimental Biology 203:3485–3494. DOI:10.1242/jeb.203.22.3485.
- Harland DP, Jackson RR. 2001. Prey classification by Portia fimbriata, a salticid spider that specializes at preying on other salticids: Species that elicit cryptic stalking. Journal of Zoology 255:445–460. DOI:10.1017/S0952836901001534.
- Harland DP, Jackson RR. 2002. Influence of cues from the anterior medial eyes of virtual prey on Portia fimbriata, an araneophagic jumping spider. Journal of Experimental Biology 205:1861–1868. DOI:10.1242/jeb.205.13.1861.
- Harland DP, Jackson RR. 2004. Portia perceptions: The Umwelt of an araneophagic jumping spider. In: Prete FR, editor. Complex worlds from simpler nervous systems. Cambridge: MIT Press. pp. 5–40.
- Harland DP, Li D, Jackson RR. 2012. How jumping spiders see the world. In: Lazareva OF, Shimizu T, Wasserman EA, editors. How animals see the world: Comparative behavior, biology, and evolution of vision. New York: Oxford University Press. pp. 133–163.
- Ingle DJ. 1983. Brain mechanisms of visual localization by frogs and toads. In: Ewert J-P, Capranica RR, Ingle DJ, editors. Advances in vertebrate neuroethology. New York: Plenum. pp. 177–226.
- Jackson RR. 1988a. The biology of Jacksonoides queenlandicus, a jumping spider (Araneae: Salticidae) from Queensland: Intraspecific interactions, web-invasion, predators, and prey. New Zealand Journal of Zoology 15:1–37. DOI:10.1080/03014223.1988.10422606.
- Jackson RR. 1988b. The biology of Tauala lepidus, a jumping spider (Araneae: Salticidae) from Queensland: Display and predatory behaviour. New Zealand Journal of Zoology 15:347–364. DOI:10.1080/03014223.1988.10422960.
- Jackson RR. 1990. Predatory and nesting behaviour of Cocalus gibbosus, a spartaeine jumping spider (Araneae: Salticidae) from Queensland. New Zealand Journal of Zoology 17:483–490. DOI:10.1080/03014223.1990.10422947.
- Jackson RR, Hallas SEA. 1986. Predatory versatility and intraspecific interactions of spartaeine jumping spiders (Araneae: Salticidae): Brettus adonis. B. cingulatus, Cyrba algerina and Phaeacius sp. indet. New Zealand Journal of Zoology 13:491–520. DOI:10.1080/03014223.1986.10422979.
- Jackson RR, Nelson X. 2012. Attending to detail by communal spider-eating spiders. Animal Cognition 15:461–471. DOI:10.1007/s10071-012-0469-y.
- Jackson RR, Nelson XJ, Sune GO. 2005. A spider that feeds indirectly on vertebrate blood by choosing female mosquitoes as prey. Proceedings of the National Academy of Sciences of the United States of America, 102:15155–15160. DOI:10.1073/pnas.0507398102.
- Jackson RR, Pollard S. 1996. Predatory behavior of jumping spiders. Annual Review of Entomology 41:287–308. DOI:10.1146/annurev.en.41.010196.001443.
- Jakob E, Skow C, Long S. 2011. Plasticity, learning and cognition. In: Herberstein M, editor. Spider behaviour, flexibility and versatility. Cambridge: Cambridge University Press. pp. 307–447.
- Kimchi R. 1992. Primacy of wholistic processing and global/local paradigm: A critical review. Psychological Bulletin 112:24. DOI:10.1037/0033-2909.112.1.24.
- Kourtzi Z, Connor CE. 2011. Neural representations for object perception: Structure, category, and adaptive coding. Annual Review of Neurosciences 34:45–67. DOI:10.1146/annurev-neuro-060909-153218.
- Kral K, Prete FR. 2004. In the mind of a hunter: The visual world of the praying mantis. In: Prete FR, editor. Complex worlds from simpler nervous systems. Cambridge: MIT Press. pp. 75–116.
- Land MF. 1969. Movements of the retinae of jumping spiders (Salticidae: Dendryphantinae) in response to visual stimuli. Journal of Experimental Biology 51:471–493. DOI:10.1242/jeb.51.2.471.
- Land MF, Nilsson D-E. 2001. Animal eyes. Oxford, UK: Oxford University Press.
- Li D, Jackson RR, Cutler B. 1996. Prey-capture techniques and prey preferences of Habrocestum pulex (Hentz), an ant-eating jumping spider (Araneae, Salticidae) from North America. Journal of Zoology 240:551–562. DOI:10.1111/j.1469-7998.1996.tb05305.x.
- Li D, Jackson RR, Harland DP. 1999. Prey-capture techniques and prey preferences of Aelurillus aeruginosus, A. cognatus and A. kochi, ant-eating jumping spiders (Araneae: Salticidae) from Israel. Israel Journal of Zoology 45:341–359.
- Nagata T, Koyanagi M, Tsukamoto H, Saeki S, Isono K, Shichida Y, Tokunaga F, Kinoshita M, Arikawa K, Terakita A. 2012. Depth perception from image defocus in a jumping spider. Science 335:469–471. DOI:10.1126/science.1211667.
- Nagelkerke NJD. 1991. A note on a general definition of the coefficient of determination. Biometrika 78:691–692. DOI:10.1093/biomet/78.3.691.
- Nelson XJ. 2010. Visual cues used by ant-like jumping spiders to distinguish conspecifics from their models. Journal of Arachnology 38:27–34. DOI:10.1636/Hi09-35.1.
- Nelson XJ, Card A. 2016. Locomotory mimicry in ant-like spiders. Behavioral Ecology 27(3):700–707. DOI:10.1093/beheco/arv218.
- Nelson XJ, Jackson RR. 2006. A predator from East Africa that chooses malaria vectors as preferred prey. PLoS ONE 1:e132. DOI:10.1371/journal.pone.0000132.
- Nelson XJ, Jackson RR. 2007. Complex display behaviour during the intraspecific interactions of myrmecomorphic jumping spiders (Araneae, Salticidae). Journal of Natural History 41(25–28):1659–1678. DOI:10.1080/00222930701450504.
- Nelson X, Jackson RR. 2011. Flexibility in the foraging strategies of spiders. In: Herberstein M, editor. Spider behaviour, flexibility and versatility. Cambridge: Cambridge University Press. pp. 31–56.
- Nelson XJ, Jackson RR. 2012a. Fine-tuning of vision-based prey-choice decisions by a predator that targets malaria vectors. Journal of Arachnology 40:23–33. DOI:10.1636/Hill-61.1.
- Nelson XJ, Jackson RR. 2012b. The discerning predator: Decision rules underlying prey classification by a mosquito-eating jumping spider. Journal of Experimental Biology 215:2255–2261. DOI:10.1242/jeb.069609.
- Nelson XJ, Jackson RR, Sune GO. 2005. Use of Anopheles-specific prey-capture behavior by the small juveniles of Evarcha culicivora, a mosquito-eating jumping spider. Journal of Arachnology 33:541–548. DOI:10.1636/05-3.1.
- Peaslee AG, Wilson G. 1989. Spectral sensitivity in jumping spiders (Araneae, Salticidae). Journal of Comparative Physiology A 164:359–363. DOI:10.1007/BF00612995.
- Prete FR. 1992a. Discrimination of visual stimuli representing prey versus non-prey by the praying mantis Sphodromantis lineola (Burr.). Brain, Behavior & Evolution 39:285–288. DOI:10.1159/000114125.
- Prete FR. 1992b. The effects of background pattern and contrast on prey discrimination by the praying mantis Sphodromantis lineola (Burr.). Brain, Behavior & Evolution 40:311–320. DOI:10.1159/000113921.
- Prete FR. 1993. Stimulus configuration and location in the visual field affect appetitive responses by the praying mantis. Sphodromantis lineola (Burr.). Visual Neuroscience 10:997–1005. DOI:10.1017/S0952523800010105.
- Prete FR. 1999. Prey recognition. In: Prete FR, Wells H, Wells PH, Hurd LE, editors. The praying mantids. Baltimore: Johns Hopkins University Press. pp. 141–179.
- Prete FR, Mahaffey RJ. 1993. Appetitive responses to computer-generated visual stimuli by the praying mantis Sphodromantis lineola. (Burm.) Visual Neuroscience 10:669–679. DOI:10.1017/S0952523800005368.
- Prete FR, McLean T. 1996. Responses to moving small-field stimuli by the praying mantis, Sphodromantis lineola (Burmeister). Brain Behavior & Evolution 47:42–54. DOI:10.1159/000113228.
- Richman D, Jackson RR. 1992. A review of the ethology of jumping spiders (Araneae, Salticidae). Bulletin of the British Arachnology Society 9:33–37.
- Rößler DC, De Agrò M, Kim K, and Shamble PS. 2022. Static visual predator recognition in jumping spiders. Functional Ecology 36:561–571. DOI:10.1111/1365-2435.13953.
- Shamble PS, Hoy RR, Cohen I, Beatus T. 2017. Walking like an ant: A quantitative and experimental approach to understanding locomotor mimicry in the jumping spider Myrmarachne formicaria. Proceedings of the Royal Society B 284:20170308. DOI:10.1098/rspb.2017.0308.
- Soto FA, Wasserman EA. 2014. Mechanisms of object recognition: What we have learned from pigeons. Frontiers in Neural Circuits 8:1–22. DOI:10.3389/fncir.2014.00122.
- Spano L, Long SM, Jakob EM. 2012. Secondary eyes mediate the response to looming objects in jumping spiders (Phidippus audax. Salticidae). Biology Letters 8:949–951. DOI:10.1098/rsbl.2012.0716.
- Uhl G, Elias D. 2011. Communication. In: Herberstein M, editor. Spider behaviour, flexibility and versatility. Cambridge: Cambridge University Press. pp. 127–189.
- Wachowitz S, Ewert J-P. 1996. A key by which the Toad’s visual system gets access to the domain of prey. Physiology & Behavior 60:877–887. DOI:10.1016/0031-9384(96)00070-4.
- Wagemans J, Elder JH, Kubovy M, Palmer SE, Peterson MA, Singh M, von der Heydt R. 2012. A century of Gestalt psychology in visual perception: I. Perceptual grouping and figure-ground organization. Psychological Bulletin 138:1172–1217. DOI:10.1037/a0029333.
- Williams D, McIntyre P. 1980. The principal eyes of a jumping spider have a telephoto component. Nature 288:578–580. DOI:10.1038/288578a0.
- World Spider Catalog. 2022. World spider catalog. Version 23.0. Natural history museum Bern. http://wsc.nmbe.ch. Accessed 2022 02 09.
- Zurek DB, Nelson XJ. 2012a. Saccadic tracking of targets mediated by the anterior-lateral eyes of jumping spiders. Journal of Comparative Physiology A 198:411–417. DOI:10.1007/s00359-012-0719-0.
- Zurek DB, Nelson XJ. 2012b. Hyperacute motion detection by the lateral eyes of jumping spiders. Vision Research 66:26–30. DOI:10.1016/j.visres.2012.06.011.
- Zurek DB, Taylor AJ, Evans CS, Nelson XJ. 2010. The role of the anterior lateral eyes in the vision-based behaviour of jumping spiders. Journal of Experimental Biology 213:2372–2378. DOI:10.1242/jeb.042382.