ABSTRACT
Amblyopia is a common perceptual disorder resulting from abnormal visual input during development. The clinical presentation and visual deficits associated with amblyopia are well characterized. Less is known however, about amblyopia’s impact on the central nervous system (CNS). While early insights into the neuropathophysiology of amblyopia have been based on findings from animal models and postmortem human studies, recent advances in noninvasive magnetic resonance imaging (MRI) techniques have enabled the study of amblyopia’s effects in vivo. We review recent retinal and neuroimaging research documenting amblyopia’s structural and functional impact on the CNS. Clinical imaging provides some evidence for retinal and optic nerve abnormalities in amblyopic eyes, although the overall picture remains inconclusive. Neuroimaging studies report clearer changes in both structure and function of the visual pathways. In the optic nerves, optic tracts, and optic radiations of individuals with amblyopia, white-matter integrity is decreased. In the lateral geniculate nuclei, gray matter volume is decreased and neural activity is reduced. Reduced responses are also seen in the amblyopic primary visual cortex and extrastriate areas. Overall, amblyopia impacts structure and function at multiple sites along the visual processing hierarchy. Moreover, there is some evidence that amblyopia’s impact on the CNS depends on its etiology, with different patterns of results for strabismic and anisometropic amblyopia. To clarify the impact of amblyopia on the CNS, simultaneous collection of retinal, neural, and perceptual measures should be employed. Such an approach will help (1) distinguish cause and effect of amblyopic impairments, (2) separate the impact of amblyopia from other superimposed conditions, and (3) identify the importance of amblyopic etiology to specific neural and perceptual deficits.
Introduction
Amblyopia is caused by poor or poorly coordinated retinal input during childhood. It is characterized by a unilateral, or less commonly, bilateral reduction in best-corrected visual acuity. The prevalence of amblyopia in North America is 1–4%,Citation1,Citation2 and ~2% globally.Citation3 It is the most common cause of vision loss in children.Citation4
While amblyopia is caused by abnormal retinal input during childhood, the underlying neural deficits are primarily found in the central nervous system (CNS). The chronic abnormal retinal input leads to an atypical developmental trajectory, which is reflected in both structural and functional CNS abnormalities.
Historically, most of what we know about the impact of amblyopia on the CNS is based on animal models and postmortem histology. However, noninvasive magnetic resonance imaging (MRI) techniques have become available to study the impact of amblyopia on the microstructure and function of the human visual system in vivo.
Here, we review recent neuroimaging research that documents the impact of amblyopia on the central nervous system. We start with a brief overview of the behavioral consequences of amblyopia, which extend well beyond the clinical criterion of reduced visual acuity. Mirroring the behavioral consequences, amblyopia seems to impact microstructure and function at multiple sites along the visual processing hierarchy. Importantly, both behavioral and neural impairments differ to some extent based on the type of retinal input disruption – anisometropic and strabismic amblyopia produce divergent patterns of abnormalities. We conclude that future work should take etiology into account when considering the impact of amblyopia on the CNS.
Behavioral consequences
While amblyopia is clinically defined as a two-line reduction in visual acuity between eyes, it impacts overall visual function much more broadly. Deficits range from reductions in fixational stability, to reduced spatial acuityCitation5,Citation6 and contrast sensitivity,Citation7–9 through impairments in form,Citation10–13 motionCitation14,Citation15 and depthCitation5,Citation16 perception. Some studies have even reported a negative impact on reading.Citation17–19
Effects are not exclusive to the amblyopic eye. The non-amblyopic (fellow) eye actively suppresses input from the amblyopic eye,Citation20,Citation21 and there are measurable deficits of spatial, position, and motion sensitivity in the fellow eye.Citation21–23
Amblyopia can be caused by a range of abnormalities,Citation1,Citation24 with strabismus and anisometropia being most common.Citation25 While amblyopia can have other causes, such as input deprivation, we limit discussion of these other types, because few human studies have been conducted on their perceptual and neural correlates.
Importantly, the exact pattern of perceptual deficits appears to differ depending on etiology. In anisometropic amblyopia, binocular function is more likely to be preserved. Specifically, stereoacuity and binocular motion sensitivity are usually less affected and can improve with treatment.Citation22,Citation26 Furthermore, anisometropic amblyopia is associated with greater fixation stability.Citation27
In strabismic amblyopia on the other hand, there is often no measurable stereoacuity, even if visual acuity has improved after treatment.Citation22,Citation28 However, contrast sensitivity is more likely to be preserved,Citation26 which might suggest that individuals with strabismic amblyopia retain better monocular spatial resolution at the expense of reduced binocularity.
In conclusion, the broad impact of amblyopia on visual function points to neural impairments at multiple stages of the visual hierarchy. Moreover, different amblyopia etiologies may be associated with partially non-overlapping neural abnormalities.
Impact of amblyopia on the CNS: The classical view
Much of our knowledge of the neurodevelopmental impact of abnormal visual input comes from animal studies. Research on the impact of deprivation amblyopia in cats showed that extended eye closure through eyelid suturing during a so-called “critical period” in early life (from 4 weeks to about 3 months of age) results in significant structural and functional changes in both the lateral geniculate nuclei (LGN) and striate cortex, commonly referred to as primary visual cortex, or V1, in humans.Citation29
The visual system is highly sensitive during this critical period, and just a few days of monocular deprivation are sufficient to significantly disrupt neural development. In the LGN, cells responsive to the sutured eye were noticeably smaller than those of the open eye. In striate cortex, direct electrophysiological recordings showed that the number of binocularly responsive cells (and cells showing any responsiveness to the sutured eye) were significantly reduced. Moreover, even if normal binocular visual input was restored after a period of extended deprivation (3 months), these physiological changes were largely irreversible. Thus, while the visual system is highly plastic early in development, in the absence of timely intervention, these early structural changes become permanent.
In the macaque monkey, monocular deprivation during a critical period extending from birth to about 10 weeks of life significantly disrupted the development of typical ocular dominance columns in striate cortex.Citation30 Assessing the potential for recovery from this deprivation, a subset of animals were reverse-sutured, such that the sutured eye was opened and the previously open eye was sutured shut. When this reverse suture was performed around six weeks of age, some recovery of the normal ocular dominance column structure was possible. However, when the reverse suture was performed later in life, at 1 year of age, no physiological recovery was possible, again illustrating reduced plasticity following early development.
In addition to the more severe case of full monocular deprivation via lid suturing, disrupted visual input has also been studied in cats with artificial squint or induced anisometropia. Such animals typically exhibit reduced numbers of binocularly responsive cells in early visual cortexCitation31 and altered sensitivity to spatial frequency.Citation32,Citation33 As in earlier deprivation studies, these physiological effects are age-dependent, such that abnormal visual input early on (in the first 3 months of life) led to lasting structural changes, with the largest effects resulting from induced strabismus between 4 and 7 weeks.Citation34 This pattern of results has also been found in more recent studies with non-human primates.(Citation31,Citation33)
Early neuroanatomical and electrophysiological studies of disrupted visual input in animal models have guided the clinical understanding of amblyopia in humans. While these animal studies provided strong preliminary evidence for the basic etiologies of amblyopia and other neurodevelopmental disorders, the characterization of central nervous system changes due to amblyopia in humans remained imprecise. However, the advent of advanced functional and structural in vivo neuroimaging techniques have presented an opportunity to characterize the neural impact of these disorders in humans in more detail.
Impact of amblyopia on the CNS: Human studies
The dependence on noninvasive methods has traditionally limited the study of neural abnormalities in human amblyopia to retinal and postmortem studies. Structural integrity in the most anterior stages of the visual pathway (i.e. the retina and optic nerve head) can be assessed with standard clinical techniques, such as fundus imaging and optical coherence tomography (OCT). Functional integrity has traditionally been determined using electroretinography (ERG) in the retina and visually evoked potentials (VEPs) in brain structures that are close to the skull.
However, in recent decades, advances in magnetic resonance imaging (MRI) methods have enabled more precise in vivo assessment of the structure and function of the brain beyond the retina. Functional integrity can be assessed using functional MRI (fMRI) in which neural activity is estimated based on metabolic demand via blood oxygen level-dependent (BOLD) signals.Citation35 Structural integrity can be assessed with voxel-based morphometry (VBM) or diffusion MRI (dMRI). VBM is based on MRI intensity differences produced by different kinds of brain tissue and can assess local gray and white-matter volume. Diffusion MRI measures the diffusion of water molecules in tissue, enabling the identification of white-matter pathways in the brain and the estimation of their structural integrity.
An abnormal developmental trajectory can reduce the structural integrity of white-matter tissue in two ways. It can reduce the local density of tissue leading to greater water diffusion, or it can increase local disorganization of tissue, leading to more uniform diffusion. These properties are reflected in two primary dMRI measures: mean diffusivity (MD) and fractional anisotropy (FA). MD reflects overall diffusivity, such that greater values indicate lower white-matter density. FA reflects directional diffusivity, where greater values indicate greater white-matter directionality. In general, decreased white-matter integrity (as indicated by increased MD and/or decreased FA) can be thought of in terms of decreased fiber organization, decreased myelination, and degeneration of white-matter pathways.Citation36,Citation37
If one thinks of amblyopia as a singular disorder with reduced visual acuity as the primary behavioral deficit, one might expect a neural impairment isolated to a single neural site. However, as discussed previously, amblyopia is associated with multiple behavioral impairments, which appear to depend on etiology to some extent. Compared to experimental manipulation in animal models, the observational nature of human amblyopia research makes it more challenging to distinguish the cause and effect of these deficits. However, as we will see below, the pattern of behavioral deficits is mirrored by neural impairments at multiple sites along the visual pathway. The primary findings have been summarized in .
Figure 1. Impact of amblyopia on the central nervous system. Amblyopia produces a pattern of structural and functional impairments across the visual processing hierarchy. The primary neural changes are summarized here. Abbreviations: FA, fractional anisotropy; GM, gray matter; BOLD, blood oxygen level dependent; MD, mean diffusivity; VEP, visually evoked potentials; ILF, inferior longitudinal fasciculus; SLF, superior longitudinal fasciculus
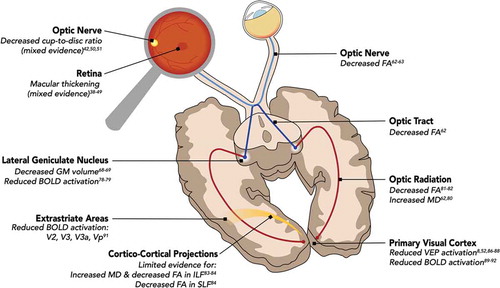
Anterior visual system
Retina and optic nerve head
Evidence for structural differences at the level of the retina and optic nerve has been mixed. Some studies have reported significant differences in macular and retinal nerve fiber layer thickness.Citation38–42 However, others have reported no significant retinal differences.Citation43–47 An earlier meta-analysis indicated increased macular thickness in amblyopic retinas.Citation48 However, a recent comprehensive review of OCT findings in amblyopia concluded that evidence for macular and RNFL changes in amblyopia remains mixed.Citation49 Evidence is similarly mixed for changes to the optic nerve head, with some of the studies showing decreased cup-to-disc ratios of amblyopic and fellow eyes,Citation42 while others report no difference.Citation50,Citation51
Evidence that retinal measures differ based on etiology are inconclusive as well. Some studies have reported that RNFL thickness is affected in anisometropic amblyopia, but not in strabismic amblyopia,Citation38 while others have reported the exact opposite result.Citation40 Perhaps not surprisingly then, a meta-analysis found no significant etiology-based differences.Citation48
Finally, evidence for impairments of retinal function are similarly mixed. Some groups have reported reduced amplitude and/or delayed latency of ERGs.Citation52–55 However, other groups have reported no difference in the retinal responses of anisometropic and strabismic subjects.Citation56
One factor that may contribute to the mixed pattern of results is that other disorders may either be misdiagnosed as amblyopia or superimposed on amblyopia. For example, optic nerve hypoplasia has been reported in presumed amblyopes.Citation57 That is, the optic discs tend to be smaller in both the affected and fellow eyes of amblyopes when compared to those in controls.Citation58 While most pediatric ophthalmologists no longer entertain the possibility that optic nerve hypoplasia underlies unilateral amblyopia, it is often difficult to determine if retinal abnormalities are the direct result of amblyopia or are based on some other cause, especially when those causes tend to co-occur.
A second factor, which may contribute to the mixed pattern of etiology-based results specifically, is that strabismus and anisometropia frequently co-occur as well. Such mixed cases may well be misclassified as exclusively strabismic or anisometropic, further complicating our ability to isolate the retinal measures associated with either etiology.
A third complication in assessing retinal impairments associated with amblyopia is the question of cause and effect. The retinal impairments may either be the cause of amblyopia, or may instead result from compensatory mechanisms due to neural impairments further along the visual processing pathway, which may for example cause abnormal eye movements. Delayed saccades, absence of smooth pursuits, and inaccurate vergence movement has been reported in both amblyopic eyes and their non-amblyopic fellow eyes.Citation17,Citation22 Amblyopic eyes have poor fixation stability, but their fellow non-amblyopic eyes seem to retain this ability.Citation22,Citation59,Citation60 Accommodation is also less accurate in amblyopic eyes than their fellow eyes.Citation22,Citation61
In conclusion, the exact relationship between amblyopia and retinal structure and function remains unclear. Some studies have provided evidence for RNFL thickening in amblyopic eyes, but the overall pattern of results remains inconclusive. While it is possible that certain retinal changes are associated with amblyopia, it is unclear what, if any pathological role these changes may play. Further research is needed to clarify whether retinal changes play any causal role in disease progression, and whether changes noted in previous studies have been simply idiopathic or part of an unrelated ocular pathology.
Optic nerve, optic chiasm, and optic tract
While studies of retinal structure can rely on imaging methods performed in the clinic (OCT, fundus photography, etc.), in vivo assessment of subsequent sub-cortical stages of the visual pathway requires other techniques such as magnetic resonance imaging (MRI). Relatively few studies have explored the structural integrity of the optic nerve, optic chiasm, and optic tract in amblyopia because they are small structures located near the nasal cavity, which distorts MRI signals. However, the handful of studies that do exist provide evidence for structural white-matter abnormalities in these anterior portions of the visual pathway.
Compared to normally sighted controls, fractional anisotropy (FA) in the optic nerves and optic tracts of amblyopes is decreased (averaged for both hemispheres), indicating decreased structural integrity.Citation62 Also of note is the presence of structural changes in the optic tract.Citation62 FA is reduced in the optic tracts of amblyopic patients compared to controls, similar to the reduction in FA noted in amblyopic optic nerves. Given the well-established structural abnormalities of the LGNs in amblyopes (and the partial evidence for retinal changes), it is reasonable that structural changes would occur in the anterior white-matter pathways as well.
Gümüstas, et al. used dMRI with manually drawn regions of interest to sample diffusion measures from prechiasmatic and chiasmatic regions of the visual pathway in unilateral and bilateral amblyopes.Citation63 Similar to the findings of Allen et al.Citation62 in the unilateral amblyopia group, they noted decreased FA in the prechiasmatic portion of affected and fellow optic nerves, as well as increased mean diffusivity in fellow optic nerves compared to controls. Interestingly, they did not find any significant differences in MD or FA in the prechiasmatic or chiasmatic regions of the optic nerves of bilateral amblyopes. The reason for this divergence in structural changes in the anterior pathways of unilateral and bilateral amblyopes is unclear. It is possible that the underlying pathology differs, and that the structural changes noted in the unilateral group (and in the patient population studied by Allen et al.Citation62) is driven by the difference in retinal input between the two eyes, compared to bilateral amblyopia where acuity is poor in both eyes.
Thalamus
Lateral geniculate nucleus (LGN)
Changes in both LGN microstructure and function are a well-documented feature of amblyopic pathology in animal models. The LGN layers receiving input from the affected eye show histological abnormalities.Citation64–67 Similar structural changes have been found in human subjects as well. Two postmortem dissections of human brains from individuals with confirmed strabismic and anisometropic amblyopia noted reduced cell sizes in the parvocellular layers of LGN innervated by sensory afferents from the amblyopic eye.Citation67,Citation68
While the spatial resolution of MRI is insufficient to allow for direct interrogation of the individual layers of LGN, it is sufficient to detect gross differences between patients and controls. Accordingly, in vivo structural neuroimaging has shown decreased gray matter (GM) volumes in LGNs of amblyopic patients compared to normally sighted control subjects.Citation68,Citation69
When it comes to the functional properties of LGN, animal studies indicate response reductions at high spatial frequencies in the LGN.Citation70–74 However, others have suggested that LGN function is preserved.Citation75–77
In humans, several studies have assessed neural activation using fMRI, noting reduced responses in amblyopes compared to controls. Hess et al. measured BOLD responses in a cohort of six amblyopes with different etiologies (anisometropic, strabismic, and deprivation amblyopia) and found bilaterally reduced BOLD activation in the LGNs when stimuli were presented to the amblyopic eyes compared to fellow eyes.Citation78 A case report of a single anisometropic amblyopia patient found absent LGN activation when stimuli were presented to the amblyopic eye compared to the fellow eye.Citation79
In conclusion, changes in LGN microstructure and function in animal models appear to be mirrored in human subjects, as evidenced in both postmortem dissection and in vivo structural neuroimaging studies. While the functional impact of these apparent structural changes is not as well characterized, human fMRI studies provide evidence for disrupted functional activation in response to input from the amblyopic eye. However, a precise characterization of these functional deficits as they relate to amblyopia subtype is currently lacking, and remains an important avenue for future study.
Optic radiation
There are also notable changes to white-matter structure in the optic radiations, the major visual pathways connecting LGN to the primary visual cortex (V1). In general, structural integrity of the optic radiations appears to be reduced compared to those of control subjects, as revealed by changes in mean diffusivity (MD) and fractional anisotropy (FA). Allen et al. reported significantly increased MD in the optic radiations (averaged across both hemispheres) of amblyopes compared to controls.Citation62,Citation80 Other studies have noted bilateral FA reductions in the optic radiations compared to controls.Citation81,Citation82 Duan, et al. reported increased MD in the optic radiation; however, their results were hemisphere-specific, with significant increases in the left optic radiation, but not in the right optic radiation.Citation83 Li, et al. reported decreased FA in the optic radiation, however their effects were restricted to the right, rather than the left optic radiation.Citation84
Primary visual cortex
A number of studies have investigated structural and functional changes in the primary visual cortex (V1) as a result of amblyopia. Structurally, amblyopia is associated with a reduction of gray matter volume in V1.Citation85 Functionally, visual evoked potentials (VEPs) show a reduction in response amplitude and increase in latency.Citation8,Citation52,Citation86–88 Similarly, in humans, fMRI has demonstrated reduced activation in V1Citation89–92 as well as an enlargement of receptive field size, which may reflect either a loss of spatial resolution or decreased spatial organization.Citation93
Cortico-cortical projections
Structural changes have been found in larger cortico-cortical projections, including increased mean diffusivity in the left inferior longitudinal fasciculus (ILF), a major pathway connecting occipital cortex (striate and extrastriate regions) to anterior temporal cortex.Citation83,Citation94 This study described similar structural changes in the right vertical occipital fasciculus (VOF), a little-known (and not thoroughly characterized) pathway connecting dorsal and ventral occipital cortex.Citation95,Citation96 Li et al. found structural changes (decreased FA) in the left ILF as well, in addition to the right superior longitudinal fasciculus (SLF),Citation84 a large pathway that connects portions of the frontal, parietal, occipital, and temporal cortices.Citation97,Citation98
One study reported that projections from subcortical (thalamic) structures directly to extrastriate areas might be affected as well. Mean diffusivity is increased in a pathway connecting LGN and the pulvinar nucleus of the thalamus to extrastriate area hMT+ in amblyopes compared to controls.Citation80 However, given the proximity of this pathway to the optic radiation, some care should be taken in interpreting this result.
Structural white matter (WM) changes have also been found in neural pathways not typically associated with visual function, including the anterior corpus callosum (ACC).Citation83 However, it should be noted that DeMorsier syndrome (septo-optic dysplasia) frequently includes both corpus callosum abnormalities as well optic nerve hypoplasia.Citation99 Therefore, it is possible that this specific finding is the result of an unrelated disorder with neurological and optic nerve features, rather than a structural change resulting from true amblyopia.
Extrastriate cortex
Cortical areas beyond the primary visual cortex are affected as well. Functional activity as measured by BOLD fMRI is reduced in areas V2, V3, Vp, and V3a in the brains of amblyopes (when stimuli are presented to the amblyopic eye) compared to both the dominant and non-dominant eyes of normally sighted controls.Citation91 The reductions in BOLD activity in extrastriate regions appear to be driven, at least in part, by reductions in striate activation, as the responses are highly correlated (V1-V2, V1-V3, etc.).
Functional changes have also been reported in the visual motion-sensitive area hMT+ (sometimes referred to as V5). Comparing activation in response to motion stimuli, Thompson et al. showed functional differences between amblyopes and controls. Control subjects and amblyopic subjects viewing with their fellow eyes exhibited significantly decreased hMT+ activation in response to incoherent motion stimuli, whereas amblyopic participants viewing with their affected eyes did not display differential hMT+ activations.Citation100 Overall, extrastriate cortical areas display functional abnormalities similar to those noted in V1 and to an extent, earlier pre-cortical areas.
Discussion
We reviewed work assessing the impact of amblyopia on the human central nervous system. While the most widely supported effects are thalamocortical, abnormalities are present at multiple stages of visual processing, perhaps beginning at the retina. Deficits are apparent across different imaging modalities, with OCT, fMRI, and dMRI revealing various structural and functional alterations.
With respect to pre-thalamic impact, there is some evidence for microstructural changes in the optic nerves and optic tracts of amblyopic patients. What remains unclear is whether these structural changes follow retinal pathology, result from anterograde degeneration from later portions of the visual system, or are unrelated to the pathophysiology of amblyopia. One fruitful avenue for further research would be to consider the potential relevance of subtype (anisometropia, strabismus, or mixed) to amblyopia’s pathophysiology. This would help establish whether structural changes in the anterior portion of the visual pathways are a necessary feature of amblyopia, or a side effect of a subtype-specific property.
With respect to thalamo-cortical impact, there is substantial evidence for disrupted structure of the optic radiations of amblyopes. It remains unclear, however, to what extent these results (1) are the consequence of earlier stages of dysfunction and (2) vary according to subtype of amblyopia. Additionally, while some studies suggest lateralized effects, the reason for this apparent lateralization is unclear. There is not an obvious pathophysiological explanation for such an effect, as the retinal signals from the amblyopic eyes are mixed with those from the fellow eyes in both optic radiations. It is possible that this is simply an artifact of the specific MRI protocol or patient population under consideration. While it is clear that amblyopia has a significant thalamo-cortical impact, the existing MRI studies have relied on relatively small populations with heterogenous etiology. A larger scale study or the combination of datasets collected at multiple sites based on emergent open science initiatives would help refine our understanding.
With respect to extrastriate impact, there is fairly strong evidence for functional changes in the extrastriate cortex of human amblyopes, with abnormal activation patterns in V2, V3, Vp, V3a, and hMT+. Moreover, these functional abnormalities are reflected in both direct thalamocortical projections (as in the LGN+pulvinar to hMT+ pathway) as well as larger cortico-cortical projections (ILF, SLF, VOF). More interestingly, there is some evidence for structural changes that extend beyond the immediate visual system, with decreased white-matter integrity in the anterior callosal projections (although these findings should be interpreted cautiously). Studies that simultaneously assess the evidence for amblyopia and hypoplasia would help isolate the impact of amblyopia on the function and connectivity of extrastriate regions, from other potentially superimposed disorders.
Treatment options and evidence for plasticity
Most treatments for amblyopia are aimed at increasing the use of the amblyopic eye by depriving the fellow eye of visual input. Depending on the etiology of amblyopia, different treatment methods are utilized with varying efficacy. Research suggests that outcomes are better when children begin treatment at earlier ages, but most studies suspect this is due to compliance difficulties among older patients, rather than differences in plasticity.Citation101,Citation102
While refractive correction, occlusion, and penalization therapy are generally effective at treating amblyopia, few studies have examined the evidence for central nervous system plasticity after completion of treatment. Carbidopa-levodopa has been found to enhance visual function in amblyopia, but fMRI results conflict on where activation is seen in the CNS after administration of this medication.Citation103–105 Additionally, studies have found regression of visual acuity once carbidopa-levodopa is stopped, due to the central nervous system’s resistance to change.Citation106–108
In animal models where invasive manipulations can be made, a number of studies have suggested an extension or reactivation of the critical period beyond the typical timeframe.Citation109–111 However, the jump to human treatment has been challenging.Citation112 Despite the variety of treatment options available, most have not led to results indicating significant plasticity of the visual system after the critical period for development. One issue is that the upper limit on plasticity in the visual system has not been well-established, though an in progress study on recovery from congenital cataract might provide some insight.Citation113
Perceptual learning and software-based active treatment are newer modalities for addressing amblyopia, but the improvements seen with these novel methods are largely similar to those seen in patching alone. One contributing factor is that the achievable improvement in behavioral performance past the critical period is limited. Studies investigating CNS changes after perceptual learning treatment using neuroimaging are underway, but as of now, there is no clear picture of if and how perceptual learning impacts CNS structure and function.Citation114,Citation115
In conclusion, the results from animal studies have not translated to humans as well as researchers hoped, and more studies are needed investigating CNS changes after amblyopia treatment. Overall, the central nervous system is quite resistant to change, posing a challenge for effective amblyopia treatment after the critical period for vision development.
Conclusion and future directions
The behavioral consequences of amblyopia extend well beyond the clinical criterion of reduced visual acuity. Mirroring the behavioral consequences, amblyopia seems to impact microstructure and function at multiple sites along the visual processing hierarchy. Importantly, both behavioral and neural impairments differ depending on the cause of retinal input disruption with anisometropic and strabismic amblyopia, producing different patterns of abnormalities.
Most of what we know is based on work in relatively small samples of adult patients in which amblyopia was either not treated, or unsuccessfully treated. To date, no large-scale study exists of the impact of amblyopia treatment on the CNS in childhood. We are optimistic that such a study will see significant results. Based on the studies conducted so far, we expect that given the heterogeneous nature of amblyopia, impact will be distributed differently across neural sites depending on etiology.
The field of MRI is seeing substantial technological and methodological advances. A particularly promising avenue is provided by the increasingly widespread adoption of open science approaches providing access to large datasets, and the potential to track neural changes across the lifespan. This work may facilitate diagnosis through a combination of behavioral and neuroimaging methods. While the primary clinical criterion focuses on a two-line difference in visual acuity between the eyes, it is clear that there are other behavioral deficits. Perhaps the treatment efficacy of behavioral deficits may be tracked by associated CNS changes and the potential for personalized prognoses will help motivate compliance. In any case, the field at large is ripe for further exploration.
Disclosure of interest
The authors report no conflicts of interest.
Acknowledgments
We would like to thank Philip Rodenbough for manuscript comments.
Additional information
Funding
References
- DeSantis D. Amblyopia. Pediatr Clin North Am. 2014;61(3):505–518. doi:10.1016/j.pcl.2014.03.006.
- Quinlan EM, Lukasiewicz PD. Amblyopia: challenges and opportunities the lasker/IRRF initiative for innovation in vision science. Vis Neurosci. 2018;35. doi:10.1017/S0952523817000384.
- Hashemi H, Pakzad R, Yekta A, et al. Global and regional estimates of prevalence of amblyopia: A systematic review and meta-analysis. Strabismus. 2018;26:168–183. doi:10.1080/09273972.2018.1500618.
- Amblyopia (Lazy Eye). In: national eye institute [Internet]. https://www.nei.nih.gov/learn-about-eye-health/eye-conditions-and-diseases/amblyopia-lazy-eye. Published July 2, 2019. Accessed September 27, 2020.
- Holmes JM, Clarke MP. Amblyopia. Lancet. 2006;367:1343–1351. doi:10.1016/S0140-6736(06)68581-4.
- Levi DM, Klein S. Hyperacuity and amblyopia. Nature. 1982;298:268–270. doi:10.1038/298268a0.
- Hess RF, Howell ER. The threshold contrast sensitivity function in strabismic amblyopia: evidence for a two type classification. Vision Res. 1977;17:1049–1055.
- Levi DM, Harwerth RS. Contrast evoked potentials in strabismic and anisometropic amblyopia. Invest Ophthalmol Vis Sci. 1978;17:571–575.
- Howell ER, Mitchell DE, Keith CG. Contrast thresholds for sine gratings of children with amblyopia. Invest Ophthalmol Vis Sci. 1983;24:782–787.
- Hess RF, McIlhagga W, Field DJ. Contour integration in strabismic amblyopia: the sufficiency of an explanation based on positional uncertainty. Vision Res. 1997;37:3145–3161. doi:10.1016/S0042-6989(96)00281-7.
- Hess RF, Demanins R. Contour integration in anisometropic amblyopia. Vision Res. 1998;38:889–894. doi:10.1016/S0042-6989(97)00233-2.
- Levi DM, Li RW, Klein SA. “Phase capture” in amblyopia: the influence function for sampled shape. Vision Res. 2005;45:1793–1805. doi:10.1016/j.visres.2005.01.021.
- Mirabella G, Hay S, Wong AMF. Deficits in perception of images of real-world scenes in patients with a history of amblyopia. Arch Ophthal. 2011;129:176. doi:10.1001/archophthalmol.2010.354.
- Simmers AJ, Ledgeway T, Hess RF, McGraw PV. Deficits to global motion processing in human amblyopia. Vision Res. 2003;43:729–738. doi:10.1016/S0042-6989(02)00684-3.
- Simmers AJ, Ledgeway T, Hess RF. The influences of visibility and anomalous integration processes on the perception of global spatial form versus motion in human amblyopia. Vision Res. 2005;45:449–460. doi:10.1016/j.visres.2004.08.026.
- Li J, Thompson B, Lam CSY, et al. The role of suppression in amblyopia. Invest Ophthalmol Vis Sci. 2011;52:4169–4176. doi:10.1167/iovs.11-7233.
- Niechwiej-Szwedo E, Colpa L, Wong AMF. Visuomotor behaviour in amblyopia: deficits and compensatory adaptations. Neural Plast. 2019;2019:6817839. doi:10.1155/2019/6817839.
- Kanonidou E, Proudlock FA, Gottlob I. Reading strategies in mild to moderate strabismic amblyopia: an eye movement investigation. Invest Ophthalmol Vis Sci. 2010;51:3502–3508. doi:10.1167/iovs.09-4236.
- Kelly KR, Jost RM, De La Cruz A, Birch EE. Amblyopic children read more slowly than controls under natural, binocular reading conditions. J Aapos. 2015;19:515–520. doi:10.1016/j.jaapos.2015.09.002.
- Meier K, Giaschi D. Unilateral amblyopia affects two eyes: fellow eye deficits in amblyopia. Invest Ophthalmol Vis Sci. 2017;58:1779–1800. doi:10.1167/iovs.16-20964.
- Farivar R, Thompson B, Mansouri B, Hess RF. Interocular suppression in strabismic amblyopia results in an attenuated and delayed hemodynamic response function in early visual cortex. J Vis. 2011;11:16. doi:10.1167/11.14.16.
- Webber AL. The functional impact of amblyopia. Clin Exp Optom. 2018;101:443–450. doi:10.1111/cxo.12663.
- Simons K. Amblyopia characterization, treatment, and prophylaxis. Surv Ophthalmol. 2005;50:123–166. doi:10.1016/j.survophthal.2004.12.005.
- Jefferis JM, Connor AJ, Clarke MP. Amblyopia. BMJ. 2015;351:h5811. doi:10.1136/bmj.h5811.
- Stanković B. Clinical aspects of different types of amblyopia. Vojnosanit Pregl. 2011;68:696–698. doi:10.2298/VSP1108696S.
- McKee SP, Levi DM, Movshon JA. The pattern of visual deficits in amblyopia. J Vis. 2003;3:380–405. doi:10.1167/3.5.5.
- Kelly KR, Cheng-Patel CS, Jost RM, Wang Y-Z, Birch EE. Fixation instability during binocular viewing in anisometropic and strabismic children. Exp Eye Res. 2019;183:29–37. doi:10.1016/j.exer.2018.07.013.
- Levi DM, Knill DC, Bavelier D. Stereopsis and amblyopia: A mini-review. Vision Res. 2015;114:17–30. doi:10.1016/j.visres.2015.01.002.
- Hubel DH, Wiesel TN. The period of susceptibility to the physiological effects of unilateral eye closure in kittens. J Physiol. 1970;206:419–436. doi:10.1113/jphysiol.1970.sp009022.
- LeVay S, Wiesel TN, Hubel DH. The development of ocular dominance columns in normal and visually deprived monkeys. J Comp Neurol. 1980;191:1–51. doi:10.1002/cne.901910102.
- Yinon U, Auerbach E. The ocular dominance of cortical neurons in cats developed with divergent and convergent squint. Vision Res. 1975;15:1251–1256. doi:10.1016/0042-6989(75)90170-4.
- Eggers HM, Blakemore C. Physiological basis of anisometropic amblyopia. Science. 1978;201:264–267. doi:10.1126/science.663654.
- Kiorpes L, Kiper DC, O’Keefe LP, Cavanaugh JR, Movshon JA. Neuronal correlates of amblyopia in the visual cortex of macaque monkeys with experimental strabismus and anisometropia. J Neurosci. 1998;18:6411–6424. doi:10.1523/JNEUROSCI.18-16-06411.1998.
- Yinon U. Age dependence of the effect of squint on cells in kittens’ visual cortex. Exp Brain Res. 1976;26:151–157. doi:10.1007/BF00238279.
- Dumoulin SO, Knapen T. How visual cortical organization is altered by ophthalmologic and neurologic disorders. Annu Rev Vis Sci. 2018;4:357–379. doi:10.1146/annurev-vision-091517-033948.
- Alexander AL, Lee JE, Lazar M, Field AS. Diffusion tensor imaging of the brain. Neurotherapeutics. 2007;4:316–329. doi:10.1016/j.nurt.2007.05.011.
- Rokem A, Takemura H, Bock AS, et al. The visual white matter: the application of diffusion MRI and fiber tractography to vision science. J Vis. 2017;17:4. doi:10.1167/17.2.4.
- Yen M-Y, Cheng C-Y, Wang A-G. Retinal nerve fiber layer thickness in unilateral amblyopia. Invest Ophthalmol Vis Sci. 2004;45:2224–2230. doi:10.1167/iovs.03-0297.
- Yoon SW, Park WH, Baek S-H, Kong SM. Thicknesses of macular retinal layer and peripapillary retinal nerve fiber layer in patients with hyperopic anisometropic amblyopia. Korean J Ophthalmol. 2005;19:62–67. doi:10.3341/kjo.2005.19.1.62.
- Dickmann A, Petroni S, Salerni A, Dell’Omo R, Balestrazzi E. Unilateral amblyopia: an optical coherence tomography study. J Aapos. 2009;13:148–150. doi:10.1016/j.jaapos.2008.10.009.
- Huynh SC, Samarawickrama C, Wang XY, et al. Macular and nerve fiber layer thickness in amblyopia: the Sydney childhood eye study. Ophthalmology. 2009;116:1604–1609. doi:10.1016/j.ophtha.2009.03.013.
- Araki S, Miki A, Yamashita T, et al. A comparison between amblyopic and fellow eyes in unilateral amblyopia using spectral-domain optical coherence tomography. Clin Ophthalmol. 2014;8:2199–2207. doi:10.2147/OPTH.S69501.
- Kusbeci T, Karti O, Karahan E, Oguztoreli M. The evaluation of anatomic and functional changes in unilateral moderate amblyopic eyes using optical coherence tomography and pupil cycle time. Curr Eye Res. 2017;42:1725–1732. doi:10.1080/02713683.2017.1349153.
- Singh N, Rohatgi J, Gupta VP, Kumar V. Measurement of peripapillary retinal nerve fiber layer thickness and macular thickness in anisometropia using spectral domain optical coherence tomography: a prospective study. Clin Ophthalmol. 2017;11:429–434. doi:10.2147/OPTH.S123273.
- Kantarci FA, Tatar MG, Uslu H, et al. Choroidal and peripapillary retinal nerve fiber layer thickness in adults with anisometropic amblyopia. Eur J Ophthalmol. 2015;25:437–442. doi:10.5301/ejo.5000594.
- Kee S-Y, Lee S-Y, Lee Y-C. Thicknesses of the fovea and retinal nerve fiber layer in amblyopic and normal eyes in children. Korean J Ophthalmol. 2006;20:177–181. doi:10.3341/kjo.2006.20.3.177.
- Repka MX, Goldenberg-Cohen N, Edwards AR. Retinal nerve fiber layer thickness in amblyopic eyes. Am J Ophthalmol. 2006;142:247–251. doi:10.1016/j.ajo.2006.02.030.
- Li J, Ji P, Yu M. Meta-analysis of retinal changes in unilateral amblyopia using optical coherence tomography. Eur J Ophthalmol. 2015;25:400–409. doi:10.5301/ejo.5000583.
- Gaier ED, Gise R, Heidary G. Imaging amblyopia: insights from Optical Coherence Tomography (OCT). Semin Ophthalmol. 2019;34:303–311. doi:10.1080/08820538.2019.1620810.
- Yakar K, Kan E, Alan A, Alp MH, Ceylan T. Retinal nerve fibre layer and macular thicknesses in adults with hyperopic anisometropic amblyopia. J Ophthalmol. 2015;2015:946467. doi:10.1155/2015/946467.
- Miki A, Shirakashi M, Yaoeda K, et al. Optic disc measurements using the Heidelberg Retina Tomograph in amblyopia. Clin Ophthalmol. 2010;4:1025–1028. doi:10.2147/OPTH.S13143.
- Sokol S, Nadler D. Simultaneous electroretinograms and visually evoked potentials from adult amblyopes in response to a pattern stimulus. Invest Ophthalmol Vis Sci. 1979;18:848–855.
- Arden GB, Hogg CR, Powell DJ, Carter RM. Pattern ERGs are abnormal in many amblyopes. Trans Ophthalmol Soc UK. 1980;100:453–460.
- Persson HE, Wanger P. Pattern-reversal electroretinograms in squint amblyopia, artificial anisometropia and simulated eccentric fixation. Acta Ophthalmol. 1982;60:123–132. doi:10.1111/j.1755-3768.1982.tb05788.x.
- Arden GB, Wooding SL. Pattern ERG in amblyopia. Invest Ophthalmol Vis Sci. 1985;26:88–96.
- Hess RF, Baker CL Jr, Verhoeve JN, Keesey UT, France TD. The pattern evoked electroretinogram: its variability in normals and its relationship to amblyopia. Invest Ophthalmol Vis Sci. 1985;26:1610–1623.
- Lempert P. Optic nerve hypoplasia and small eyes in presumed amblyopia. J Aapos. 2000;4:258–266. doi:10.1067/mpa.2000.106963.
- Lempert P. Retinal area and optic disc rim area in amblyopic, fellow, and normal hyperopic eyes: a hypothesis for decreased acuity in amblyopia. Ophthalmology. 2008;115:2259–2261. doi:10.1016/j.ophtha.2008.07.016.
- González EG, Wong AMF, Niechwiej-Szwedo E, Tarita-Nistor L, Steinbach MJ. Eye position stability in amblyopia and in normal binocular vision. Invest Ophthalmol Vis Sci. 2012;53:5386–5394. doi:10.1167/iovs.12-9941.
- Subramanian V, Jost RM, Birch EE. A quantitative study of fixation stability in amblyopia. Invest Ophthalmol Vis Sci. 2013;54:1998–2003. doi:10.1167/iovs.12-11054.
- Ukai K, Ishhii M, Ishikawa S. A quasi-static study of accommodation in amblyopia. Ophthalmic Physiol Opt. 1986;6:287–295.
- Allen B, Schmitt MA, Kushner BJ, Rokers B. Retinothalamic white matter abnormalities in amblyopia. Invest Ophthalmol Vis Sci. 2018;59:921–929.
- Gümüstas S, Altintas Ö, Anik Y, et al. Anterior visual pathways in amblyopia: quantitative assessment with diffusion tensor imaging. J Pediatr Ophthalmol Strabismus. 2013;50:369–374. doi:10.3928/01913913-20131125-04.
- Guillery RW. Binocular competition in the control of geniculate cell growth. J Comp Neurol. 1972;144:117–129. doi:10.1002/cne.901440106.
- Einon G, Ikeda H, Tremain KE. Perikaryal size of cells in the lateral geniculate nucleus and amblyopia in cats reared with convergent squint [proceedings]. J Physiol. 1978;278:50P.
- Tremain KE, Ikeda H. Relationship between amblyopia, LGN cell “shrinkage”and cortical ocular dominance in cats. Exp Brain Res. 1982;45:243–252.
- von Noorden GK, Crawford ML. The lateral geniculate nucleus in human strabismic amblyopia. Invest Ophthalmol Vis Sci. 1992;33:2729–2732.
- Von Noorden GK, Crawford ML, Levacy RA. The lateral geniculate nucleus in human anisometropic amblyopia. Invest Ophthalmol Vis Sci. 1983;24:788–790.
- Barnes GR, Li X, Thompson B, Singh KD, Dumoulin SO, Hess RF. Decreased gray matter concentration in the lateral geniculate nuclei in human amblyopes. Invest Ophthalmol Vis Sci. 2010;51:1432–1438. doi:10.1167/iovs.09-3931.
- Sherman SM, Wilson JR, Guillery RW. Evidence that binocular competition affects the postnatal development of Y-cells in the cat’s lateral geniculate nucleus. Brain Res. 1975;100:441–444. doi:10.1016/0006-8993(75)90498-9.
- Ikeda H, Tremain KE, Einon G. Loss of spatial resolution of lateral geniculate nucleus neurones in kittens raised with convergent squint produced at different stages in development. Exp Brain Res. 1978;31:207–220. doi:10.1007/BF00237600.
- Chino YM, Cheng H, Smith III EL, Garraghty PE, Roe AW, Sur M. Early discordant binocular vision disrupts signal transfer in the lateral geniculate nucleus. Proc Natl Acad Sci U S A. 1994;91:6938–6942. doi:10.1073/pnas.91.15.6938.
- Yin ZQ, Crewther SG, Pirie B, Crewther DP. Cat-301 immunoreactivity in the lateral geniculate nucleus and visual cortex of the strabismic amblyopic cat. Aust N Z J Ophthalmol. 1997;25:107–109. doi:10.1111/j.1442-9071.1997.tb01773.x.
- Levitt JB, Schumer RA, Sherman SM, Spear PD, Movshon JA. Visual response properties of neurons in the LGN of normally reared and visually deprived macaque monkeys. J Neurophysiol. 2001;85:2111–2129. doi:10.1152/jn.2001.85.5.2111.
- Derrington AM, Hawken MJ. Spatial and temporal properties of cat geniculate neurones after prolonged deprivation. J Physiol. 1981;314:107–120. doi:10.1113/jphysiol.1981.sp013694.
- Blakemore C, Vital-Durand F. Organization and post-natal development of the monkey’s lateral geniculate nucleus. J Physiol. 1986;380:453–491. doi:10.1113/jphysiol.1986.sp016297.
- Sasaki Y, Cheng H, Smith III EL, Chino Y. Effects of early discordant binocular vision on the postnatal development of parvocellular neurons in the monkey lateral geniculate nucleus. Exp Brain Res. 1998;118:341–351. doi:10.1007/s002210050288.
- Hess RF, Thompson B, Gole G, Mullen KT. Deficient responses from the lateral geniculate nucleus in humans with amblyopia. Eur J Neurosci. 2009;29:1064–1070. doi:10.1111/j.1460-9568.2009.06650.x.
- Miki A, Liu GT, Goldsmith ZG, Liu C-SJ, Haselgrove JC. Decreased activation of the lateral geniculate nucleus in a patient with anisometropic amblyopia demonstrated by functional magnetic resonance imaging. Ophthalmologica. 2003;217:365–369. doi:10.1159/000071353.
- Allen B, Spiegel DP, Thompson B, Pestilli F, Rokers B. Altered white matter in early visual pathways of humans with amblyopia. Vision Res. 2015;114:48–55. doi:10.1016/j.visres.2014.12.021.
- Qi S, Mu Y-F, Cui L-B, et al. Association of optic radiation integrity with cortical thickness in children with anisometropic amblyopia. Neurosci Bull. 2016;32:51–60. doi:10.1007/s12264-015-0005-6.
- Li Q, Jiang Q, Guo M, Li Q, Cai C, Yin X. Grey and white matter changes in children with monocular amblyopia: voxel-based morphometry and diffusion tensor imaging study. Br J Ophthalmol. 2013;97:524–529. doi:10.1136/bjophthalmol-2012-302218.
- Duan Y, Norcia AM, Yeatman JD, Mezer A. The structural properties of major white matter tracts in strabismic amblyopia. Invest Ophthalmol Vis Sci. 2015;56:5152–5160. doi:10.1167/iovs.15-17097.
- Li Q, Zhai L, Jiang Q, et al. Tract-based spatial statistics analysis of white matter changes in children with anisometropic amblyopia. Neurosci Lett. 2015;597:7–12. doi:10.1016/j.neulet.2015.04.027.
- Mendola JD, Conner IP, Roy A, et al. Voxel-based analysis of MRI detects abnormal visual cortex in children and adults with amblyopia. Hum Brain Mapp. 2005;25:222–236. doi:10.1002/hbm.20109.
- Arden GB, Barnard WM, Mushin AS. Visually evoked responses in amblyopia. Br J Ophthalmol. 1974;58:183–192. doi:10.1136/bjo.58.3.183.
- Sokol S. Abnormal evoked potential latencies in amblyopia. Br J Ophthalmol. 1983;67:310–314. doi:10.1136/bjo.67.5.310.
- Levi DM, Walters JW. Visual evoked responses in strabismic and anisometropic amblyopia: effects of check size and retinal locus. Am J Optom Physiol Opt. 1977;54:691–698.
- Barnes GR, Hess RF, Dumoulin SO, Achtman RL, Pike GB. The cortical deficit in humans with strabismic amblyopia. J Physiol. 2001;533:281–297. doi:10.1111/j.1469-7793.2001.0281b.x.
- Muckli L, Kiess S, Tonhausen N, Singer W, Goebel R, Sireteanu R. Cerebral correlates of impaired grating perception in individual, psychophysically assessed human amblyopes. Vision Res. 2006;46:506–526. doi:10.1016/j.visres.2005.10.014.
- Li X, Dumoulin SO, Mansouri B, Hess RF. Cortical deficits in human amblyopia: their regional distribution and their relationship to the contrast detection deficit. Invest Ophthalmol Vis Sci. 2007;48:1575–1591. doi:10.1167/iovs.06-1021.
- Goodyear BG, Nicolle DA, Humphrey GK, Menon RS. BOLD fMRI response of early visual areas to perceived contrast in human amblyopia. J Neurophysiol. 2000;84:1907–1913. doi:10.1152/jn.2000.84.4.1907.
- Clavagnier S, Dumoulin SO, Hess RF. Is the cortical deficit in amblyopia due to reduced cortical magnification, loss of neural resolution, or neural disorganization? J Neurosci. 2015;35:14740–14755. doi:10.1523/JNEUROSCI.1101-15.2015.
- Tusa RJ, Ungerleider LG. The inferior longitudinal fasciculus: a reexamination in humans and monkeys. Ann Neurol. 1985;18:583–591. doi:10.1002/ana.410180512.
- Jitsuishi T, Hirono S, Yamamoto T, Kitajo K, Iwadate Y, Yamaguchi A. White matter dissection and structural connectivity of the human vertical occipital fasciculus to link vision-associated brain cortex. Sci Rep. 2020;10:820. doi:10.1038/s41598-020-57837-7.
- Yeatman JD, Weiner KS, Pestilli F, Rokem A, Mezer A, Wandell BA. The vertical occipital fasciculus: a century of controversy resolved by in vivo measurements. Proc Natl Acad Sci U S A. 2014;111:E5214–23. doi:10.1073/pnas.1418503111.
- Wang X, Pathak S, Stefaneanu L, Yeh F-C, Li S, Fernandez-Miranda JC. Subcomponents and connectivity of the superior longitudinal fasciculus in the human brain. Brain Struct Funct. 2016;221:2075–2092. doi:10.1007/s00429-015-1028-5.
- Martino J, De Witt Hamer PC, Berger MS, et al. Analysis of the subcomponents and cortical terminations of the perisylvian superior longitudinal fasciculus: a fiber dissection and DTI tractography study. Brain Struct Funct. 2013;218:105–121. doi:10.1007/s00429-012-0386-5.
- Dwyer JA, Newton TH, Hoyt WF. Radiologic features of septooptic dysplasia: de Morsier syndrome. AJNR Am J Neuroradiol. 1980;1:443–447.
- Thompson B, Villeneuve MY, Casanova C, Hess RF. Abnormal cortical processing of pattern motion in amblyopia: evidence from fMRI. Neuroimage. 2012;60:1307–1315. doi:10.1016/j.neuroimage.2012.01.078.
- Chen AM, Cotter SA. The amblyopia treatment studies: implications for clinical practice. Adv Ophthalmol Optom. 2016;1:287–305. doi:10.1016/j.yaoo.2016.03.007.
- Holmes JM, Lazar EL, Melia BM, et al. Effect of age on response to amblyopia treatment in children. Arch Ophthalmol. 2011;129:1451–1457. doi:10.1001/archophthalmol.2011.179.
- Yang C-I, Yang M-L, Huang J-C, et al. Functional MRI of amblyopia before and after levodopa. Neurosci Lett. 2003;339:49–52. doi:10.1016/S0304-3940(02)01465-9.
- Rogers GL. Functional magnetic resonance imaging (fMRI) and effects of L-dopa on visual function in normal and amblyopic subjects. Trans Am Ophthalmol Soc. 2003;101:401–415.
- Algaze A, Leguire LE, Roberts C, Ibinson JW, Lewis JR, Rogers G. The effects of L-dopa on the functional magnetic resonance imaging response of patients with amblyopia: a pilot study. J Aapos. 2005;9:216–223. doi:10.1016/j.jaapos.2005.01.014.
- Yeh KC, August TF, Bush DF, et al. Pharmacokinetics and bioavailability of Sinemet CR: a summary of human studies. Neurology. 1989;39:25–38.
- Leguire LE, Rogers GL, Bremer DL, Walson P, Hadjiconstantinou-Neff M. Levodopa and childhood amblyopia. J Pediatr Ophthalmol Strabismus. 1992;29:290–298. discussion 299.
- Pandey PK, Chaudhuri Z, Kumar M, Satyabala K, Sharma P. Effect of levodopa and carbidopa in human amblyopia. J Pediatr Ophthalmol Strabismus. 2002;39:81–89.
- He H-Y, Ray B, Dennis K, Quinlan EM. Experience-dependent recovery of vision following chronic deprivation amblyopia. Nat Neurosci. 2007;10:1134–1136. doi:10.1038/nn1965.
- Duffy KR, Mitchell DE. Darkness alters maturation of visual cortex and promotes fast recovery from monocular deprivation. Curr Biol. 2013;23:382–386. doi:10.1016/j.cub.2013.01.017.
- Fong M-F, Mitchell DE, Duffy KR, Bear MF. Rapid recovery from the effects of early monocular deprivation is enabled by temporary inactivation of the retinas. Proc Natl Acad Sci U S A. 2016;113:14139–14144. doi:10.1073/pnas.1613279113.
- Wiedeman R. For better vision, living in the dark. The New Yorker. https://www.newyorker.com/magazine/2016/12/19/for-better-vision-living-in-the-dark. Published December 11, 2016. Accessed July 15 2020.
- Miller NP, Gandhi T, Sinha P, Rokers B. White-Matter plasticity following sight-restoration in congenitally blind patients. J Vis. 2019;19:277d. doi:10.1167/19.10.277d.
- Schwartz S, Maquet P, Frith C. Neural correlates of perceptual learning: a functional MRI study of visual texture discrimination. Proc Natl Acad Sci U S A. 2002;99:17137–17142.
- Zhai J, Chen M, Liu L, et al. Perceptual learning treatment in patients with anisometropic amblyopia: a neuroimaging study. Br J Ophthalmol. 2013;97:1420–1424. doi:10.1136/bjophthalmol-2013-303778.
