Abstract
Tardigrades have been recorded from a variety of habitats including mosses, lichens, leaflitter, streams, and marine sediments; however, reports from rock pools are still scarce. Rock pools across the world are known to host diverse invertebrate communities and endemisms are common. We provide the description by integrative taxonomy of Mesobiotus huecoensis sp. nov., found in the sediment of an ephemeral rock pool in Box Canyon Recreational Area in New Mexico (USA), and placed it in the M. montanus morphogroup based on the presence of eggs with hemispherical processes. This new species has elongated claws, particularly on the fourth pair of legs. Elongated claws are typical of freshwater tardigrades, and could represent an adaptation that allows the new species to better move in the substrate when the rock pool is fully inundated. We also provide information on the sperm morphology and mating behaviour of this new species. The finding of this new species highlights the importance of ephemeral rock pools for the discovery of new taxa and the need for their study and conservation.
http://zoobank.org/urn:lsid:zoobank.org:pub:2EE663A1-684B-4BE9-A11A-AD40B46802A2
Introduction
Tardigrades, a phylum of microinvertebrates that includes over 1400 species (Degma & Guidetti Citation2023), are water-dependent animals that need at least a film of water to survive (Nelson et al. Citation2018). However, many species have the ability to enter a state called cryptobiosis, where they can resist harsh conditions, such as drying out or freezing (Rebecchi et al. Citation2007; Hengherr & Schill Citation2018; Schill & Hengherr Citation2018). Due to this ability, tardigrades can be found in a wide range of environments including terrestrial, freshwater, and marine habitats all over the world (Nelson et al. Citation2018). As a result of their small size and cryptobiotic capabilities, tardigrades colonize a variety of environments, ranging from limno-terrestrial (leaf litter, soil, mosses, lichens) to aquatic habitats (periphyton, sediment) (Nelson et al. Citation2018) and climatic conditions from glaciers (Zawierucha et al. Citation2016) to deserts (Darby & Neher Citation2012).
Tardigrade records in ephemeral freshwater rock pools are extremely limited, having been recorded only a few times in the scientific literature (De Vries Citation1996; Koste Citation1996; Spencer et al. Citation1999; Jocqué et al. Citation2007; Boix et al. Citation2016; Velasco-González et al. Citation2020). However, the potential of this particular habitat for hosting rich and diverse tardigrades communities is evident from recent studies (Vecchi et al. Citation2022), where a new endemic species from a globally rare genus was discovered (Vecchi et al. Citation2023).
The family Macrobiotidae is a group of limno-terrestrial tardigrades, within which 14 distinct genera have been recognized so far. However, most of the species’ diversity in this family are found in only four genera (Macrobiotus, Mesobiotus, Minibiotus, and Paramacrobiotus) (Degma & Guidetti Citation2023). These genera have been historically recognized as informal species groups within the genus Macrobiotus, but were later elevated to the genus rank (Schuster et al. Citation1980; Guidetti et al. Citation2009; Vecchi et al. Citation2016). The genus Mesobiotus is the focus of this study and currently comprises 75 nominal species, four of which are designated as nomina inquirenda. The genus was erected by Vecchi et al. (Citation2016) and supported by morphological and genetic data. Subsequent studies have shown that the genus is monophyletic, but there is no support for the two traditionally recognized species groups in the genus (harmsworthi group and furciger group). These two species groups are characterized by distinctive morphologies of the eggs processes (dichotomous branching on the tip of the process present in the furciger group and absent in the harmsworthi group). However due to their lack of reciprocal monophyly, Short et al. (Citation2022) proposed to abolish the use of these two informal groupings. Stec (Citation2022) argued instead to maintain the usage of these morphogroups as they are useful for taxonomists and name-users in aiding identification and communication regarding taxa. Stec (Citation2022) provided new explicit and clear definitions of the harmsworthi and furciger morphogroups and proposed the institution of an additional montanus morphogroup (defined by the presence of dome-shaped egg processes).
Here we describe, by means of integrative taxonomy, a new species of Mesobiotus belonging to the montanus morphogroup from ephemeral freshwater rock pools from the northern portion of the U.S. Chihuahuan Desert. In addition, we provide data on its sperm morphology and mating behaviour.
Materials and methods
Study area
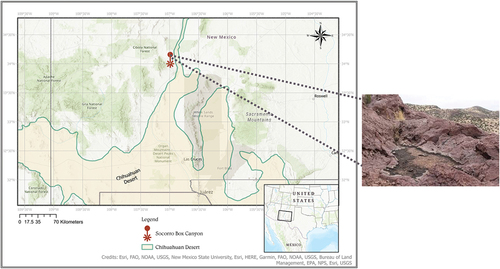
The Box Canyon Recreational Area is 18 km southwest of the city of Socorro, NM. It is managed by New Mexico’s Bureau of Land Management and covers an area of 2.6 km2 with an maximum elevation of 1800 m. There are five cliffs that surround the canyon, and the origin of the rock is volcanic rhyolite (New Mexico Energy, Minerals, and Natural Resources Department). Throughout these rock formations there are many small spatially separated temporary rock pools found near canyon’s floor and on top of the surrounding cliffs. The area’s vegetation is a mix of Chihuahuan Desert canyon shrubland and Juniper Pinyon Woodlands (McDaniel Citation2022). Freebird rock pool (; created with the software ArcGis Pro 3.1 using maps from Jornada Basin Spatial Data Laboratory Citation2006; Esri Citation2017) is located on top of one rocky plateau. It is a shallow rock pool of approximately 1.25 m in maximum length and 1 m in maximum width, with a depth of 4 cm. The rock pool fills during monsoonal rains and is hydrologically connected to a several rock pools in the drainage basin. When dried, there is a blackish algal/biofilm mat in part of the pool.
Figure 1. Type locality of Mesobiotus sp. nov, Freebird rock pool, Socorro Box Canyon located in near Socorro, New Mexico. Map was made using ArcGIS pro 3.1.1 (ESRI Citation2017) with Chihuahuan Desert boundary from Jornada Basin Spatial Data Laboratory (Citation2006).
Sampling and samples processing
A dry sample was taken from the sediment surface (~2.5 cm of thickness) with a clean trowel. The sample was kept desiccated until processing. Tardigrades extraction was performed with the Ludox protocol recommended by Bartels and Nelson (Citation2006) with the modifications by Vecchi et al. (Citation2022) but without the boiling water killing step.
Microscopy and imaging
Specimens for light microscopy were mounted on microscope slides in a small drop of Hoyer’s (~200 mg) medium, secured with a cover slip (22 × 22 mm) and dried at 60°C for a week. Additional individuals were stained with Orcein (Bertolani Citation1971) to identify males and the sperm maturation pattern.
Slides were examined under a Leica DMLB light microscope with phase contrast (PCM), associated with a digital camera (5440 × 3648 pix). For structures that could not be satisfactorily focused on a single light microscope photograph, a stack of 2–5 images were taken with an equidistance of ca. 0.2 μm and assembled manually into a single deep-focus image in GIMP v.2–10 (GIMP Development Team Citation2019).
Specimens for Scanning Electron Microscopy (SEM) were asphyxiated for 30 minutes at 60°C and subjected to an ascending concentration gradient of ethanol (20%, 50%, 70%, 90%, 95%, 100%, 100%) for 15 minutes each. The specimens were critical point dried in CO2, mounted on stubs, and coated with gold. The SEM observations were carried out with a Nova Nano SEM 450 (FEI Company—Oxford Instruments, 1536 × 1103pix), available at the “Centro Interdipartimentale Grandi Strumenti” at University of Modena and Reggio Emilia (Modena, Italy).
All figures were assembled in Figure J (Mutterer & Zinck Citation2013).
Sperm staining and measuring
One male individual (with moving sperm in the gonad) was dissected on a polylysinated slide in 5 μl of 0.1X PBS (Phosphate Buffer Saline) with tungsten needles to release the sperm. The slide was incubated for 10 minutes at room temperature (21°C) in a humidity chamber and then fixed with 50 μl of 1% formaldehyde in 0.1X PBS. After fixing, the solution was removed and the slide was left to air-dry. Staining was performed by adding 32 μl of staining solution (10 μM Phalloidin-TRITC and 0.25% Triton-X100 in 0.1X PBS) for 1 hour in the dark at room temperature. Staining solution was then quickly rinsed with distilled water and replaced with 15 μl of Fluoromount mounting medium with 40 μg/ml Hoechst 33,342. A glass coverslip was then applied and sealed with nail polish. The slide was image 30 minutes after preparation on a Leica S18 Falcon confocal microscope. Phalloidin-TRITC stains actin (acrosome, midpiece, and tail) and was imaged with excitation at 551 nm and emission at 570-750 nm with an image size of 2048 × 2048 px. Hoechst 33,342 stains the chromatin in the nucleus and was imaged with excitation at 405 nm and emission at 410-480 nm. A 4.5 μm Z-stack of ten equidistant focal planes was acquired. The focal planes were combined by using a maximum intensity Z projection in ImageJ (Schneider et al. Citation2012), and the sperm components were measured with the same software. The raw sperm measurements are provided in SM.02.
Morphometrics and morphological nomenclature
All measurements are given in micrometers (μm). Structures were measured only if their orientation was suitable. Body length was measured from the anterior extremity to the posterior end of the body, excluding the hind legs. Buccal tube length and the level of the stylet support insertion point were measured according to Pilato (Citation1981). The pt index is the ratio of the length of a given structure to the length of the buccal tube (Pilato Citation1981). Measurements of buccal tube widths, heights of claws and eggs, as well as the terminology used to describe the Oral Cavity Armature (OCA) and eggshell morphology follow Kaczmarek et al. (Citation2020). Morphometric data were handled using the “Parachela” ver. 1.7 template available from the Tardigrada Register. The raw morphometric data are provided as Supplementary Materials (SM.01).
Thorpe’s normalization of morphometric data was performed according to Bartels et al. (Citation2011) with the R script provided by Vecchi and Stec (Citation2021). The results of Thorpe’s normalization are provided in SM.03.
Tardigrade taxonomy follows Bertolani et al. (Citation2014), Stec (Citation2022) and Stec et al. (Citation2021).
Comparative material
Photographs of eggs of members of the Mesobiotus montanus morphogroups were obtained from the Pilato & Binda collection at the University of Catania, Catania, Italy (M. mottai type series 4471 from Antarctica), the Maucci collection hosted at the University of Modena and Reggio Emilia, Modena, Italy (M. lusitanicus type series 11,242 from Portugal and M. montanus from Italy), and the Tardigrade collection of the Institute of Systematics and Evolution of Animals (Polish Academy of Sciences), Krakow, Poland (M. peterseni GL.002 from Greenland).
Genotyping
DNA was extracted from individual animals following a Chelex® 100 resin (BioRad) extraction method by Casquet et al. (Citation2012) with modifications described in detail in Stec et al. (Citation2020). Carcasses were recovered and mounted as hologenophores (voucher codes provided in the new species description section). We attempted to sequence four DNA fragments, three nuclear (18S rRNA, 28S rRNA, ITS2) and one mitochondrial (COI). All fragments were amplified and sequenced according to the primers and protocols described in Stec et al. (Citation2020). Sequencing products were read with an ABI 3130xl sequencer at the Department of Biological and Environmental Sciences (University of Jyväskylä, Finland).
Phylogenetic analysis
The multilocus phylogenetic analysis was conducted using concatenated 18S rRNA + 28S rRNA+ITS-2+COI sequences. We used the same dataset as in Stec (Citation2022) (), with the addition of the sequences from the new species.
Table I. Sequences used for phylogenetic analysis.
The 18S rRNA, 28S rRNA and ITS-2 sequences were aligned using MAFFT ver. 7 (Katoh Citation2002; Katoh & Toh Citation2008) with the G-INS-i method (thread = 4, threadtb = 5, threadit = 0, reorder, adjust direction, any symbol, max iterate = 1000, retree 1, global pair input). The COI sequences were aligned according to their amino acid sequences (translated using the invertebrate mitochondrial code) with the MUSCLE algorithm (Edgar Citation2004) in MEGA7 with default settings (i.e., all gap penalties = 0, max iterations = 8, clustering method=UPGMB, lambda = 24). Alignments were visually inspected and trimmed in MEGA7. Sequences were concatenated with the R package “concatipede” v1.0.0 (Vecchi & Bruneaux Citation2021).
Model selection was performed for each alignment partition (6 in total: 18S rRNA, 28S rRNA, ITS-2 and three COI codons) with PartitionFinder2 (Lanfear et al. Citation2016).
Bayes inference (BI) phylogenetic reconstruction was done with MrBayes v3.2.6 (Ronquist et al. Citation2012). Two runs with one cold chain and three heated chains were run for 20 million generations with a burn-in of 2 million generations, sampling a tree every 1000 generations. Posterior distributions were checked with Tracer v1.7 (Rambaut et al. Citation2018). MrBayes input file with the input alignment is available as Supplementary Materials (SM.04).
The phylogenetic tree was visualized with FigTree v1.4.4 (Rambaut Citation2007) and the image was edited with Inkscape 0.92.3 (Inkscape Project Citation2020). The complete phylogenetic tree is available in SM.05.
Mating behaviour observation
Individual tardigrades were kept isolated for at least one week in wells of a 24-wells plate filled with mineral water at 21°C. The animals were fed ad libitum with algae (Chlorella sp. and Chlorococcum hypnosporum) and nematodes (Panagrellus pycnus). Five pairs of sexually mature individuals (females with large and evident oocytes in the gonad and males with gonad filled with motile sperm) were used. Each pair was placed in a well of a Ibidi µ-Slide 15 Well 3D slide (4 mm on diameter), with the bottom covered with 10 µl of 1% agar in mineral water. Each couple was then recorded under an inverted microscope Carl Zeiss Cell Observer HS (2009/2009) linked to a Zeiss AxioCam MRm (1388 × 1040 pix, pix size 6.45 µm) for at least 20 minutes. Video were analysed with the software BORIS (Friard & Gamba Citation2016) using the following ethogram: (i) contact: contact between both individuals (touching of any body part at the exclusion of cloacas); (ii) follow: male follows the female; (iii) mount: male aligns his cloaca with that of the female; (iv) touch: male touches the female cloaca; (v) ejaculation: visible sperm is released by the male. Plots of the tardigrade behaviour observed in each video are provided in SM.06.
Results
Phylogenetic reconstruction
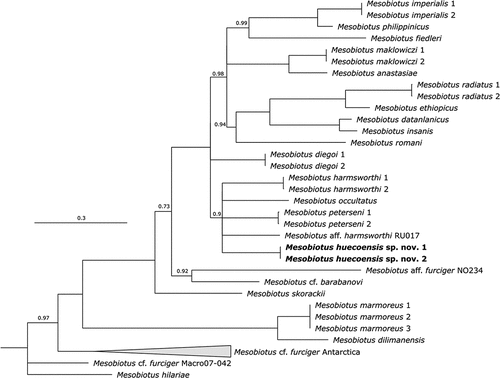
The BI phylogenetic reconstruction () of the genus Mesobiotus yielded a generally poorly supported tree, but with a topology that is still in agreement with that of Stec (Citation2022). The new species is placed in an unresolved clade comprising M. harmsworthi (the type species of the genus), M. occultatus, M. aff. Harmsworthi RU017, and M. peterseni.
Figure 2. Bayesian phylogenetic reconstruction of the genus Mesobiotus. Numbers above nodes indicate bayesian posterior probability (pp) (shown when pp = 1). Nodes with pp < 0.70 were collapsed. The new described species is highlighted in bold. Scale bar indicates the number of substitutions/site. Outrgoup taxa are not shown. For a complete version of the phylogenetic reconstruction including nodes with pp < 0.70 and outgroups see SM.03.
Taxonomic account
Phylum: Tardigrada Doyère, Citation1840
Class: Eutardigrada Richters, Citation1926
Order: Parachela Schuster, Nelson, Grigarick and Christenberry, Citation1980
Superfamily: Macrobiotoidea Thulin, Citation1928 (in Marley et al., Citation2011)
Family: Macrobiotidae Thulin, Citation1928
Genus: Mesobiotus Vecchi, Cesari, Bertolani, Jönsson, Rebecchi and Guidetti, Citation2016
Mesobiotus huecoensis Vecchi, McDaniel & Walsh Citation2023 sp. nov.
https://urn:lsid:zoobank.org:act:D935E484-2E38-47E4-BF84-BD9F9E12E2B0
(; ; SM.01–3)
Figure 3. Mesobiotus huecoensis sp. nov. habitus and leg structures under PCM. (a) Holotype habitus. (b) Claws on leg I from paratype on slide SL1. (c) Claws on leg IV from holotype. (d) Granulation on external side of leg I from paratype on slide SL1. (e) Cuticular bulge on the internal side of leg III from paratype on slide SL1. (f) Granulation on legs IV from paratype on slide SL1. Arrowhead: continuous cuticular bar with with shadowed extensions toward the double muscle attachments. Arrow: flexible primary branch on claws IV. Indented arrowhead: horseshoe-shaped structure connects the anterior and posterior lunulae on claws IV. Empty arrowhead: granulation on legs. Empty indented arrowhead: cuticular bulge on internal leg surface. Images a- e were assembled from multiple focus stacks. Scalebars: 10 µm.
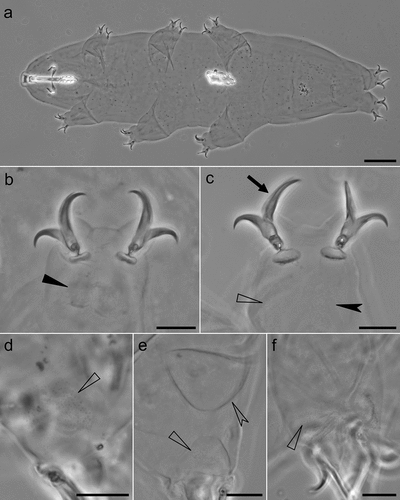
Figure 4. Mesobiotus huecoensis sp. nov. buccopharyngeal apparatus under PCM. (a) Buccopharyngeal apparatus of the holotype. (b) Dorsal view of the placoids row of the holotype. (c) Ventral view of the placoids row of the holotype. (d) Dorsal part of the oral cavity armature (OCA) of the holotype. (e) Dorsal part of the OCA of a paratype on slide SL1. (f) Ventral part of the OCA of the holotype. (g) Ventral part of the OCA of a paratype on slide SL1.Arrowhead: constriction in the third macroplacoid. Arrow: first (anterior) OCA band. Indented arrowhead: second (middle) OCA band. Empty indented arrowhead: third (posterior) OCA band. Empty arrowhead: mucrone behind the median part of the ventral third (posterior) OCA. Image a was assembled from a multiple focus stack. Scalebars: 10 µm.
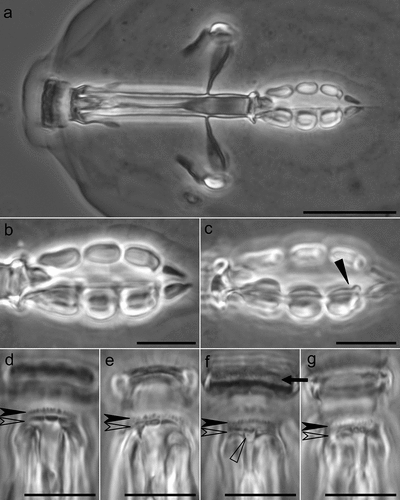
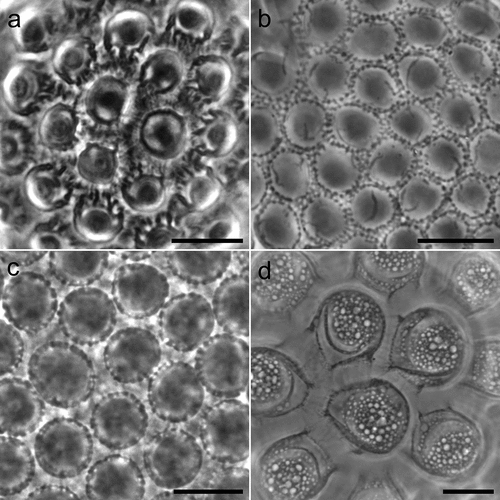
Figure 5. Mesobiotus huecoensis sp. nov. eggs under PCM. (a) General view of the egg showing rounded processes and reticulation on the chorion. (b, c) Sections of egg processes. (d) Egg chorion covered by a reticulum. (e – j) Dorsal view of individual egg processes showing their variability. Arrowheads: pores on the processes. Image a was assembled from a multiple focus stack. Scalebars: (a) 10 µm, (b – j) 5 µm.
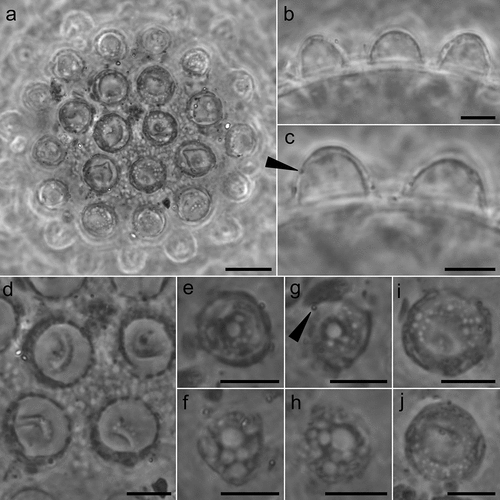
Figure 6. Mesobiotus huecoensis sp. nov. egg under SEM. (a) In toto view. (b) Egg processes showing pores on their lower half. (c) Focus on the reticulated eggshell chorion. Arrowheads: pores on the processes. Indented arrowhead: hole in the chorion reticulum. Scalebars:10 µm.
Table II. Measurements [in μm] and pt values of selected morphological structures of individuals of Mesobiotus huecoensis sp. nov.; specimens mounted in Hoyer’s medium.
Table III. Measurements [in μm] of the eggs of Mesobiotus huecoensis sp. nov.; eggs mounted in Hoyer’s medium; process base/height ratio is expressed as percentage.
Table IV. Measurements [in μm] of the sperms of Mesobiotus huecoensis sp. nov. stained with Phalloidin-TRITC and Hoechst 33,342.
Material examined
29 animals and 19 eggs mounted on microscope slides in Hoyer’s medium, two animals stained with Orcein, one egg examined by SEM, one specimen was processed for sperm measurement and two specimens were processed for DNA sequencing and the carcasses were mounted on microscope slides in Hoyer’s medium.
Type locality
34°00’02.9”N 106°59’22.6”W; 1730 m asl: Freebird rock pool, Socorro Box Canyon, New Mexico (U.S.); sediment from ephemeral rock pool; collected 5th January 2022.
Etymology
From the Spanish, “hueco” means rock pool. In reference to the habitat where the new species has been found.
Type depositories
Holotype: slide S2027_SL3 with 5 paratypes, 24 paratypes (on slides S2027_SL1–4, 6), 19 eggs (on slide S2027_SL7) and 1 hologenophore (Voucher_S2027_Meb.1) are deposited at the Institute of Systematics and Evolution of Animals, Polish Academy of Sciences, Sławkowska 17, 31–016, Kraków, Poland, whereas one SEM stub with 1 egg (S2027_Stub1), 5 paratypes (S2027_SL5) and 1 hologenopore voucher (Voucher_S2027_Meb.4) are deposited in the Bertolani collection in the Laboratory of Evolutionary Zoology, Department of Life Sciences, University of Modena and Reggio Emilia, Via Campi 213/d, 41125 Modena, Italy (Slides codes in Bertolani collection: paratypes C5102_S1 and hologenophore voucher C5102_V01).
Animals
(measurements and statistics in , raw morphometric data in SM.01) Body almost transparent in small specimens, whitish in adults (). Eyes present in alive animals and dissolved by Hoyer’s medium in approximately 70% of mounted animals. Body cuticle smooth and without pores.
Claws of the Mesobiotus type (), with a peduncle connecting the claw to the lunula, a basal septum, and well-developed accessory points situated parallel to the primary branch. Claws on legs IV with elongated main branches (). A single continuous cuticular bar with shadowed extensions toward double muscle attachments is present below claws I–III (), while a faintly visible horseshoe shaped structure connects the anterior and posterior lunulae on claws IV (). A fine granulation is present on the external surface and on the internal surface of legs I–III (). Granulation is also present on the lateral and dorsal surfaces of legs IV (). A cuticular bulge, similar to a pulvinus, is present on the internal surface of legs I–III (). Lunulae under all claws smooth ().
Mouth antero-ventral. Bucco-pharyngeal apparatus of the Macrobiotus type (), with ventral lamina, and ten small peribuccal lamellae. The oral cavity armature is well-developed and composed of three bands of teeth (). The first band of teeth is composed of numerous small granules situated anteriorly in the oral cavity, just behind the bases of the peribuccal lamellae (). The second band of teeth is located between the ring fold and the third band of teeth is composed of ridges parallel to the main axis of the buccal tube that are larger than those in the first band (). The teeth of the third band are located within the posterior portion of the oral cavity, between the second band of teeth and the opening of the buccal tube (). The third band of teeth is discontinuous and divided into a dorsal and ventral portion. Under PCM, dorsal and ventral teeth are visible as two lateral ridges and one median transverse ridge (). Sometimes, the median ridge (both dorsal and ventral) is subdivided in two parts (). An additional mucrone can present behind the median part of the ventral third OCA band (). The pharyngeal bulb is subspherical (), with triangular apophyses, three rod-shaped macroplacoids, and a drop-shaped microplacoid placed close to the third macroplacoid ). The macroplacoid length sequence is 2 < 3 < 1. The first macroplacoid is anteriorly narrowed and the third has a clearly defined subterminal constriction ().
Eggs
(measurements and statistics in , raw morphometric data in SM.01) White – yellowish, laid free, spherical in shape, and equipped with evenly spaced processes in the shape of domes (). Egg surface between the processes without areolation. In PCM the egg surface between processes appears covered by an irregular reticulum (). In SEM, the state is intermediate between pores and reticulum, as nodes and bars are of the same size (or bigger) than pores (). Faint dark thickenings, visible under PCM, are present around the bases of the processes (). The labyrinthine layer is visible under PCM as irregular bubbles with usually, but not always, a bigger bubble in the apical part of the process (). Pores on the processes surface (mostly concentrated in the bottom half of the processes) are present and visible both in LM () and SEM ().
Reproduction
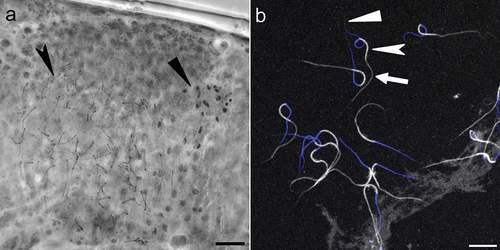
The new species is dioecious. In males, the testes, filled with sperm, are clearly visible under PCM after orcein staining (). The new species does not exhibit male secondary sexual dimorphism traits such as lateral gibbosities on legs IV. The presence in the testis of both mature spermatozoa and cell at earlier spermatogenesis stages suggests a continuous pattern of maturation (Rebecchi & Bertolani Citation1994).
Figure 7. Mesobiotus huecoensis sp. nov. male gonad and spermatozoa. (a) Male gonad under PCM stained with orcein showing different maturation stages of the male gamets. (b) Stained spermatozoa under Confocal Microscopy, blue represents stained DNA (nucleus), whereas white represents Phalloidin-stained actin (acrosome, midpiece, tail). Black arrowhead: spermatids. Black indented arrowhead: mature spermatozoa. White arrowhead: acrosome. White indented arrowhead: midpiece. White arrow: tail. Scalebars:10 µm.
Mating behaviour
The following behaviour and mounting attempts were observed in four out of five mating trials. However, ejaculation was only recorded in one case (SM.07). The mating behaviour sequence of this species is concordant with the observations of Sugiura and Matsumoto (Citation2021a) on another undescribed Mesobiotus species: tracking (i.e., moving around and physical interaction), touching (i.e., the male touches the female cloaca), standstill (i.e., the female stops moving), and mounting/ejaculation (i.e., the male aligns his cloaca with the female’s one and may release sperm). Two distinct sperm release events were observed, both occurring at about one body length distance between male and female individuals and within the first ten minutes of the trial (SM.07).
Sperm
(measurements and statistics in ) Typical morphology of Macrobiotoidea sperm () (Sugiura & Matsumoto Citation2021a, Citation2021b) with an average total length of 52.7 µm [SD 0.6 µm]. Composed of an acrosome (average 5 µm [SD 0.6 µm]), followed by nucleus and midpiece (average 15.5 µm [SD 0.7 µm] and 9.5 µm [SD 0.7 µm], respectively) and tail (average 22.7 µm [SD 1.2 µm]).
DNA sequences
The sequences were obtained for only two (SSU and COI) out of the four molecular markers tested (SSU, LSU, COI and ITS2). LSU and ITS2 failed to amplify.
SSU: Voucher S2027_Meb.1 OQ756248; Voucher S2027_Meb.4 OQ756249
COI: Voucher S2027_Meb.1 OQ756246; Voucher S2027_Meb.1 OQ756247
Additional notes
this species was found with the rotifer Adineta vaga (Davis, Citation1873) and unidentified nematodes.
Differential diagnosis
By having egg processes in the shape of hemispherical or mammillate-like domes, Mesobiotus huecoensis sp. nov. belongs to the montanus morpho-group. The new species differs from all the other species in the montanus morpho-group by the elongated primary branch in claws IV (not elongated in all other species). Specifically, the new species differs from:
Mesobiotus lusitanicus (Maucci and Durante Pasa, 1984) by the presence of a labyrinthine layer in the egg processes walls (not visible in M. lusitanicus, vs. visible in M. huecoensis sp. nov. . Maucci and Durante-Pasa (Citation1984) reported a high variability in the shape M. lusitanicus egg processes, however we considered in this differential diagnosis only the egg morphotype that is most similar to the eggs of M. huecoensis sp. nov.
Mesobiotus montanus (Murray, Citation1910) by the presence of a labyrinthine layer in the egg processes walls (not visible in M. montanus, vs. visible in M. huecoensis sp. nov. ) and by the spacing between processes (very close by in M. montanus, vs. spaced in M. huecoensis sp. nov. ).
Mesobiotus mottai (Binda & Pilato, Citation1994) by the presence of a reticulation between the egg processes (not visible in M. mottai, vs. visible in M. huecoensis sp. nov. ), by the presence of a labyrinthine layer in the egg processes walls (not visible in M. mottai, vs. visible in M. huecoensis sp. nov. ), and by the spacing between processes (very close by in M. mottai, vs. spaced in M. huecoensis sp. nov. ).
Mesobiotus peterseni (Maucci, Citation1991) by the presence of a reticulation between the egg processes (not visible in M. peterseni, vs. visible in M. huecoensis sp. nov. ), and by more numerous processes on the egg circumference (21 – 30 in M. huecoensis, 13 – 15 in M. peterseni).
By having elongated primary claw IV branches, M. huecoensis is similar to Mesobiotus altitudinalis and Mesobiotus barabanovi, but differs from:
Mesobiotus altitudinalis (Biserov, Citation1997/8) by the egg processes shape (domes in M. huecoensis sp. nov. vs. pointed cones in M. altitudinalis).
Mesobiotus barabanovi (Tumanov, Citation2005) by the egg processes shape (domes in M. huecoensis sp. nov. vs. pointed cones in M. barabanovi), the presence of smooth lunulae (dentate in M. barabanovi) and shorted claws IV (pt of posterior primary branch of claws IV 37.9 – 49.9 in M. huecoensis sp. nov. vs. 51.7 – 66.7 in M. barabanovi)
Discussion
Tardigrade records from New Mexico are very scarce, as so far in only four studies records of tardigrades are provided (Beasley Citation1988; Mehlen Citation1969; Meyer & Hinton Citation2010; Pilato et al. Citation2007; records also reviewed in; Meyer Citation2013; Kaczmarek et al. Citation2016). Of the 21 species recorded, one of them (Hypsibius macrocalcaratus Beasley, Citation1988) has its type locality in this state, whereas two others have been described with material that included specimens from New Mexico (Echiniscus viridianus Pilato, Fontoura & Lisi, Citation2007 and Milnesium zsalakoae Meyer & Hinton, Citation2010). Regarding the genus Mesobiotus, only Beasley (Citation1988) recorded it, making the present contribution the second record for this genus in New Mexico.
Claws elongation in tardigrades is hypothesized to be an adaptation to aquatic and glacial habitats, as longer claws help grip the substrate to avoid being dislodged by water currents; this trait has evolved multiple times convergently in tardigrades (Bertolani Citation1981; Zawierucha et al. Citation2018; Guidetti et al. Citation2019; Stec et al. Citation2022; Stec & Morek Citation2022). The phylogenetic position of this new species, completely unrelated to M. cf. barabanovi (another species with elongated claws), represents the first evidence of multiple independent evolution of elongated claws within a tardigrade genus. The claws of the new Mesobiotus species can be considered intermediate between standard Mesobiotus claws and properly elongated ones (pt of posterior primary branch of claws IV in two typical Mesobiotus species: M. diegoi and M. maklowiczi do not exceed 36%, and it ranges from 37% and 50% in the new species, and between 51% and 67% in M. barabanovi). This intermediate claw elongation could hint to its adaptation to the peculiar habitat in which it has been found with periodic inundations followed by desiccation. However, a mechanicistic explanations on how exactly claw elongation relates to substrate type and inundation frequency are still elusive.
The sperm of the new species shows similarities with the two other Mesobiotus species for which sperm data are available, namely Mesobiotus harmsworthi (Murray Citation1907; Rebecchi et al. Citation2011) and Mesobiotus sp (Sugiura & Matsumoto Citation2021a). These new data on Mesobiotus sperm, together with the data on other Macrobiotidae genera sperm morphometry presented by Rebecchi (Citation1997), Rebecchi et al. (Citation2011) and Sugiura and Matsumoto (Citation2021a, Citation2021b) allow us to hypothesize a genus level constant sperm size, with the model Mesobiotus sperm of intermediate size (range within Mesobiotus 45.6–52.7 µm, average values for N = 3 species) between Macrobiotus (range 18–38 µm, N = 7 species) and Paramacrobiotus (range 82.9–117 µm, N = 3 species); as noted by Sugiura and Matsumoto (Citation2021a). Mesobiotus sperm is also easily differentiated from the other two considered Macrobiotidae genera sperm by the longer midpiece (Mesobiotus 4–11.5 µm, N = 3 species; Macrobiotus 2.3–4 µm, N = 5; Paramacrobiotus 3.4–4.5 µm, N = 3) (data on sperm morphometric from Guidi & Rebecchi Citation1996; Rebecchi Citation1997; Rebecchi & Guidi Citation1991; Rebecchi et al. Citation2000, Citation2011; Sugiura & Matsumoto Citation2021a, Citation2021c). This trend of uniformity in sperm morphometry within genera can be confirmed by examining additional species and will be helpful in providing more informative characters for delimitation of genera and overall taxonomy, as well as provide insight into the evolution of sperm in tardigrades. The mating behaviour also shows similarities with what described for another undescribed Mesobiotus species (Sugiura & Matsumoto Citation2021a). In particular multiple ejaculations at distance from the female seem to be a characteristic behavioral trait. The mounting behaviour also cannot be considered as an accidental cloaca contact, as the male curled up in the classic position observed in mounting males (Sugiura & Matsumoto Citation2021a).
Tardigrades from freshwater rock pools have been largely neglected. The very recent description of another species from this habitat type (Acutuncus giovanniniae Vecchi et al., Citation2023), together with the species described here, support the idea that these ephemeral water basins could host a high biodiversity and endemism. The same patterns have been noted for rotifers in the same habitat (Schröder et al. Citation2007; Brown et al. Citation2020). The presence of endemic meiofauna (of which probably many more species are yet to be discovered) highlights the need for the study and preservation of this peculiar habitat and calls for efforts in overcoming the biodiversity knowledge shortfalls (Hortal et al. Citation2015) that prevent a comprehensive understanding of their biodiversity.
CRediT author statement
VM: Conceptualization, Methodology, Formal analysis, Data curation, Writing - Original Draft, Writing - Review & Editing, Visualization, Supervision, Funding acquisition. MJL: Conceptualization, Methodology, Writing - Original Draft, Writing - Review & Editing, Visualization. CJ: Methodology, Writing - Original Draft, Writing - Review & Editing, Visualization. VT: Methodology, Writing - Review & Editing. WEJ: Conceptualization, Resources, Writing - Original Draft, Writing - Review & Editing, Visualization, Supervision, Project administration, Funding acquisition. CS: Resources, Writing - Review & Editing, Project administration, Funding acquisition.
Supplemental Material
Download MS Word (12 KB)Supplemental Material
Download MS Word (282.2 KB)Supplemental Material
Download MS Word (16.9 KB)Supplemental Material
Download MS Word (31.1 KB)Supplemental Material
Download MS Word (12 KB)Supplemental Material
Download MS Excel (11.1 KB)Supplemental Material
Download MS Excel (135.5 KB)Acknowledgments
We thank Matthew Atencio, New State Bureau of Land Management, for facilitating collections at Socorro Box Canyon. We are grateful to Daniele Camarda (University of Catania, Italy) and Daniel Stec (Institute of Systematics and Evolution of Animals, Polish Academy of Sciences, Poland) for acquiring microphotographs of the specimens belonging to the species of the Mesobiotus montanus morphogroup and of the new species. We also thank Roberto Guidetti (University of Modena and Reggio Emilia, Italy) for acquiring the egg SEM photograph. Tristan Chavez-Poeschel produced the map.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data that support the findings of this study are openly available in Figshare at DOI:10.6084/m9.figshare.22656586 under CC-BY 4.0 license and in GenBank (see Table 1 for accession numbers).
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/24750263.2023.2263033.
Additional information
Funding
References
- Bartels PJ Nelson DR. 2006. A large-scale, multihabitat inventory of the Phylum Tardigrada in the Great Smoky Mountains National Park, USA: A preliminary report. Hydrobiologia 558(1):111–118. DOI:10.1007/s10750-005-1405-9.
- Bartels PJ, Nelson DR Exline RP. 2011. Allometry and the removal of body size effects in the morphometric analysis of tardigrades. Journal of Zoological Systematics and Evolutionary Research 49(s1):17–25. DOI:10.1111/j.1439-0469.2010.00593.x.
- Beasley CW. 1988. Altitudinal distribution of Tardigrada of new Mexico with the description of a new species. The American Midland Naturalist 120(20):436–440. DOI:10.2307/2426016.
- Bertolani R. 1971. Contributo alla cariologia dei Tardigradi. Osservazioni su Macrobiotus hufelandii. Atti Della Accademia Nazionale dei Lincei: Classe di Scienze Fisiche, Matematiche e Naturali: Rendiconti 50(6):772–775.
- Bertolani R. 1981. A new genus and five new species of Italian freshwater tardigrades. Bollettino del Museo Civico di Storia Naturale di Verona 8:249–254.
- Bertolani R, Guidetti R, Marchioro T, Altiero T, Rebecchi L Cesari M. 2014. Phylogeny of Eutardigrada: New molecular data and their morphological support lead to the identification of new evolutionary lineages. Molecular Phylogenetics and Evolution 76(1):110–126. DOI:10.1016/j.ympev.2014.03.006.
- Binda MG Pilato G. 1994. Macrobiotus mottai, nuova specie di eutardigrado dell’Antartide. Animalia 21:53–56.
- Biserov VI. 1997/98. Tardigrades of the Caucasus with a taxonomic analysis of the genus Ramazzottius (Parachela: Hypsibiidae). Zoologischer Anzeiger 236:139–159.
- Boix D, Kneitel J, Robson BJ, Duchet C, Zúñiga L, Day J, Gascón S, Sala J, Quintana XD Blaustein L. 2016. Invertebrates of freshwater temporary ponds in Mediterranean climates. In: Invertebrates in freshwater wetlands. Springer International Publishing. pp. 141–189. DOI:10.1007/978-3-319-24978-0_5.
- Brown PD, Schröder T, Ríos-Arana JV, Rico-Martinez R, Silva-Briano M, Wallace RL Walsh EJ. 2020. Patterns of Rotifer diversity in the Chihuahuan desert. Diversity 12(10):393. DOI:10.3390/d12100393.
- Casquet J, Thebaud C Gillespie RG. 2012. Chelex without boiling, a rapid and easy technique to obtain stable amplifiable DNA from small amounts of ethanol‐stored spiders. Molecular Ecology Resources 12(1):136–141. DOI:10.1111/j.1755-0998.2011.03073.x.
- Coughlan K Stec D. 2019. Two new species of the Macrobiotus hufelandi complex (Tardigrada: Eutardigrada: Macrobiotidae) from Australia and India, with notes on their phylogenetic position. European Journal of Taxonomy 573:Article 573. DOI:10.5852/ejt.2019.573.
- Darby BJ Neher DA. 2012. Stable isotope composition of microfauna supports the occurrence of biologically fixed nitrogen from cyanobacteria in desert soil food webs. Journal of Arid Environments 85:76–78. DOI: 10.1016/j.jaridenv.2012.06.006.
- Davis H. 1873. A new Callidina: With the result of experiments on the desiccation of rotifers. The Monthly Microscopical Journal 9(5):201–209. DOI:10.1111/j.1365-2818.1873.tb02268.x.
- De Vries CH. 1996. Invertebrate community structure and dynamics in Korannaberg rock pools. Bloemfontein, South Africa: University of the Orange Free State.
- Degma P, and Guidetti R. 2023. Actual checklist of Tardigrada species (2009-2023, 42 th Edition: 09-01-2023). Modena, Italy: Università di Modena e Reggio Emilia.
- Doyère L. 1840. Mémoire sur les Tardigrades. Annales des Sciences Naturelles 14:269–361.
- Edgar RC. 2004. MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Research 32(5):1792–1797. DOI:10.1093/nar/gkh340.
- Esri. 2017. Topographic [basemap]. Scale not given. “World topographic Map”. Oct 26. Available: https://esri.maps.arcgis.com/home/item.html?id=7dc6cea0b1764a1f9af2e679f642f0f5. Accessed Mar 2023 9.
- Friard O Gamba M. 2016. BORIS: A free, versatile open‐source event‐logging software for video/audio coding and live observations. Methods in Ecology and Evolution 7(11):1325–1330. DOI:10.1111/2041-210X.12584.
- GIMP Development Team. 2019. GIMP. Available: https://www.gimp.org.
- Guidetti R, Massa E, Bertolani R, Rebecchi L Cesari M. 2019. Increasing knowledge of Antarctic biodiversity: New endemic taxa of tardigrades (Eutardigrada; Ramazzottiidae) and their evolutionary relationships. Systematics and Biodiversity 17(6):573–593. DOI:10.1080/14772000.2019.1649737.
- Guidetti R, Schill RO, Bertolani R, Dandekar T Wolf M. 2009. New molecular data for tardigrade phylogeny, with the erection of Paramacrobiotus gen. nov. Journal of Zoological Systematics and Evolutionary Research 47(4):315–321. DOI:10.1111/j.1439-0469.2009.00526.x.
- Guidi A Rebecchi L. 1996. Spermatozoan morphology as a character for tardigrade systematics: Comparison with sclerified parts of animals and eggs in eutardigrades. Zoological Journal of the Linnean Society 116(1–2):101–113. DOI:10.1111/j.1096-3642.1996.tb02336.x.
- Hengherr S Schill RO. 2018. Environmental adaptations: Cryobiosis. In: Schill R, editor. Water bears: The biology of Tardigrades. Zoological monographs. Vol. 2. Cham: Springer International Publishing. pp. 295–310. DOI:10.1007/978-3-319-95702-9_11.
- Hortal J, de Bello F, Diniz-Filho JAF, Lewinsohn TM, Lobo JM Ladle RJ. 2015. Seven shortfalls that beset large-scale knowledge of biodiversity. Annual Review of Ecology, Evolution, and Systematics 46(1):523–549. DOI:10.1146/annurev-ecolsys-112414-054400.
- Inkscape Project. 2020. Inkscape. Available: https://inkscape.org.
- Itang LAM, Stec D, Mapalo MA, Mirano-Bascos D Michalczyk Ł. 2020. An integrative description of Mesobiotus dilimanensis, a new tardigrade species from the Philippines (Eutardigrada: Macrobiotidae: Furciger group). The Raffles Bulletin of Zoology 68:19–31.
- Jocqué M, Graham T Brendonck L. 2007. Local structuring factors of invertebrate communities in ephemeral freshwater rock pools and the influence of more permanent water bodies in the region. Hydrobiologia 592(1):271–280. DOI:10.1007/s10750-007-0766-7.
- Jornada Basin Spatial Data Laboratory, NSF Jornada Basin LTER/USDA ARS Jornada Experimental Range. 2006 Mar. Chihuahuan desert boundary. Available: http://jornada-www.nmsu.edu/gis/giscat.php. Accessed Apr 2022 21.
- Kaczmarek Ł, Bartylak T, Stec D, Kulpa A, Kepel M, Kepel A Roszkowska M. 2020. Revisiting the genus Mesobiotus (Eutardigrada, Macrobiotidae)–remarks, updated dichotomous key and an integrative description of new species from Madagascar. Zoologischer Anzeiger 287:121–146. DOI:10.1016/j.jcz.2020.05.003.
- Kaczmarek Ł, Michalczyk Ł McInnes SJ. 2016. Annotated zoogeography of non-marine Tardigrada. Part III: North America and Greenland. Zootaxa 4203(1):1–249. DOI:10.11646/zootaxa.4203.1.1.
- Kaczmarek Ł, Zawierucha K, Buda J, Stec D, Gawlak M, Michalczyk Ł, Roszkowska M, and Rubal M. 2018. An integrative redescription of the nominal taxon for the Mesobiotus harmsworthi group (Tardigrada: Macrobiotidae) leads to descriptions of two new Mesobiotus species from Arctic. PLoS One 13(10):e0204756. DOI:10.1371/journal.pone.0204756.
- Katoh K. 2002. MAFFT: A novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Research 30(14):3059–3066. DOI:10.1093/nar/gkf436.
- Katoh K Toh H. 2008. Recent developments in the MAFFT multiple sequence alignment program. Briefings in Bioinformatics 9(4):286–298. DOI:10.1093/bib/bbn013.
- Kayastha P, Roszkowska M, Mioduchowska M, Gawlak M, and Kaczmarek Ł. 2021. Integrative descriptions of two new tardigrade species along with the new record of Mesobiotus skorackii Kaczmarek et al., 2018 from Canada. Diversity 13(8):394. DOI:10.3390/d13080394.
- Koste W. 1996. On soil Rotatoria from a lithotelma near Halali Lodge in Etosha National Park in N-Namibia, South Africa. Internationale Revue Der Gesamten Hydrobiologie Und Hydrographie 81(3):353–365. DOI:10.1002/iroh.19960810305.
- Lanfear R, Frandsen PB, Wright AM, Senfeld T, and Calcott B. 2016. PartitionFinder 2: New Methods for selecting partitioned models of evolution for molecular and morphological phylogenetic analyses. Molecular Biology and Evolution msw260. DOI: 10.1093/molbev/msw260.
- Mapalo MA, Stec D, Mirano-Bascos D Michalczyk Ł. 2016. Mesobiotus philippinicus sp. nov., the first limnoterrestrial tardigrade from the Philippines. Zootaxa 4126(3):Article 3. DOI:10.11646/zootaxa.4126.3.6.
- Mapalo MA, Stec D, Mirano-Bascos D, and Michalczyk Ł. 2017. An integrative description of a limnoterrestrial tardigrade from the Philippines, Mesobiotus insanis, new species (Eutardigrada: Macrobiotidae: Harmsworthi group). The Raffles Bulletin of Zoology 65:440–454.
- Marley NJ, Mcinnes SJ Sands CJ. 2011. Phylum Tardigrada: A re-evaluation of the Parachela. Zootaxa 64(2819):Article 2819. DOI:10.11646/zootaxa.2819.1.2.
- Maucci W. 1991. Tre nuove specie di Eutardigradi della Groenlandia Meridionale. Bollettino del Museo Civico di Storia Naturale di Verona 15:279–286.
- Maucci W Durante-Pasa M. 1984. Macrobiotus lusitanicus sp. nov., nuova pecie di Eutardigrado del Portogallo nord-occidentale (Tardigrada, Macrobiotidae). Bollettino del Museo Civico di Storia Naturale di Verona 11:319–326.
- McDaniel J. 2022. Chihuahuan desert rock pool community assemblages: Patterns of taxonomic diversity. El Paso, Texas, USA: University of Texas at El Paso.
- Mehlen RH. 1969. New Tardigrada from Texas. The American Midland Naturalist 81(2):395–404. DOI:10.2307/2423979.
- Meyer HA. 2013. Terrestrial and freshwater Tardigrada of the Americas. Zootaxa 3747(1):1–71. DOI:10.11646/zootaxa.3747.1.1.
- Meyer HA Hinton JG. 2010. Milnesium zsalakoae and M. jacobi, two new species of Tardigrada (Eutardigrada: Apochela: Milnesiidae) from the southwestern United States. Proceedings of the Biological Society of Washington 123(2):113–120. DOI:10.2988/09-29.1.
- Murray J. 1907. XXV.—Arctic Tardigrada, collected by Wm. S. Bruce. Earth and Environmental Science Transactions of the Royal Society of Edinburgh 45(3):669–681. DOI:10.1017/S0080456800011789.
- Murray J. 1910. Tardigrada. In: British Antarctic expedition 1907—1909: Reports on the scientific Investigations: Vol. I: Biology, Part V. pp. 115–256.
- Mutterer J Zinck E. 2013. Quick-and-clean article figures with FigureJ. Journal of Microscopy 252(1):89–91. DOI:10.1111/jmi.12069.
- Nelson DR, Bartels PJ Guil N. 2018. Tardigrade Ecology. In: Schill R, editor. Water bears: The biology of Tardigrades. Zoological monographs. Vol. 2. Cham: Springer International Publishing. pp. 163–210. DOI:10.1007/978-3-319-95702-9_7.
- Nowak B Stec D. 2018. An integrative description of Macrobiotus hannae sp. nov. (Tardigrada: Eutardigrada: Macrobiotidae: Hufelandi group) from Poland. Turkish Journal of Zoology 42(3):Article 3. DOI:10.3906/zoo-1712-31.
- Pilato G. 1981. Analisi di nuovi caratteri nello studio degli Eutardigradi. Animalia 8:51–57.
- Pilato G, Paulo F Lisi O. 2007. Remarks on the Echiniscus viridis group, with the description of a new species (Tardigrada, Echiniscidae). Journal of Limnology 66:33–39. DOI:10.4081/jlimnol.2007.s1.33.
- Rambaut A. 2007. FigTree, a graphical viewer of phylogenetic trees. http://tree.bio.ed.ac.uk/software/figtree/.
- Rambaut A, Drummond AJ, Xie D, Baele G, Suchard MA Susko E. 2018. Posterior summarization in bayesian phylogenetics using Tracer 1.7. Systematic Biology 67(5):901–904. DOI:10.1093/sysbio/syy032.
- Rebecchi L. 1997. Ultrastructural study of spermiogenesis and the testicular and spermathecal spermatozoon of the gonochoristic tardigrade Xerobiotus pseudohufelandi (Eutardigrada, Macrobiotidae). Journal of Morphology 234(1):11–24. DOI:10.1002/(SICI)1097-4687(199710)234:1<11:AID-JMOR2>3.0.CO;2-Q.
- Rebecchi L, Altiero T Guidetti R. 2007. Anhydrobiosis: The extreme limit of desiccation tolerance. Invertebrate Survival Journal 4(2):65–81.
- Rebecchi L, Altiero L Guidi A. 2011. The ultrastructure of the tardigrade spermatozoon: A comparison between Paramacrobiotus and Macrobiotus species (Eutardigrada). Invertebrate Zoology 8(1):63–67. DOI:10.15298/invertzool.08.1.08.
- Rebecchi L Bertolani R. 1994. Maturative pattern of ovary and testis in eutardigrades of freshwater and terrestrial habitats. Invertebrate Reproduction & Development 26(2):107–117. DOI:10.1080/07924259.1994.9672407.
- Rebecchi L Guidi A. 1991. First SEM studies on tardigrade spermatozoa. Invertebrate Reproduction & Development 19(2):151–156. DOI:10.1080/07924259.1991.9672169.
- Rebecchi L, Guidi A, and Bertolani R. 2000. Tardigrada. In: Adiyodi KG, Adiyodi RG, editors. Reproductive biology of invertebrates. Progress in male gamete ultrastructure and phylogenyOxford & IBH Publishing CO PVT LTD. Vol. 9. pp. 267–291.
- Richters F. 1926. Tardigrada. In: Kükenthal W, and Krumbach, editors. Handbuch der Zoologie. Vol. 3. Berlin, Germany: T. Walter de Gruyter & Co. pp. 58–61.
- Ronquist F, Teslenko M, van der Mark P, Ayres DL, Darling A, Höhna S, Larget B, Liu L, Suchard MA Huelsenbeck JP. 2012. MrBayes 3.2: Efficient bayesian phylogenetic inference and model choice across a large model space. Systematic Biology 61(3):539–542. DOI:10.1093/sysbio/sys029.
- Roszkowska M, Stec D, Gawlak M Kaczmarek Ł. 2018. An integrative description of a new tardigrade species Mesobiotus romani sp. nov. (Macrobiotidae: Harmsworthi group) from the Ecuadorian Pacific coast. Zootaxa 4450(5):Article 5. DOI:10.11646/zootaxa.4450.5.2.
- Schill RO Hengherr S. 2018. Environmental adaptations: Desiccation tolerance. In: Schill, R., editor. Water bears: The biology of Tardigrades. Zoological monographs. Vol. 2. Cham: Springer International Publishing. pp. 273–293. DOI:10.1007/978-3-319-95702-9_10.
- Schneider CA, Rasband WS Eliceiri KW. 2012. NIH image to ImageJ: 25 years of image analysis. Nature Methods 9(7):671–675. DOI:10.1038/nmeth.2089.
- Schröder T, Howard S, Arroyo ML Walsh EJ. 2007. Sexual reproduction and diapause of Hexarthra sp. (Rotifera) in short-lived ponds in the Chihuahuan desert. Freshwater Biology 52(6):1033–1042. DOI:10.1111/j.1365-2427.2007.01751.x.
- Schuster RO, Nelson DR, Grigarick AA Christenberry D. 1980. Systematic criteria of the Eutardigrada. Transactions of the American Microscopical Society 99(3):284. DOI:10.2307/3226004.
- Short KA, Sands CJ, McInnes SJ, Pisani D, Stevens MI Convey P. 2022. An ancient, antarctic-specific species complex: Large divergences between multiple antarctic lineages of the tardigrade genus Mesobiotus. Molecular Phylogenetics and Evolution 170:107429. DOI:10.1016/j.ympev.2022.107429.
- Spencer M, Blaustein L, Schwartz S Cohen J. 1999. Species richness and the proportion of predatory animal species in temporary freshwater pools: Relationships with habitat size and permanence. Ecology Letters 2:157–166. DOI:10.1046/j.1461-0248.1999.00062.x.
- Stec D. 2019. Mesobiotus datanlanicus sp. nov., a new tardigrade species (Macrobiotidae: Mesobiotus harmsworthi group) from Lâm ồng Province in Vietnam. Zootaxa 4679(1):Article 1. DOI:10.11646/zootaxa.4679.1.10.
- Stec D. 2021. Integrative descriptions of two new Mesobiotus species (Tardigrada, Eutardigrada, Macrobiotidae) from Vietnam. Diversity 13(11):605. DOI:10.3390/d13110605.
- Stec D. 2022. Integrative descriptions of two new Mesobiotus species (Tardigrada, Eutardigrada, Macrobiotidae) from Vietnam. Zoological Studies 61:605. DOI:10.6620/ZS.2022.61-85.
- Stec D Kristensen RM. 2017. An integrative description of Mesobiotus ethiopicus sp. nov.(Tardigrada: Eutardigrada: Parachela: Macrobiotidae: Harmsworthi group) from the Northern Afrotropic region. Turkish Journal of Zoology 41(5):Article 5. DOI:10.3906/zoo-1701-47.
- Stec D, Kristensen RM, and Michalczyk Ł. 2020. An integrative description of minibiotus ioculator sp. nov. from the Republic of South Africa with notes on Minibiotus pentannulatus Londoño et al., 2017 (Tardigrada: Macrobiotidae). Zoologischer Anzeiger 286:117–134. DOI:10.1016/j.jcz.2020.03.007.
- Stec D, and Morek W. 2022. Reaching the monophyly: Re-evaluation of the enigmatic species Tenuibiotus hyperonyx (Maucci, 1983) and the genus Tenuibiotus (Eutardigrada). Animals 12(3):404. DOI:10.3390/ani12030404.
- Stec D, Roszkowska M, Kaczmarek Ł Michalczyk Ł. 2018. An integrative description of a population of Mesobiotus radiatus (Pilato, Binda & Catanzaro, 1991) from Kenya. Turkish Journal of Zoology 42(5):Article 5. DOI:10.3906/zoo-1802-43.
- Stec D, Vecchi M, Calhim S Michalczyk Ł. 2021. New multilocus phylogeny reorganises the family Macrobiotidae (Eutardigrada) and unveils complex morphological evolution of the Macrobiotus hufelandi group. Molecular Phylogenetics and Evolution 160:160. DOI:10.1016/j.ympev.2020.106987.
- Stec D, Vončina K, Møbjerg Kristensen R Michalczyk Ł. 2022. The Macrobiotus ariekammensis species complex provides evidence for parallel evolution of claw elongation in macrobiotid tardigrades. Zoological Journal of the Linnean Society 195(4):1067–1099. DOI:10.1093/zoolinnean/zlab101.
- Sugiura K Matsumoto M. 2021a. Reproduction of Mesobiotus: Comparison of morphology and behavior in the family Macrobiotidae (Tardigrada: Eutardigrada). Zoological Science 38(5). DOI:10.2108/zs210045.
- Sugiura K Matsumoto M. 2021b. Sexual reproductive behaviours of tardigrades: A review. Invertebrate Reproduction & Development 65(4):279–287. DOI:10.1080/07924259.2021.1990142.
- Sugiura K Matsumoto M. 2021c. Spermatozoa morphology changes during reproduction and first observation of acrosomal contact in two dioecious species of Macrobiotidae (Tardigrada: Eutardigrada). Zygote 29(1):42–48. DOI:10.1017/S0967199420000490.
- Thulin G. 1928. Über die Phylogenie und das System der Tardigraden. Hereditas 11(2–3):207–266. DOI:10.1111/j.1601-5223.1928.tb02488.x.
- Tumanov DV. 2005. Two new species of Macrobiotus (Eutardigrada, Macrobiotidae) from Tien Shan (Kirghizia), with notes on Macrobiotus tenuis group. Zootaxa 46(1043):Article 1043. DOI:10.11646/zootaxa.1043.1.3.
- Tumanov DV. 2020. Integrative description of Mesobiotus anastasiae sp. nov. (Eutardigrada, Macrobiotoidea) and first record of Lobohalacarus (Chelicerata, Trombidiformes) from the Republic of South Africa. European Journal of Taxonomy 726. DOI: 10.5852/ejt.2020.726.1179.
- Vecchi M Bruneaux M. 2021. Concatipede: An R package to concatenate fasta sequences easily. DOI:10.5281/zenodo.5130604.
- Vecchi M, Cesari M, Bertolani R, Ingemar Jönsson K, Rebecchi L Guidetti R. 2016. Integrative systematic studies on tardigrades from Antarctica identify new genera and new species within Macrobiotoidea and Echiniscoidea. Invertebrate Systematics 30(4):303. DOI:10.1071/IS15033.
- Vecchi M, Ferrari C, Stec D Calhim S. 2022. Desiccation risk favours prevalence and diversity of tardigrade communities and influences their trophic structure in alpine ephemeral rock pools. Hydrobiologia 849(9):1995–2007. DOI:10.1007/s10750-022-04820-0.
- Vecchi M Stec D. 2021. Integrative descriptions of two new Macrobiotus species (Tardigrada, Eutardigrada, Macrobiotidae) from Mississippi (USA) and Crete (Greece). Zoosystematics and Evolution 97(1):381–306. DOI:10.3897/zse.97.65280.
- Vecchi M, Tsvetkova A, Stec D, Ferrari C, Calhim S Tumanov D. 2023. Expanding Acutuncus: Phylogenetics and morphological analyses reveal a considerably wider distribution for this tardigrade genus. Molecular Phylogenetics and Evolution 180:107707. DOI:10.1016/j.ympev.2023.107707.
- Velasco-González I, Sanchez-Jimenez A, Singer D, Murciano A, Díez-Hermano S, Lara E Martín-Cereceda M. 2020. Rain-fed granite rock basins accumulate a high diversity of dormant microbial eukaryotes. Microbial Ecology 79(4):882–897. DOI:10.1007/s00248-019-01463-y.
- Zawierucha K, Ostrowska M, Vonnahme TR, Devetter M, Nawrot AP, Lehmann S Kolicka M. 2016. Diversity and distribution of Tardigrada in Arctic cryoconite holes. Journal of Limnology 75(3). DOI:10.4081/jlimnol.2016.1453.
- Zawierucha K, Stec D, Lachowska-Cierlik D, Takeuchi N, Li Z Michalczyk Ł. 2018. High mitochondrial Diversity in a new water bear species (Tardigrada: Eutardigrada) from Mountain Glaciers in Central Asia, with the erection of a new genus Cryoconicus. Annales Zoologici 68(1):179–201. DOI:10.3161/00034541anz2018.68.1.007.