Abstract
The swift increase in antimicrobial resistance across the world presents a significant challenge, further raised by insufficient advancement in the design of new antimicrobial agents. Over the past two decades, a variety of sources has been investigated for generating new antimicrobials. Recently, antimicrobial polymers (APs) have emerged as multifaceted agents, displaying effectiveness against a range of pathogens such as bacteria, fungi, and viruses. Their notable biocompatibility, efficiency, multifunctionality, and ease of modification make them a focal point of antimicrobial research. APs are particularly valued for their structural versatility, which allows for the integration of antimicrobial functional groups. Polymer functionalization does not only enhance or introduce antimicrobial properties but also maintains high levels of biocompatibility. This review reveals broad antimicrobial breadth of APs, which can be potentially used in antiseptics, food packaging, and in biomedical sector, as e.g. medical implants’ coatings. In laboratory settings, APs have shown promising therapeutic effects, however there remains an urgent need for computational biology to fully understand their antimicrobial behaviour and stability. Recombinant biotechnological approaches are essential for facilitating the production of APs and require industrial collaboration to effectively optimize and scale up these production processes.
1. Introduction
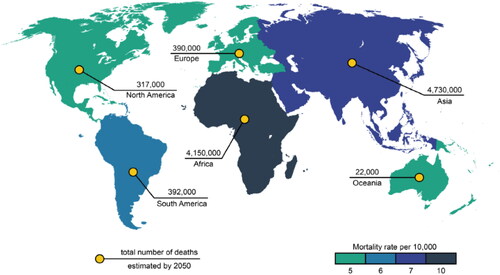
The discovery of the first synthetic antimicrobial polymer (AP) based on 2-methacryloxytropone (Cornell & Donaruma, Citation1965) geared up polymer science research, resulting in several breakthroughs in the field of APs (Palermo & Kuroda, Citation2010). Since then, several APs with a broad-spectrum antimicrobial activity have been characterized (Kenawy, Worley, & Broughton, Citation2007; Siedenbiedel & Tiller, Citation2012). The demand for alternative antimicrobial agents has substantially increased after the widespread incidence of infections and antimicrobial resistance (Brower, Citation2018) (Amin et al., Citation2021; Anowar Hosen et al., Citation2022; Mubaraki et al., Citation2024). According to the reports (Kamaruzzaman et al., Citation2019) 10 million deaths are expected due to antimicrobial resistance in the next 25 years, as shown in .
Figure 1. The worldwide probable number of mortalities due to antimicrobial resistance-in 2050. (Kamaruzzaman et al., Citation2019). Copyright (2019) MDPI.
Despite the widespread antimicrobial resistance, large pharmaceutical companies are considered the main drivers that need to give more attention to the development of new antimicrobials (Jackson, Czaplewski, & Piddock, Citation2018). The companies began withdrawing antimicrobial discovery at the end of the 2000s due to subsequent failures and low financial returns; thus, only small-scale pharmaceutical companies and university laboratories continue to develop new antimicrobials (Kamaruzzaman et al., Citation2019). However, the German Ministry of Education and Research has shown interest in providing funds to the Global Antibiotic Research and Development Partnership to work on developing antimicrobials (Kamaruzzaman et al., Citation2019).
In the last two decades, much attention has been paid to polymers as potential alternative antimicrobial agents (Lin et al., Citation2018). The COVID-19 pandemic greatly influenced antimicrobial research and ended with several publications on APs addressing the antiviral activities either in solution or APs-based coatings (Hathout & Kassem, Citation2020; Kalathiya et al., Citation2020; Milewska et al., Citation2020; Sharma et al., Citation2021; Zuniga & Cortes, Citation2020). Additionally, chemical and structural modifications enhance the effectiveness of APs and broaden their applications. In this context, functional groups are crucial as they are known to either introduce antimicrobial properties or significantly enhance the antimicrobial effectiveness (Bouazizi, Vieillard, Samir, Derf, & Le, Citation2022; González-Henríquez, Sarabia-Vallejos, & Hernandez, Citation2019; Vigliotta, Mella, Rega, & Izzo, Citation2012). Computational approaches, such as Quantitative Structure-Activity Relationship showed improvement in understanding the antimicrobial activities of chitosan. QSAR studies focused on developing equations that correlate the biological activity of APs with their numerical molecular descriptors helping to identify optimal structures with therapeutic potential (Másson, Citation2024). Such approaches integrated with other computational approaches, e.g. molecular docking (Maowa et al., Citation2021; Munia et al., Citation2022) will be helpful in providing full understanding about the antimicrobial behaviour and pharmacokinetic properties of APs.
Additionally, the advances in polymerization methods allowed the production of a library of copolymer systems characterized by antimicrobial activity and desired properties (Judzewitsch, Nguyen, Shanmugam, Wong, & Boyer, Citation2018). Such polymers have highly controlled monomer composition/distribution, functionality, and topology (Takahashi, Caputo, Vemparala, & Kuroda, Citation2017). Polymers can be further modified by the addition of various moieties (Ganewatta & Tang, Citation2015), such as amino groups (primary, secondary, tertiary), quaternary ammonium groups, guanidinium/biguanide salts, quaternary pyridinium and imidazolium groups (Zheng et al., Citation2016), as well as quaternary phosphonium and sulfonium groups (Tejero, López, López-Fabal, Gómez-Garcés, & Fernández-García, Citation2015). Such modifications are primarily applied to polymers with innate antimicrobial activities, such as chitin, chitosan, agarose, or carrageen (S. G. Liu et al., Citation2016). Methacrylate copolymers, amphiphilic methacrylate copolymers (Teratanatorn et al., Citation2017), and cationic polymers (Farshbaf et al., Citation2018) are some important classes of APs having a broad-spectrum antimicrobial potential (González-Henríquez et al., Citation2019).
Due to their degradable nature, some APs are presented as ideal polymeric materials for coatings, since they do not release toxic products upon decomposition (Piskláková et al., Citation2024). Some antimicrobial polyamine polymers are firmly adhered on substrates due to amine groups, including primary, secondary, and tertiary groups, resulting in protective layers that seal surface flaws and guard it against corrosion (Sukhareva, Chernetsov, & Burmistrov, Citation2024). Recently, a novel and dynamically reversible method was introduced, the coating of α-lipoic acid (LA) derived AP, which not only enables chemical extermination upon contact but also allows for the on-demand mechanical removal of biofilms attached to the surface (Lou & Palermo, Citation2024).
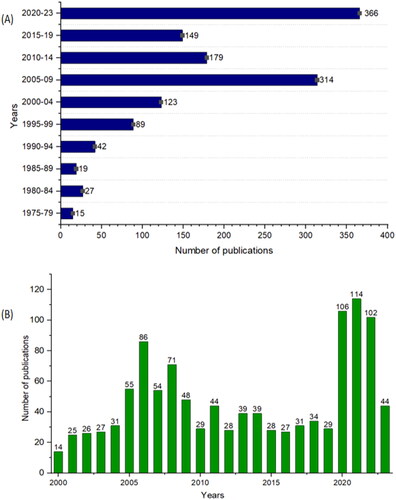
Hundreds of scientific publications have come into view addressing the diverse perspectives of synthetic APs, thus giving a broader overview of their structure and functions (Ganewatta & Tang, Citation2015), and the last decade reported a significantly higher number of publications about APs, which shows the thirst and need of APs ().
2. Search strategy and inclusion/exclusion criteria
The relevant publications were searched through different keywords such as antimicrobial polymers, synthetic antimicrobial polymers, cationic antimicrobial polymers, hydrophobic antimicrobial polymers, polymer-based antimicrobial coatings, mode of action of antimicrobial polymers, and antibacterial potential of polymers. Literature was obtained from multiple scientific databases: PubMed (http://www.ncbi.nlm.nih.gov/pubmed), Google Scholar (http://www.scholar.google.com), Elsevier (https://www.elsevier.com/en), Science Direct (http://www.sciencedirect.com), Wiley (http://www.onlinelibrary.wiley.com), Springer (http://www.springer.com), and Scopus (http://www.scopus.com) (Last accessed Sept 15th, 2023). The studies that address the antimicrobial activities of polymers, either in solutions or as surface coatings, were recruited in this summary. The studies that use other antimicrobial agents like antibiotics, antimicrobial peptides, antiseptics, metal nanoparticles, etc., combined with APs were excluded from the current manuscript. Three thousand three hundred eighty-seven scientific publications, including original articles, review articles, book chapters, and books, were manually identified from all the databases from 1975 to Sept. 15th, 2023. Of the 3387, 725 were removed as duplicates, and 1184 were removed due to non-matching with the scope of this review. We could not retrieve the full texts of 111 publications, and 44 ones were removed for other reasons i.e. language barriers. A total of 1323 publications were included in the current manuscript and are presented in a graphical form based on their year of publication. Consequently, the summary of publications on APs from 1975 to 2023, and the year-wise distribution of publications between 2000 and 2023 were presented in .
3. Antimicrobial spectrum of APs
The last two decades showed an abrupt increase in scientific publications on APs, indicating a significant interest in antimicrobial polymers (Hassan, Omer, Abbas, Baset, & Tamer, Citation2018). A first synthesized antimicrobial polymer, a derivative of 2-methacryloxytroponone, showed antibacterial activity against Staphylococcus aureus, Streptococcus pyogenes, Salmonella enterica subs. enterica sorovar Typhi, Salmonella enterica subs. enterica sorovar Chloraesuis, and Escherichia coli (Cornell & Donaruma, Citation1965). This was followed by several salicylic acid functionalized polymers that were active against bacteria (Haktaniyan & Bradley, Citation2022). The frequently explored polycationic AP is chitosan, which shows antimicrobial potential against S. aureus, E. coli, Pseudomonas aeruginosa, Cryptococcus neoformans, and Candida albicans (Teixeira-Santos, Lima, Gomes, & Mergulhão, Citation2021). The antimicrobial activity of cationic polymers is associated with a positive charge that acts on the microbial membrane, resulting in cytoplasm damage and cell lysis (Lichter & Rubner, Citation2009). Such polymers form amphiphilic conformation upon interaction with the cell membrane, which increases their bioactive performance even more. A breakthrough in the research on cationic polymers was brought up by Ikeda et al. (Ikeda, Tazuke, & Suzuki, Citation1984), which increased the researchers’ interest in APs (Arora & Mishra, Citation2018; Huang et al., Citation2016; Kamaruzzaman et al., Citation2019) and brought up several broad-spectrum APs such as poly(sodium 4-styrene sulfonate) (Jain et al., Citation2014), cationic polymethacrylates (Pu et al., Citation2017), poly(ethylene glycol)-block-poly(2-(dimethylamino)ethyl methacrylate) grafted with rosin, and methacrylate copolymers.
Another class of inherently APs includes macromolecules containing functional groups possessing nitrogen, such as quaternary ammonium compounds (e.g. benzalkonium chloride, stearalkonium chloride, cetrimonium chloride) (Sayed & Jardine, Citation2015). The length of the polymer chain is known to influence its antibacterial action, and the optimal activity is usually observed in compounds consisting of 8-18 carbon atoms. These compounds have been shown to induce cell death via electromagnetic and hydrophobic interactions, but their activity as a hemolytic agent is limited. Another example of an AP is polyethylenimine, a synthetic, nonbiodegradable polymer containing nitrogen. It induces cell death through cell membrane rupture; however, its antimicrobial activity is affected by molecular weight, with higher molecular weight forms being more efficacious (Parcheta & Sobiesiak, Citation2023). Polyguanidines, also nitrogen-containing macromolecules, exhibit a broad-spectrum antimicrobial activity and are known for their high water solubility and nontoxic nature (Stelmakh et al., Citation2021). They initially interact with microbes through electrostatic forces and show an excellent activity against gram-positive bacteria due to structural differences between gram-positive and gram-negative bacteria.
The research interest continues to explore several domains, either synthesizing new polymers or working on the enrichment of the antimicrobial activity of the existing macromolecules. APs have shown their broad spectrum therapeutic breadth against clinically essential pathogens, including viruses, and are preferred over other antimicrobials due to negligible hemolytic behavior (Exley et al., Citation2015; Mankoci, Kaiser, Sahai, Barton, & Joy, Citation2017). In a drug resistance era, APs have remained a central focus in antimicrobial research, bringing up a high number of highly active APs that find their applications in different domains, including pharmaceutical industries, surgical industries, antimicrobial coatings, surfactants and detergents, agricultural sector, protective food packaging, and filtration systems (Jain et al., Citation2014; Lim & Hudson, Citation2003). Furthermore, APs can be also used together with poly(ethylene terephthalate) to fabricate hospital textiles, which are less susceptible to colonization by pathogens (Morris & Murray, Citation2021). Some of the polymers have a broad-spectrum antimicrobial potential, such as poly(sodium 4-styrene sulfonate), which shows antimicrobial potential against human immunodeficiency (HIV-1), herpes simplex (HSV-1) viruses and Neisseria gonorrhoeae (Jain et al., Citation2014).
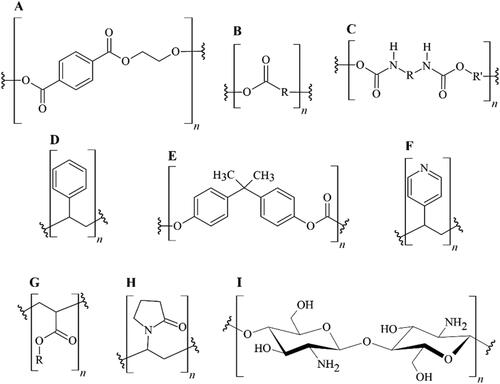
The key advantages that APs offer are ideal and optimal antimicrobial mechanisms with fewer chances of antimicrobial resistance, negligible toxicity, and prolonged antimicrobial activity compared to other small molecular antimicrobial agents. Most APs, including poly(methacrylamide), lack cytotoxic behavior or exhibit cytotoxicity at a negligible level (Paslay et al., Citation2012). The minimum hemolysis activity towards human red blood cells is a critical factor for ideal APs, and is exhibited by several polymers, including poly(methacrylamide) (Locock, Michl, Griesser, Haeussler, & Meagher, Citation2014). APs have replaced some antibiotics due to their advantages over low molecular weight agents, such as reduction in environmental contamination, toxicity (Nagaraja, Jalageri, Puttaiahgowda, Raghava Reddy, & Raghu, Citation2019), non-volatility, chemical stability, and long-term activity (Álvarez-Paino et al., Citation2015). AP-based therapy is less prone to antimicrobial resistance as APs can physically destroy the pathogens’ cell membranes (Jain et al., Citation2014). Recent times marked the increase in the use of FDA-approved disinfecting polymers, indicating the need for alternatives to antibiotics and environmentally critical disinfectants (Siedenbiedel & Tiller, Citation2012). These features pave the way for broader applications of APs, which can be used as accessible antimicrobials fixed via covalent bonds or deposited physically as surface coatings (Ren, Cheng, Wang, & Liu, Citation2017). highlights significant polymeric structures, starting points for studies described in this review paper.
Figure 3. Relevant polymeric structures as starting points for APs and related polymer-based materials. A) Poly(ethylene terephthalate); B) polyesters (general scheme); C) polyurethanes (general scheme); D) polystyrene; E) bisphenol A-derived polycarbonate; F) poly(4-vinyl pyridine); G) polyacrylates (general scheme); H) poly(vinyl pyrrolidone); I) chitosan.
4. Synthetic APs and their efficacy
Synthetic APs represent a dynamic field of research. Their versatility and effectiveness make them a promising solution in combating microbial infections and contamination in various sectors, including healthcare, food safety, and environmental protection. Synthetic APs result from advances in polymerization methods that successfully tailor the composition of copolymer systems (Y. Liu et al., Citation2020). Synthetic APs have suitable antimicrobial activity and high biocompatibility. Quaternized poly(vinyl pyridine) is one of the strong antimicrobial polymers but it displays low biocompatibility. However, its biocompatibility and antimicrobial potential can be increased through copolymerization with hydroxyethyl methacrylate and poly(ethylene glycol) methyl ether methacrylate (Sellenet, Allison, Applegate, & Youngblood, Citation2007). Chemically modified APs have broader applications than other antimicrobials due to a high level of control over chemical and structural properties, such as monomer composition/distribution, functionality, and topology (Ergene, Yasuhara, & Palermo, Citation2018) (Parcheta & Sobiesiak, Citation2023). Several approaches are used to manipulate the order of polymer blocks, representing a step towards understanding the importance of monomer domain distribution in synthetic polymers in their biological activity (Judzewitsch et al., Citation2018) (Gody, Barbey, Danial, & Perrier, Citation2015). Synthetic APs have shown promising results in in vitro studies; however, the nonbiodegradable nature of some polymers has limited their applications in vivo studies due to possible long-term toxicity (Ergene et al., Citation2018). Examples of biodegradable APs are polyesters (Chamsaz, Mankoci, Barton, & Joy, Citation2017), polyurethanes (Mankoci et al., Citation2017), and polycarbonates (Wee et al., Citation2014). Several benefits are conferred by those polymers that are degraded in a specifically triggered manner that advantages relative to passive degradation (DeWit & Gillies, Citation2009). These polymers can be cleaved with enzymes or degraded through other ways, e.g. hydrolytically, at random sites along the backbone, giving a degradation profile. The degradation kinetics can be controlled by the chemical structure of the linkages and the solvent accessibility of the microenvironment.
5. Functional group-mediated antimicrobial activity of polymers
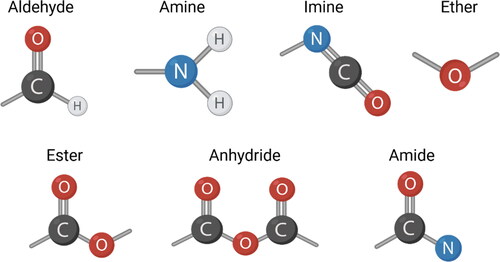
Functionalized APs have received increasing interest as antimicrobials or disinfectants. Some functional groups, such as tertiary amine groups, are reported to increase the antimicrobial activity of polystyrene-based polymers (González-Henríquez et al., Citation2019) and dendritic copolymers (Vigliotta et al., Citation2012); however, they exhibit cytotoxic behavior towards mammalian cells (Vigliotta et al., Citation2012). Phosphonium groups are less toxic cationic groups that enhance the corresponding APs’ thermal stability (Hemp, Smith, Bryson, Allen, & Long, Citation2012; Ornelas-Megiatto, Wich, & Fréchet, Citation2012). Quaternary phosphonium groups have direct association with the antimicrobial properties of polymers (Xue, Pan, Xiao, & Zhao, Citation2014). More examples of such antimicrobial groups with proven excellent antimicrobial activity are N-halamine groups, and sulfonium groups (Hirayama, Citation2011). Among those, the most biocompatible group is N-halamine, which can be regenerated and produced cheaply (Lowe, Deng, Smith, & Balkus, Citation2012). Phosphonium-containing polymeric biocides exhibit higher antimicrobial activity when compared with quaternary ammonium-based polymer salts (Jain et al., Citation2014). Quaternary phosphonium salts based on low molecular weight compounds and their corresponding polymers with different substituent groups (ethyl, n-butyl, phenyl, and n-octyl) were tested against S. aureus and indicated octyl chain as highly active (Santos et al., Citation2016). Focusing on steric structure, Palermo et al. (Palermo, Vemparala, & Kuroda, Citation2012) introduced cationic side chain spacer arms as a new strategy for modulating antibacterial activity and molecular conformation of APs. shows the antimicrobial functional groups.
High molecular weight polymers are reportedly more active than low molecular weight polymers. The association of molecular weight with antimicrobial activity also depends on the type of polymers; for example, the antimicrobial activity of cationic polypyridinium surfaces with hydrophobic alkyl or fluoroalkyl groups depends on the composition of the top few nanometers of the surface. However, optimal antimicrobial activity is exhibited by the alkyl chain length of 12–14 carbons for gram-positive bacteria, while for gram-negative bacteria, it is 14–16 carbons (Kwaśniewska, Chen, & Wieczorek, Citation2020). Polymers with longer alkyl substituents, particularly amphiphilic polymers, have improved membrane insertion capability, permeabilization ability, and potent antimicrobial activity (Ergene & Palermo, Citation2018). Vancomycin (VRSA)- and methicillin (MRSA)-resistant strains of S. aureus and C. albicans showed higher susceptibility to such polymers (Ergene & Palermo, Citation2018). Additionally, the spatial arrangement of charges, their density, and the source of cations within the polymeric architecture affect the antimicrobial activity. Polymers with butyl alkyl groups, longer alkyl tails, and primary amino groups have higher antibacterial activity (Musa, Chang, Teow, Rosli, & Mohammad, Citation2022). Adding carbohydrate pendant units reduce the cytotoxicity of polymers (Paslay et al., Citation2012). Such polymers are active against bacteria, yeast, and fungi and can retain antimicrobial efficacy even after immobilizing onto a substrate matrix (Hung, Citation2018). The other factor that influences the effectiveness of APs is the presence of a quaternized nitrogen (Krishnan et al., Citation2006). The quaternization with perfluorooctyl-1-bromohexane is reported to significantly improve the antibacterial activity of fluorinated pyridinium block copolymers by influencing the surface compositions and molecular organizations, increasing the surface charge. The electrostatic interactions between the cells and the surface are strong—thus the copolymer can efficiently eliminate bacterial cells. It has been found that the surface obtained from fluorinated pyridinium block copolymers causes membrane disruption within 15 min of contact (Krishnan et al., Citation2006).
5.1. Amino groups in APs
Amines are a group of organic compounds derived from ammonia by replacement of one or more hydrogen atoms by organic groups. Amino groups play a crucial role in the design and function of APs (Bouazizi et al., Citation2022). The structure of the amino group influences the polymer’s antimicrobial activity (Carmona-Ribeiro & de Melo Carrasco, Citation2013). Antimicrobial behavior and biological properties of APs may be modulated using different amino groups such as primary, secondary, and tertiary amines (Smola-Dmochowska et al., Citation2023). Quaternary ammonium compounds (QACs) are widely reported amines where the nitrogen atom has four organic groups attached and carries a positive charge. Amines, especially QACs, exhibit antimicrobial activity primarily through disrupting microbial cell membranes. Their cationic nature allows them to bind to the negatively charged cell membranes of microbes, leading to cell lysis and death. Compared to quaternary amines, protonated primary amines and protonated tertiary amines of polyacrylates express high antimicrobial potential and lower hemolytic behavior (Palermo & Kuroda, Citation2009). Poly(methacrylamides) and poly(diallylammonium chloride) show a similar behavior; the protonated primary amines in poly(aminohexyl acrylate) have antimicrobial activity (Thoma, Boles, & Kuroda, Citation2014). Quaternization can improve the antimicrobial activity of tertiary amines of polyacrylate (Álvarez-Paino et al., Citation2015). It has been revealed that quaternary amines cannot show antimicrobial effects after losing hydrophobicity, indicating the role of hydrophobicity in the antimicrobial mechanism (Grace et al., Citation2016). Primary ammonium ethyl methacrylate homopolymers showed antimicrobial efficacy against S. aureus in in vivo studies (Santos et al., Citation2016), while N-[3-(dimethylamino)propyl]methacrylamide or N-[3-(diethylamino)propyl]methacrylamide copolymerized N-(3-aminopropyl)methacrylamide was found to be highly active against E. coli and Bacillus subtilis (Nagaraja et al., Citation2019).
On the other hand, polystyrene-incorporated protonated tertiary amines of polyacrylate exhibited high antimicrobial potential. Polymethacrylamide and polynorbornene-linked guanidine units and primary amines have been tested for antimicrobial potential, and the results revealed that polymethacrylamide-linked primary amines exhibited higher antimicrobial activity compared to guanidine units (Exley et al., Citation2015). In addition to antimicrobial activity, guanidine copolymers lacked hemolytic behavior (Locock et al., Citation2013). On the other hand, polynorbornene and polyacrylate (Locock et al., Citation2013) conjugated guanidine units showed higher antimicrobial potential, while condensation polymers linked quaternary amines and guanidine showed similar antimicrobial potential (Zhang et al., Citation2016). The high amine content in polyethylenimine (PEI) is considered meaningful for its antimicrobial performance. Terminal primary amines and secondary amines of linear PEI showed strong antimicrobial potential against S. aureus (Wiegand, Bauer, Hipler, & Fischer, Citation2013), while primary, secondary, and tertiary amines of branched PEI did not exhibit sufficient antimicrobial potential. Wiegand et al. (Wiegand et al., Citation2013) found that PEI of 0.8 and 5 kDa are highly biocompatible. However, PEI was found to induce cytotoxicity when molecular weight was increased above 5 kDa. The presence of amino groups in APs offers a promising avenue for controlling microbial growth in various settings. Ongoing research aims to enhance their effectiveness and safety profile while minimizing environmental impact.
5.2. Hydrophobic and hydrophilic groups in APs
Hydrophobic groups in APs play a crucial role in determining their effectiveness and mode of action against microorganisms. These non-polar groups tend to repel water and can significantly influence the behavior and properties of APs (Smola-Dmochowska et al., Citation2023). The presence of hydrophobic groups can increase the affinity of the APs for microbial cell membrane. This closer interaction can enhance the antimicrobial efficacy of the polymer (Kozon-Markiewicz et al., Citation2023). Hydrophobicity of a polymer influences its solubility in water and organic solvents, which in turn affects its application. For instance, more hydrophobic polymers might be suitable for coatings and films where water resistance is needed (Frank & Matzger, Citation2019). Hydrophobic compounds are considered one of the most potent membrane-disruptive agents (Pham, Oliver, & Boyer, Citation2023), since they can interact with pathogen membranes after adsorption of antimicrobial cationic polymers, which then leak the cytoplasm and result in the cell being lysed (Yang, Cai, Huang, Tang, & Zhang, Citation2018) (). Membrane disruptive or antimicrobial mechanism of a hydrophobis moiety is influenced by the length of hydrophobic alkyl chain. The optimal chain length, which gives effective antimicrobial results, is from 3 to 8. Growing from 3 to 8, the spacer chain length decreases the minimum inhibitory concentration (MIC) against various pathogenic microbes (Pham et al., Citation2023). The excessive increase in the chain length reduces the antimicrobial potential and increases the hemolytic behavior (Pham et al., Citation2023). The antimicrobial efficiency of hydrophobic moiety also depends on the type of hydrophobic group; for example, cyclohexane groups are reported to exhibit higher antimicrobial activity and weaker hemolytic effect. (Chakraborty et al., Citation2013). Poly(ɛ-caprolactone)-connected resin acids are reportedly used as hydrophobic materials, showing a considerable and broad-spectrum antimicrobial potential against several bacterial pathogens such as S. aureus, S. pyogenes, Corynebacterium xerosis, Salmonella enterica subs. enterica serovar Typhimurium, E. coli, Proteus vulgaris and Mycobacterium smegmatis (J. Wang et al., Citation2012). Hydrophobicity significantly influences the efficacy of APs, as it has been demonstrated that quaternary amines cannot disrupt the membranes of pathogens after losing hydrophobicity. Antimicrobial efficacy increases with increased hydrophobicity.
Figure 5. The schematic illustrations of the effects of APs bearing hydrophilic and hydrophobic groups: bacterial membrane disrupting ability, potent antimicrobial coatings, and less haemolytic activity. Created with BioRender.com.
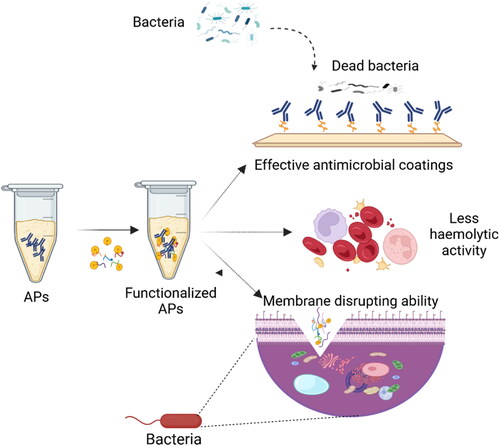
Furthermore, it has been found that the antimicrobial activity of cationic polymer surfaces is tuned by hydrophobicity and charge density (Correia et al., Citation2011). PEI can be chemically modified due to the abundance of reactive amino groups (Samal et al., Citation2012). It was observed that its antimicrobial potential is influenced by hydrophobicity and positive charge density that can be potentiated by incorporating alkyl groups. (Pham et al., Citation2023) found that cationic/hydrophobic balance is crucial for modulating and optimizing the hemolytic activity and antimicrobial activity of APs. High hydrophobicity can also reduce the antimicrobial potential due to a predisposition for self-interactions that indicates that self-assembly of polymers removes the antimicrobial activities. Adding less hydrophobic monomers recovers the antimicrobial potential by unblocking the structures (Kuroki et al., Citation2017), which shows the importance of hydrophobic monomers with highly hydrophobic functional groups, such as phenylethyl, for antimicrobial activity (Judzewitsch et al., Citation2018).
Hydrophilic groups are also incorporated to improve the selectivity and to reduce the toxicity of APs, as some studies (Nguyen et al., Citation2017) reported the reduction of undesired protein complexation through the introduction of hydrophilic groups. Hydrophilic groups offer several advantages, such as sustaining antimicrobial activity, increasing biocompatibility, and reducing hemolytic activity (Judzewitsch et al., Citation2018; Nguyen et al., Citation2017). Hydrophilic groups improve the solubility of APs in water, which is crucial for applications where the polymer needs to be dispersed or dissolved in an aqueous environment. Hydrophilic ends initiate antimicrobial activity by making pores in the pathogens’ membrane, eventually leading to cell death (Qiu et al., Citation2020). Hydrophilic-incorporated AP chains also have a better availability to interact with bacterial membranes as they prevent the formation of aggregates in aqueous media by limiting the interactions between hydrophobic groups (Figureg et al., 2018). Hydrophilic groups can enhance the interaction of the polymer with microbial cells. This interaction is significant for targeting and penetrating the outer layers of microorganisms, such as the cell wall or membrane. The optimization of chain length, flexibility, and hydrophilicity of functional groups are the main structural features that maximize biocompatibility without negatively impacting the antibacterial effect (Pham et al., Citation2023). Moreover, cytotoxicity towards mammalian cells, associated with several APs, can be reduced by incorporating hydrophilic functionality without interrupting the antimicrobial activity (Nguyen et al., Citation2017). Hydrophilic groups preserve the antimicrobial activity of polymers by reducing the unwanted protein complexation. However, hydrophilic/cationic residues without hydrophobic domains cannot show antimicrobial activity.
In the light of the above, it is clear that one of the main challenges in designing APs is achieving the right balance of hydrophobicity to ensure effectiveness without causing unwanted toxicity or environmental impact (Z. Liu, Zhang, Zhou, & Jiao, Citation2010). Another consideration is the potential for resistance development. Polymers with an optimal balance of hydrophilic and hydrophobic properties might reduce the likelihood of resistance development in microorganisms. Hydrophobic groups are a crucial feature in the design of APs, contributing significantly to their effectiveness, application, and overall behavior. The interplay between hydrophobic and hydrophilic properties in these polymers is critical in determining their mode of action and suitability for various applications, as shown in .
Table 1. The antimicrobial breadth of APs against pathogenic bacteria, fungi and viruses.
6. Mode of action of APs
The range of modes of action of APs is based on the type of polymers and functional groups. APs can eliminate or inhibit pathogens’ growth in several ways; however, they mostly interact with negatively charged bacterial cell walls (Xue, Xiao, & Zhang, Citation2015), disrupting bacterial cell walls and cytoplasmic membranes (). Cationic APs have a potent antimicrobial activity against gram-negative bacteria due to the negative charge of phosphate and pyrophosphate groups of lipopolysaccharides that attract the positive surface of polymers (Kara, Aksoy, Yuksekdag, Hasirci, & Aksoy, Citation2014). In contrast, surface-coated APs can also kill pathogens via electrostatic or hydrophobic interaction (Ren et al., Citation2017) and form pores that disrupt the cytoplasmic membranes. The electrostatic interactions mediate the attraction between highly cationic polymers and negatively charged lipids of bacterial membranes. This result is also verified by computer molecular dynamics simulations to investigate the interaction of dimethyldecylammonium chitosan (with high quaternization)-graft-poly(ethylene glycol) methacrylate with bacterial membranes. APs use different mechanisms, such as barrel-stave, toroidal pores, and carpet, to form pores (Ren et al., Citation2017); however, some polymers disrupt the pathogen’s membrane without leading to pore formation. Cationic polymers, such as polyhexamethylene, indirectly kill the pathogens by modulating the immune responses (Qiu et al., Citation2020).
Figure 6. The schematic of the mode of action of APs: membrane lysis by forming pores; production of oxidative stress, which interferes with damage to the cell wall and cell membrane, inhibits protein synthesis, and interferes with enzymatic activity; condensation of bacterial membrane; indirect inhibition of pathogens through immunomodulatory effects. Created with BioRender.com.
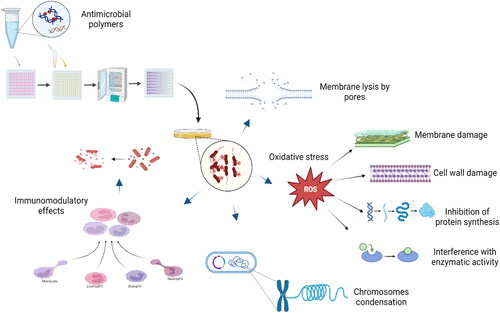
APs can easily differentiate between zwitterionic and microbial cell membranes, as the mammalian cells are rich in phosphatidylcholine and phosphatidylserine, and are less negatively charged. Therefore, APs are primarily attracted to strongly negatively charged bacterial membranes (Tew, Scott, Klein, & Degrado, Citation2010). Poly(hexamethylene biguanide) is also reported to have differential selectivity towards bacterial chromosomes and is known to stop cell division and to condense the chromosomes; however, it cannot reach mammalian cells nuclei. Such an unanticipated paradigm for antimicrobial action avoids the development of resistance to poly(hexamethylene biguanide) (Chindera et al., Citation2016). Poly(norbornene) that contains guanidine units targets the bacterial and mycobacterium DNA (Ren et al., Citation2017). Dissolved APs inhibit pathogens but APs also show antimicrobial behavior when coated on the surface (Gabriel et al., Citation2008). Poly(p-phenylene-ethynylene) are important APs that lyse the bacterial cell by forming needle-like structures (Y. Wang et al., Citation2013).
The antimicrobial activity of chitosan is associated with its amino groups that bind to the microorganism’s cell walls, particularly those negatively charged (Yan et al., Citation2021). Chitosan also has the chelating capacity, as well as the ability to pass through cell membranes. After passing, it interacts with genetic material and inhibits transcription and protein synthesis (Raafat & Sahl, Citation2009). Similarly, the antimicrobial activity of ε-polylysine (ε-PL) is also associated with the presence of amino groups. When tested against E. coli K-12 cells, ε-PL was found to be adsorbed at the bacterium cell wall’s surface, disrupting the outer layer and causing pore formation (Chang et al., Citation2014). Once ε-PL reaches inside, it generates reactive oxygen species (ROS) which interact with DNA, thus leading to cell death (). The exact mechanism of antimicrobial action of ε-PL was also reported by Ye et al. (Ye et al., Citation2013) against E. coli O157:H7. APs based on benzimidazole derivatives inhibit the cytochrome P-450 monooxygenase of Plasmodium falciparum malaria parasites (Leshabane et al., Citation2021). The antimicrobial mechanism of APs containing N-halamine groups is unique. It lyses the bacterial cell by releasing the oxidative halogen (Cl+ or Br+) on the cell wall. Kenawy’s group modified APs with amino groups by combining them with aromatic aldehydes phenolic ester derivatives as modified polymers, which significantly inhibited the growth of bacteria and fungi. It was demonstrated that phenolic moieties are responsible for antimicrobial action through damage in the cell membranes and intracellular coagulation of cytoplasmic constituents (Kenawy, Imam Abdel-Hay, Abou El-Magd, & Mahmoud, Citation2006).
7. APs as a tool for developing antimicrobial-functionalized coatings
APs-based surfaces actively interfere with bacterial attachment and proliferation; however, their efficacy depends on the chemistry, topology, and morphology of APs fixed on the surfaces. APs can be used to coat the surface through several techniques, such as a layer-by-layer deposition (Qiao et al., Citation2012), grafting techniques, and plasma polymerization. However, fabricating some polymer coatings requires many steps, like surface grafting of poly-4-vinyl-N-hexylpyridinium on glass. Before poly-4-vinyl-N-hexylpyridinium can be grafted, the surface needs to be acrylated with aminosilane and acryloyl chloride, which is followed by copolymerization with 4-vinylpyridine followed by alkylation of the final product with hexylbromide (Tiller, Liao, Lewis, & Klibanov, Citation2001a). Similarly, methylation and alkylation with long-chain alkyl halides are also required for the aminoglass-attached PEI. The polymers-based contact-active antimicrobial surfaces are also fabricated with short-chain APs or surface-attached biocidal groups (Bouloussa, Rondelez, & Semetey, Citation2008; Gottenbos, Van Der Mei, Klatter, Nieuwenhuis, & Busscher, Citation2002). Hernández-Montelongo et al. (Hernández-Montelongo et al., Citation2016) used chitosan and hyaluronic acid to prepare AP-based nanofilms through a layer-by-layer assembly technique. Nanofilms showed antimicrobial activity against Xylella fastidiosa, and the film’s surface rich with amino groups was highly active against pathogens. The layer-by-layer assembly technique is also helpful in fabricating chitosan and alginate-based cotton samples, which showed antimicrobial activity against S. aureus and Klebsiella pneumoniae (Gomes et al., Citation2013). These chitosan-based materials can have broad-spectrum applications in other surface coatings, e.g. as coatings for implants (Teixeira-Santos et al., Citation2021).
Interestingly, polymer-based antimicrobial films fabricated from poly(vinyl alcohol), ε-polylysine, and bacterial cellulose sustained their antimicrobial potential even after the two-time uses (Ren et al., Citation2017). ε-polylysine is a biodegradable, nontoxic, water-soluble AP (Wahid et al., Citation2019) isolated from the culture filtrates of Streptomyces albulus. However, it is also produced on an industrial scale through several microorganisms, such as mutated strains of S. albulus, Kitasatospora spp., and Epichloae spp. (Sayed & Jardine, Citation2015). Chitosan-coated central venous catheters showed antimicrobial and antibiofilm activities against C. albicans and Candida parapsilosis. Chitosan also showed antifungal and antibiofilm effects against C. neoformans, P. aeruginosa, and S. aureus on polystyrene surfaces (Martinez et al., Citation2010). The mobility of surfaced-coated APs is limited compared to free APs; however, APs can still interact with the cell membranes of pathogens electrostatically. Consequently, surfaced-coated APs disrupt the cell membrane through the polymeric spacer effect, the phospholipid sponge effect, and the extraction of stabilizing ions. However, layer-by-layer coated surfaces of some polymers, such as poly(ethyl or propyl or butyl acrylic acid) and polyvinylpyrrolidone, disrupt cell membranes with hydrophobic alkyl chains (Lu et al., Citation2015). Flattened AP brushes, grafted to the surface, are suggested to facilitate AP penetration through the membrane (Zou et al., Citation2015). Additionally, it was shown that a high density of the immobilized quaternary ammonium groups on the surfaces accessible to pathogens is a determining factor of their potency (H. Liu, Elkin, Chen, & Klibanov, Citation2015). Various grafting methods were used to obtain surface-immobilized poly(vinyl pyridinium)-based polymers and investigated the effect of changes in hydrophobicity on the antimicrobial activity. Polymer surfaces with short alkyl chain lengths on the pyridinium ring (propyl to hexyl) had higher antimicrobial activity than longer ones. In the case of long polyelectrolyte chains, the antimicrobial activity of polymers is shown by the needle-like structure they form on the substrate if they can stay in the form of densely grafted polymer brushes. However, they cannot stay in the form of rigid rods under physiological conditions and eventually collapse into coils (Riga, Vöhringer, Widyaya, & Lienkamp, Citation2017). Thin AP brushes of about 30-37 nm are highly bactericidal and enough for piercing pathogens’ cell walls. The surface with high molecular weight chains has better antimicrobial potential than those coated with lower molecular weight polymers. Murata et al. (Murata et al., Citation2007) observed a correlation between surface density and antimicrobial activity of 2-(dimethylamino) ethyl methacrylate. It was suggested that surface density greater than 1-5 x 1015 accessible quaternary amine units/cm2 is a critical factor for the design of polymer-based antimicrobial surfaces.
It has been observed that charge density and surface architecture influence the bactericidal mechanism of antimicrobial surfaces. APs, such as poly(2-alkyl oxazolines)s with quaternary ammonium end groups, show antimicrobial properties in dental material (Álvarez-Paino et al., Citation2017). PEI-incorporated nanoparticles of dental resins are also reported to be active against S. mutans bacteria (Zaltsman et al., Citation2016). PEI is a synthetic, nonbiodegradable, cationic polymer available in both branched and linear forms. The branched structure can be formed by acid-catalyzed polymerization of aziridine while linear through ring-opening polymerization of 2-ethyl-2-oxazoline followed by hydrolysis (Samal et al., Citation2012). The high number of reactive amino groups allows PEI for chemical modification. It was observed that its antimicrobial potential is influenced by hydrophobicity and positive charge density that can be potentiated by incorporating alkyl groups. Alkyl-PEI fabricated surfaces of different materials exhibited significant antimicrobial potential against drug-resistant pathogens and lacked cytotoxic behavior towards mammalian (monkey kidney) cells.
Talu et al. (Talu et al., Citation2010) used maleic anhydride N-vinyl-2-pyrrolidone and N-isopropyl acrylamide to prepare a water-soluble terpolymer, which showed a broad-spectrum antimicrobial potential against S. aureus, S. enteridis, Enterococcus faecalis, E. coli, K. pneumoniae, and P. aeruginosa. Additionally, several studies reported the antimicrobial potential of terpolymers against E. coli and S. epidermidis (Ajithkumar et al., Citation2018). Similarly, maleic anhydride-acrylamide copolymer and maleic anhydride-acrylamide copolymer-based ultrathin coatings showed significant inhibition of E. coli, S. aureus, M. smegmatis and C. albicans (Nagaraja et al., Citation2019).
8. APs activity against coronaviruses and other viruses
Since the initial studies of APs came into view in the 1970s, the 2000s is the rebirth of APs, possibly due to the increased incidence of infections and drug resistance. The studies on APs decreased slightly between 2010 and 2020. However, since 2020, the research on APs has observed an abrupt increase. The COVID-19 pandemic is a player behind this increase that influences the ratio of other microbial diseases; therefore, the thirst for alternative antimicrobials increases. Due to the lack of suitable antiviral drugs, controlling the spread of SARS-CoV-2 was difficult. Thus, the scientific focus was turned towards antimicrobial surface coating, for which several antimicrobial agents, including APs, were tested to prevent the spread of the virus (Nasri et al., Citation2021). Several sources and antimicrobial agents attracted the researchers. Still, APs stand out as one of the most viable candidates due to their favorable flexibility properties and simple synthesis (Mahat, Sabere, Azizi, & Amdan, Citation2021).
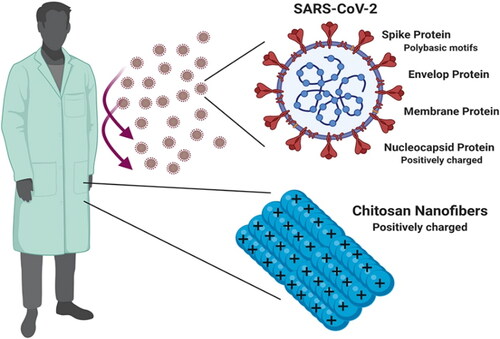
Furthermore, conducting polymers such as polyaniline (PANI), poly(aniline-co-3-aminobenzoic acid), polyaniline-g-chitosan (PANI-g-CS), polypyrrole-g-chitosan (PPy-g-CS), and polythiophene-g-chitosan (PT-g-CS) were suggested as promising antimicrobials against secondary bacterial infection among COVID-19 patients (Mahat et al., Citation2021). APs such as chitosan and chitosan derivatives (Sharma et al., Citation2021) are indicated in the prototyping of critical medical devices to increase the production of connectors for ventilators and face masks (Zuniga & Cortes, Citation2020). Srisa et al. (Srisa et al., Citation2022) comprehensively reviewed the polymers for food packaging during the pandemic. They concluded that APs, e.g. poly(butylene adipate terephthalate), polylactide, and chitosan, could be efficiently utilized to develop protective food to avoid SARS-CoV-2 contamination. The coating of personal protective equipment (PPE) of SARS-CoV-2 with positively charged chitosan nanofibers is also suggested for healthcare providers (). Chitosan with abundant cationic amino groups will likely exhibit antiviral potential (Hathout & Kassem, Citation2020).
Figure 7. A Schematic presentation of positively charged chitosan nanofibers based PPEs for health care providers to enhance protection and safety during COVID-19 (Hathout & Kassem, Citation2020). Copyrights by frontiers (2020).
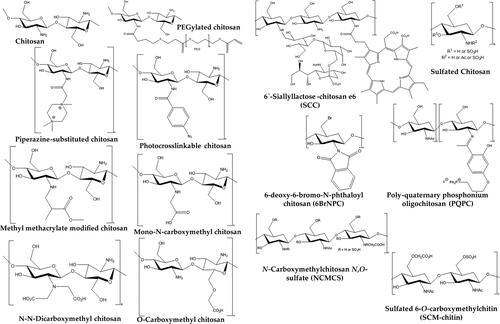
Cationically modified chitosan derivatives such as N-(2-hydroxypropyl)-3-trimethylammonium chitosan chloride (HTCC) and hydrophobically-modified HTCC are reported to inhibit the replication of human coronavirus-NL63 and murine hepatitis virus (Milewska et al., Citation2013). It was found that HTCC prevents the inhibition between cell receptors and spike protein by aggregation of spike proteins (Sharma et al., Citation2021). HTCC also showed inhibitory effects against human coronaviruses such as the Middle East respiratory syndrome Coronavirus (MERS-CoV) and severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in in vitro studies using Vero and Vero E6 cell lines and ex vivo studies, which were based on a human airway epithelium (HAE) model (Milewska et al., Citation2020). Compared to MERS-CoV, HTCC was highly effective against SARS-CoV-2. The inhibitory effects were associated with electrostatic interactions mediated prevention of SARS-CoV-2 spike protein RBD binding with ACE2. Anionic dendritic polymers, succinic acid–terminated polyamidoamine dendrimers, and cationic dendritic polymers containing primary amine end groups have shown inhibitory potential against MERS-CoV (Kandeel et al., Citation2020). SARS-CoV-2 studies of molecular dynamics showed a strong binding affinity of chitosan towards RBD of spike protein and seems to be the potential therapeutic lead for developing antiviral drugs (Kalathiya et al., Citation2020). It has been reported that β-chitosan downregulated ACE2 expression and inflammation and blocked spike-RBD binding to lung cells harboring ACE2 receptors in vivo (Alitongbieke et al., Citation2020). Among APs, chitosan is reported to be the most remarkable antiviral agent effective against several viruses. shows the chitosan derivatives with proven antiviral effects.
Figure 8. Various chitosan derivatives have a proven antiviral potential (Gopal et al., Citation2023). Copyrights (2023) MDPI.
Chitosan has been reported to protect BALB/c mice against lethal infection by influenza virus H7N9 by acting as an innate immune system stimulatory agent (Bouloussa et al., Citation2008). Chitosan exhibited strong antiviral effects ten days after the intranasal administration. In tested animals, enhanced infiltration of leukocytes and high intensities of proinflammatory cytokines were observed. Influenza virus also showed susceptibility to several other polymers, such as N, N-dodecylmethyl-polyethyleneimine (Zou et al., Citation2015), and PEI (H. Liu et al., Citation2015). N-dodecyl methyl-polyethyleneimine exhibited antiviral effects against herpes simplex virus (Larson & Klibanov, 2014). Chitosan-oligosaccharide (3–5 kDa) also showed antiviral properties against human immunodeficiency virus type 1 (HIV-1) (Artan et al., Citation2010). Chitosan-oligosaccharide inhibited the replication of HIV-1 through suppressing HIV-1-induced syncytia formation. Furthermore, it was found that this chitooligosaccharide also disrupts the binding of HIV-1 gp120 to CD4 cell surface receptors, thus avoiding viral entry and virus-cell fusion (Artan et al., Citation2010). Pauls et al. (Pauls, Citation2016) demonstrated that chitosan has antiviral effects against adenovirus. They evaluated the antiviral effects of chitosan using flow cytometry by treating the NIH-3 T3 cell lines of mouse embryo tissue infected with green fluorescent protein (GFP)-adenovirus. Compared to the control group, the chitosan-treated group displayed the highest drop in fluorescence. The infectivity of enveloped vesicular stomatitis, Simbis, and vaccinia viruses are reportedly inhibited by poly(methacrylic acid) (Jiang et al., Citation2021). The other anti-SARS polymers are poly(acrylic acid), poly(vinyl benzoic acid), poly(vinyl phosphonic acid), and poly(2-acrylamidoethyl)phosphate. Poly(vinylbenzoic acid) is highly effective against non-enveloped viruses and comparatively enveloped viruses (Jiang et al., Citation2021). Some cationic polymers, such as PEI, have been reported to exhibit contact-killing antiviral action (Sinclair et al., Citation2018). PEI-based coatings in filter membranes were reported to reduce the viral reduction in drinking water.
9. Limitations and future directions
Although antimicrobial potential of APs is well known, future studies are needed to evaluate the effects of APs on the host organism. For this reason, it is mandatory to perform large-scale in vivo experiments to provide the detailed information on the toxicity, cell viability, biodistribution, and immunogenicity of APs. The lack of this particular set of data is currently the limiting factor for a clinical application of APs. Before entering the final step, the above features need to be evaluated using the guidelines on the translational requirements to move the new antimicrobial agents toward the investigational new drug application, as demonstrated by Mignani et al. (Mignani et al., Citation2018). Besides the development of new, AP-based drugs, the wide-range applicability of APs should be also demonstrated in the context of the design of antibacterial coatings, particularly those used to modify the surface of medical implants, hospital products and textiles, as well as wound healing dressings. Due to their broad-spectrum antimicrobial potential, multifunctionality, peculiar characteristics, and flexible nature, APs may become valuable assets for biomedical engineering, as suggested by the increasing interest in APs observed in the last two decades.
Since it takes significantly longer to develop new antimicrobials, employing the existing research of APs would ensure the progress of new antimicrobials. Regulatory bodies, researchers, and biotechnological/pharmaceutical companies must collaborate to set an integral plan to enhance the thrust toward developing AP-based antimicrobial therapies. The broad-spectrum inhibitory potential of APs against bacteria, fungi, and viruses, their safety profile, and their stability to high temperatures, pH, and proteolysis represent a solid basis for developing APs as antimicrobial therapeutic agents for clinical use or antimicrobial coatings.
10. Conclusion
Antimicrobial polymers emerged as promising alternatives for antimicrobial development. Undoubtedly, the slow but steady progress in APs in the past two decades is on the edge of rapid growth, most probably due to the COVID-19 pandemic, influencing the ratio of other microbial diseases. Therefore, the thirst for alternative antimicrobials has increased. This study provides an up-to-date review of APs detailing synthetic and natural APs, their potential applications, and modes of action. The malleable structure of APs allows researchers to generate desired biological properties using particular functional groups. Adding antimicrobial functional groups enhances the antimicrobial potential and reduced the hemolytic activity and toxicity. Versatile chemical structure, low manufacturing costs, and highly reported antimicrobial activity have proven APs to be one of the most commercially attractive candidates for drug discovery. Rational design and combinatorial engineering can reduce limitations and improve antimicrobial polymers’ physiochemical and biological characteristics.
Author contributions
Conceptualization, IUH; Visualization, IUH, KK; Writing—original draft preparation, IUH, KK; Writing—review and editing, RPV, M, W.G.L., M.E.d.L; Funding acquisition, KK. All authors have read and agreed to the published version of the manuscript.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Ajithkumar, M. P., Yashoda, M. P., Prasannakumar, S., Sruthi, T. V., & Sameer Kumar, V. B. (2018). Synthesis, characterization, microstructure determination, thermal studies of poly (N-vinyl pyrrolidone-maleic anhydride-methyl methacrylate). Journal of Macromolecular Science, Part A: Pure and Applied Chemistry, 55(4), 362–368. doi:10.1080/10601325.2018.1440178
- Alitongbieke, G., Li, X., Wu, Q., Lin, Z., Huang, J., Liu, J., … Normal, M. (2020). Effect of β -chitosan on the binding interaction between. BioRxiv.
- Álvarez-Paino, M., Muñoz-Bonilla, A., & Fernández-García, M. (2017). Antimicrobial polymers in the nano-world. Nanomaterials (Basel, Switzerland), 7(2), 48. doi:10.3390/nano7020048
- Álvarez-Paino, M., Muñoz-Bonilla, A., López-Fabal, F., Gómez-Garcés, J. L., Heuts, J. P. A., & Fernández-García, M. (2015). Effect of glycounits on the antimicrobial properties and toxicity behavior of polymers based on quaternized DMAEMA. Biomacromolecules, 16(1), 295–303. doi:10.1021/bm5014876
- Amin, R., Yasmin, F., Hosen, M. A., Dey, S., Mahmud, S., Saleh, A., … Kawsar, A. (2021). Synthesis, antimicrobial, anticancer, PASS, molecular docking, molecular dynamic simulations & pharmacokinetic predictions of some methyl β-D-galactopyranoside analogs. Molecules (Basel, Switzerland), 26(22), 7016. doi:10.3390/molecules26227016
- Anowar Hosen, M., Sultana Munia, N., Al-Ghorbani, M., Baashen, M., Almalki, F. A., Ben Hadda, T., … Kawsar, S. M. A. (2022). Synthesis, antimicrobial, molecular docking and molecular dynamics studies of lauroyl thymidine analogs against SARS-CoV-2: POM study and identification of the pharmacophore sites. Bioorganic Chemistry, 125(April), 105850. doi:10.1016/j.bioorg.2022.105850
- Arora, A., & Mishra, A. (2018). Antibacterial polymers - A mini review. Materials Today: Proceedings, 5(9), 17156–17161. doi:10.1016/j.matpr.2018.04.124
- Artan, M., Karadeniz, F., Karagozlu, M. Z., Kim, M. M., & Kim, S. K. (2010). Anti-HIV-1 activity of low molecular weight sulfated chitooligosaccharides. Carbohydrate Research, 345(5), 656–662. doi:10.1016/j.carres.2009.12.017
- Bieser, A. M., & Tiller, J. C. (2011). Mechanistic considerations on contact-active antimicrobial surfaces with controlled functional group densities. Macromolecular Bioscience, 11(4), 526–534. doi:10.1002/mabi.201000398
- Bouazizi, N., Vieillard, J., Samir, B., Derf., & F., Le. (2022). Advances in amine-surface functionalization of inorganic adsorbents for water treatment and antimicrobial activities: A review. Polymers, 14(3), 378. doi:10.3390/polym14030378
- Bouloussa, O., Rondelez, F., & Semetey, V. (2008). A new, simple approach to confer permanent antimicrobial properties to hydroxylated surfaces by surface functionalization. Chemical Communications (Cambridge, England), 8(8), 951–953. doi:10.1039/b716026g
- Brower, J. L. (2018). The threat and response to infectious diseases (revised). Microbial Ecology, 76(1), 19–36. doi:10.1007/s00248-016-0806-9
- Carmona-Ribeiro, A. M., & de Melo Carrasco, L. D. (2013). Cationic antimicrobial polymers and their assemblies. International Journal of Molecular Sciences, 14(5), 9906–9946. doi:10.3390/ijms14059906
- Chakraborty, S., Liu, R., Lemke, J. J., Hayouka, Z., Welch, R. A., Weisblum, B., … Gellman, S. H. (2013). Effects of cyclic vs acyclic hydrophobic subunits on the chemical structure and biological properties of nylon-3 copolymers. ACS Macro Letters, 2(8), 753–756. doi:10.1021/mz400239r
- Chamsaz, E. A., Mankoci, S., Barton, H. A., & Joy, A. (2017). Nontoxic cationic coumarin polyester coatings prevent Pseudomonas aeruginosa biofilm formation. ACS Applied Materials & Interfaces, 9(8), 6704–6711. doi:10.1021/acsami.6b12610
- Chang, Y., McLandsborough, L., & McClements, D. J. (2014). Antimicrobial delivery systems based on electrostatic complexes ofcationic e{open}-polylysine and anionic gum arabic. Food Hydrocolloids, 35, 137–143. doi:10.1016/j.foodhyd.2013.05.004
- Chen, Y., Wilbon, P. A., Chen, Y. P., Zhou, J., Nagarkatti, M., Wang, C., … Tang, C. (2012). Amphipathic antibacterial agents using cationic methacrylic polymers with natural rosin as pendant group. RSC Advances, 2(27), 10275–10282. doi:10.1039/c2ra21675b
- Chindera, K., Mahato, M., Kumar Sharma, A., Horsley, H., Kloc-Muniak, K., Kamaruzzaman, N. F., … Good, L. (2016). The antimicrobial polymer PHMB enters cells and selectively condenses bacterial chromosomes. Scientific Reports, 6(1), 23121. doi:10.1038/srep23121
- Cornell, R. J., & Donaruma, L. G. (1965). 2-MethacryIoxytropones. Intermediates for the synthesis of biologically active polymers. Journal of Medicinal Chemistry, 8(3), 388–390. doi:10.1021/jm00327a025
- Correia, V. G., Bonifácio, V. D. B., Raje, V. P., Casimiro, T., Moutinho, G., da Silva, C. L., … Aguiar-Ricardo, A. (2011). Oxazoline-based antimicrobial oligomers: Synthesis by CROP using supercritical CO 2. Macromolecular Bioscience, 11(8), 1128–1137. doi:10.1002/mabi.201100126
- DeWit, M. A., & Gillies, E. R. (2009). A cascade biodegradable polymer based on alternating cyclization and elimination reactions. Journal of the American Chemical Society, 131(51), 18327–18334. doi:10.1021/ja905343x
- Ergene, C., & Palermo, E. F. (2018). Antimicrobial synthetic polymers: An update on structure-activity relationships. Current Pharmaceutical Design, 24(8), 855–865. doi:10.2174/1381612824666180213140732
- Ergene, C., Yasuhara, K., & Palermo, E. F. (2018). Biomimetic antimicrobial polymers: Recent advances in molecular design. Polymer Chemistry, 9(18), 2407–2427. doi:10.1039/C8PY00012C
- Exley, S. E., Paslay, L. C., Sahukhal, G. S., Abel, B. A., Brown, T. D., McCormick, C. L., … Morgan, S. E. (2015). Antimicrobial peptide mimicking primary amine and guanidine containing methacrylamide copolymers prepared by raft polymerization. Biomacromolecules, 16(12), 3845–3852. doi:10.1021/acs.biomac.5b01162
- Farshbaf, M., Davaran, S., Zarebkohan, A., Annabi, N., Akbarzadeh, A., & Salehi, R. (2018). Significant role of cationic polymers in drug delivery systems. Artificial Cells, Nanomedicine and Biotechnology, 46(8), 1872–1891. doi:10.1080/21691401.2017.1395344
- Figg, C. A., Hickman, J. D., Scheutz, G. M., Shanmugam, S., Carmean, R. N., Tucker, B. S., … Sumerlin, B. S. (2018). Color-coding visible light polymerizations to elucidate the activation of trithiocarbonates using Eosin y. Macromolecules, 51(4), 1370–1376. doi:10.1021/acs.macromol.7b02533
- Frank, D. S., & Matzger, A. J. (2019). Effect of polymer hydrophobicity on the stability of amorphous solid dispersions and supersaturated solutions of a hydrophobic pharmaceutical. Molecular Pharmaceutics, 16(2), 682–688. doi:10.1021/acs.molpharmaceut.8b00972
- Gabriel, G. J., Madkour, A. E., Dabkowski, J. M., Nelson, C. F., Nüsslein, K., & Tew, G. N. (2008). Synthetic mimic of antimicrobial peptide with nonmembrane-disrupting antibacterial properties. Biomacromolecules, 9(11), 2980–2983. doi:10.1021/bm800855t
- Ganewatta, M. S., & Tang, C. (2015). Controlling macromolecular structures towards effective antimicrobial polymers. Polymer, 63, A1–A29. doi:10.1016/j.polymer.2015.03.007
- Gelman, M. A., Weisblum, B., Lynn, D. M., & Gellman, S. H. (2004). Biocidal activity of polystyrenes that are cationic by virtue of protonation. Organic Letters, 6(4), 557–560. doi:10.1021/ol036341+
- Gody, G., Barbey, R., Danial, M., & Perrier, S. (2015). Ultrafast RAFT polymerization: Multiblock copolymers within minutes. Polymer Chemistry, 6(9), 1502–1511. doi:10.1039/C4PY01251H
- Gomes, A. P., Mano, J. F., Queiroz, J. A., & Gouveia, I. C. (2013). Layer-by-layer deposition of antimicrobial polymers on cellulosic fibers: A new strategy to develop bioactive textiles. Polymers for Advanced Technologies, 24(11), 1005–1010. doi:10.1002/pat.3176
- González-Henríquez, C. M., Sarabia-Vallejos, M. A., & Hernandez, J. R. (2019). Antimicrobial polymers for additive manufacturing. International Journal of Molecular Sciences, 20(5), 1210. doi:10.3390/ijms20051210
- Gopal, J.,Muthu, M.,Pushparaj, S. S. C., &Sivanesan, I. (2023). Anti-COVID-19 credentials of Chitosan composites and derivatives: Future scope?. Antibiotics, 12(4), 665 10.3390/antibiotics12040665.
- Gottenbos, B., Van Der Mei, H. C., Klatter, F., Nieuwenhuis, P., & Busscher, H. J. (2002). In vitro and in vivo antimicrobial activity of covalently coupled quaternary ammonium silane coatings on silicone rubber. Biomaterials, 23(6), 1417–1423. doi:10.1016/S0142-9612(01)00263-0
- Grace, J. L., Huang, J. X., Cheah, S. E., Truong, N. P., Cooper, M. A., Li, J., … Whittaker, M. R. (2016). Antibacterial low molecular weight cationic polymers: Dissecting the contribution of hydrophobicity, chain length and charge to activity. RSC Advances, 6(19), 15469–15477. doi:10.1039/C5RA24361K
- Haktaniyan, M., & Bradley, M. (2022). Polymers showing intrinsic antimicrobial activity. Chemical Society Reviews, 51(20), 8584–8611. doi:10.1039/d2cs00558a
- Hassan, M. A., Omer, A. M., Abbas, E., Baset, W. M. A., & Tamer, T. M. (2018). Preparation, physicochemical characterization and antimicrobial activities of novel two phenolic chitosan Schiff base derivatives. Scientific Reports, 8(1), 11416. doi:10.1038/s41598-018-29650-w
- Hathout, R. M., & Kassem, D. H. (2020). Positively charged electroceutical spun chitosan nanofibers can protect health care providers from COVID-19 infection : An opinion. Frontiers in Bioengineering and Biotechnology, 8(August), 885. doi:10.3389/fbioe.2020.00885
- Hemp, S. T., Smith, A. E., Bryson, J. M., Allen, M. H., & Long, T. E. (2012). Phosphonium-containing diblock copolymers for enhanced colloidal stability and efficient nucleic acid delivery. Biomacromolecules, 13(8), 2439–2445. doi:10.1021/bm300689f
- Hernández-Montelongo, J., Nascimento, V. F., Murillo, D., Taketa, T. B., Sahoo, P., Souza, A. A., … Cotta, M. A. (2016). Nanofilms of hyaluronan/chitosan assembled layer-by-layer : An antibacterial surface for Xylella fastidiosa. Carbohydrate Polymers, 136, 1–11. doi:10.1016/j.carbpol.2015.08.076
- Hirayama, M. (2011). The antimicrobial activity, hydrophobicity and toxicity of sulfonium compounds, and their relationship. Biocontrol Science, 16(1), 23–31. doi:10.4265/bio.16.23
- Huang, K. S., Yang, C. H., Huang, S. L., Chen, C. Y., Lu, Y. Y., & Lin, Y. S. (2016). Recent advances in antimicrobial polymers: A mini-review. International Journal of Molecular Sciences, 17(9), 1578. doi:10.3390/IJMS17091578
- Hung, A. Y.-T. (2018). Designing antimicrobial polymer coating to inhibit pathogenic and spoilage microorganisms Thesis, March.
- Ikeda, T., Tazuke, S., & Suzuki, Y. (1984). Biologically active polycations.4. Synthesis and antimicrobial activity of poly(trialkylvinylbenzylammonium chloride)s. Makromolekulare Chemie Macromolecular Chemistry and Physics, 185, 869–876.
- Jackson, N., Czaplewski, L., & Piddock, L. J. V. (2018). Discovery and development of new antibacterial drugs: Learning from experience? The Journal of Antimicrobial Chemotherapy, 73(6), 1452–1459. doi:10.1093/jac/dky019
- Jain, A., Duvvuri, L. S., Farah, S., Beyth, N., Domb, A. J., Khan, W., … Beyth, N. (2014). Antimicrobial polymers. Advanced Healthcare Materials, 3(12), 1969–1985. doi:10.1002/ADHM.201400418
- Jiang, X., Li, Z., Young, D. J., Liu, M., Wu, C., Wu, Y. L., & Loh, X. J. (2021). Toward the prevention of coronavirus infection: What role can polymers play? Materials Today. Advances, 10, 100140. doi:10.1016/j.mtadv.2021.100140
- Judzewitsch, P. R., Nguyen, T. K., Shanmugam, S., Wong, E. H. H., & Boyer, C. (2018). Towards sequence-controlled antimicrobial polymers: Effect of polymer block order on antimicrobial activity. Angewandte Chemie International Edition, 57(17), 4559–4564. doi:10.1002/anie.201713036
- Kalathiya, U., Padariya, M., Mayordomo, M., Lisowska, M., Nicholson, J., Singh, A., … Alfaro, J. A. (2020). Highly conserved homotrimer cavity formed by the sars-cov-2 spike glycoprotein: A novel binding site. Journal of Clinical Medicine, 9(5), 1473. doi:10.3390/jcm9051473
- Kamaruzzaman, N. F., Tan, L. P., Hamdan, R. H., Choong, S. S., Wong, W. K., Gibson, A. J., … De Fatima Pina, M. (2019). Antimicrobial polymers: The potential replacement of existing antibiotics? International Journal of Molecular Sciences, 20(11), 2747. doi:10.3390/ijms20112747
- Kandeel, M., Al-Taher, A., Park, B. K., Kwon, H. J., & Al-Nazawi, M. (2020). A pilot study of the antiviral activity of anionic and cationic polyamidoamine dendrimers against the Middle East respiratory syndrome coronavirus. Journal of Medical Virology, 92(9), 1665–1670. doi:10.1002/jmv.25928
- Kara, F., Aksoy, E. A., Yuksekdag, Z., Hasirci, N., & Aksoy, S. (2014). Synthesis and surface modification of polyurethanes with chitosan for antibacterial properties. Carbohydrate Polymers, 112, 39–47. doi:10.1016/j.carbpol.2014.05.019
- Kenawy, E. R., Imam Abdel-Hay, F., Abou El-Magd, A., & Mahmoud, Y. (2006). Synthesis and antimicrobial activity of some polymers derived from modified amino polyacrylamide by reacting it with benzoate esters and benzaldehyde derivatives. Journal of Applied Polymer Science, 99(5), 2428–2437. doi:10.1002/app.22249
- Kenawy, E. R., Worley, S. D., & Broughton, R. (2007). The chemistry and applications of antimicrobial polymers: A state-of-the-art review. Biomacromolecules, 8(5), 1359–1384. doi:10.1021/BM061150Q/ASSET/IMAGES/LARGE/BM061150QF00031.JPEG
- Kozon-Markiewicz, D., Kopiasz, R. J., Głusiec, M., Łukasiak, A., Bednarczyk, P., & Jańczewski, D. (2023). Membrane lytic activity of antibacterial ionenes, critical role of phosphatidylcholine (PC) and cardiolipin (CL). Colloids and Surfaces. B, Biointerfaces, 229(July), 113480. doi:10.1016/j.colsurfb.2023.113480
- Krishnan, S., Ward, R. J., Hexemer, A., Sohn, K. E., Lee, K. L., Angert, E. R., … Ober, C. K. (2006). Surfaces of fluorinated pyridinium block copolymers with enhanced antibacterial activity. Langmuir: The ACS Journal of Surfaces and Colloids, 22(26), 11255–11266. doi:10.1021/la061384v
- Kügler, R., Bouloussa, O., & Rondelez, F. (2005). Evidence of a charge-density threshold for optimum efficiency of biocidal cationic surfaces. Microbiology (Reading, England), 151(Pt 5), 1341–1348. doi:10.1099/mic.0.27526-0
- Kuroki, A., Sangwan, P., Qu, Y., Peltier, R., Sanchez-Cano, C., Moat, J., … Perrier, S. (2017). Sequence control as a powerful tool for improving the selectivity of antimicrobial polymers. ACS Applied Materials & Interfaces, 9(46), 40117–40126. doi:10.1021/acsami.7b14996
- Kwaśniewska, D., Chen, Y. L., & Wieczorek, D. (2020). Biological activity of quaternary ammonium salts and their derivatives. Pathogens (Basel, Switzerland), 9(6), 459. doi:10.3390/pathogens9060459
- Larson, A. M., Oh, H. S., Knipe, D. M., & Klibanov, A. M. (2014). Decreasing herpes simplex viral infectivity in solution by surface-immobilized and suspended N, N-Dodecyl, methylpolyethylenimine. Pharmaceutical Research, 30(1), 25–31. doi:10.1007/s11095-012-0825-2.Decreasing
- Leshabane, M., Dziwornu, G. A., Coertzen, D., Reader, J., Moyo, P., Watt, V., … Birkholtz, L. (2021). Benzimidazole derivatives are potent against multiple life cycle stages of plasmodium falciparum malaria parasites. ACS Infectious Diseases, 7(7), 1945–1955. doi:10.1021/acsinfecdis.0c00910
- Li, G., & Shen, J. (2000). Study of pyridinium-type functional polymers. IV. Behavioral features of the antibacterial activity of insoluble pyridinium-type polymers. Journal of Applied Polymer Science, 78(3), 676–684. doi:10.1002/1097-4628(20001017)78:3<676::AID-APP240>3.0.CO;2-E
- Lichter, J. A., & Rubner, M. F. (2009). Polyelectrolyte multilayers with intrinsic antimicrobial functionality: The importance of mobile polycations. Langmuir: The ACS Journal of Surfaces and Colloids, 25(13), 7686–7694. doi:10.1021/la900349c
- Lim, S. H., & Hudson, S. M. (2003). Review of chitosan and its derivatives as antimicrobial agents and their uses as textile chemicals. Journal of Macromolecular Science - Polymer Reviews, 43(2), 223–269. doi:10.1081/MC-120020161
- Lin, J., Chen, X., Chen, C., Hu, J., Zhou, C., Cai, X., … Liu, H. (2018). Durably antibacterial and bacterially antiadhesive cotton fabrics coated by cationic fluorinated polymers. ACS Applied Materials & Interfaces, 10(7), 6124–6136. doi:10.1021/acsami.7b16235
- Lin, J., Tiller, J. C., Lee, S. B., Lewis, K., & Klibanov, A. M. (2002). Insights into bactericidal action of surface-attached poly(vinyl-N-hexylpyridinium) chains. Biotechnology Letters, 24(10), 801–805. doi:10.1023/A:1015584423358
- Liu, H., Elkin, I., Chen, J., & Klibanov, A. M. (2015). Why do some immobilized N-Alkylated polyethylenimines far surpass others in inactivating influenza viruses? Biomacromolecules, 16(1), 351–356. doi:10.1021/bm5015427
- Liu, S. G., Li, N., Ling, Y., Kang, B. H., Geng, S., Li, N. B., & Luo, H. Q. (2016). pH-mediated fluorescent polymer particles and gel from hyperbranched polyethylenimine and the mechanism of intrinsic fluorescence. Langmuir: The ACS Journal of Surfaces and Colloids, 32(7), 1881–1889. doi:10.1021/acs.langmuir.6b00201
- Liu, Y., Song, L., Feng, N., Jiang, W., Jin, Y., & Li, X. (2020). Recent advances in the synthesis of biodegradable polyesters by sustainable polymerization: Lipase-catalyzed polymerization. RSC Advances, 10(59), 36230–36240. doi:10.1039/d0ra07138b
- Liu, Z., Zhang, Z., Zhou, C., & Jiao, Y. (2010). Hydrophobic modifications of cationic polymers for gene delivery. Progress in Polymer Science, 35(9), 1144–1162. doi:10.1016/j.progpolymsci.2010.04.007
- Locock, K. E. S., Michl, T. D., Griesser, H. J., Haeussler, M., & Meagher, L. (2014). Structure–activity relationships of guanylated antimicrobial polymethacrylates. Pure and Applied Chemistry, 86(8), 1281–1291. doi:10.1515/pac-2014-0213
- Locock, K. E. S., Michl, T. D., Valentin, J. D. P., Vasilev, K., Hayball, J. D., Qu, Y., … Haeussler, M. (2013). Guanylated polymethacrylates: A class of potent antimicrobial polymers with low hemolytic activity. Biomacromolecules, 14(11), 4021–4031. doi:10.1021/bm401128r
- Lou, Y., & Palermo, E. F. (2024). Dynamic antimicrobial poly(disulfide) coatings exfoliate biofilms on demand via triggered depolymerization. Advanced Healthcare Materials, 13(11), e2303359. doi:10.1002/adhm.202303359
- Lowe, A., Deng, W., Smith, D. W., & Balkus, K. J. (2012). Acrylonitrile-based nitric oxide releasing melt-spun fibers for enhanced wound healing. Macromolecules, 45(15), 5894–5900. doi:10.1021/ma300913w
- Lu, Y., Wu, Y., Liang, J., Libera, M. R., & Sukhishvili, S. A. (2015). Self-defensive antibacterial layer-by-layer hydrogel coatings with pH-triggered hydrophobicity. Biomaterials, 45, 64–71. doi:10.1016/j.biomaterials.2014.12.048
- Mahat, M. M., Sabere, A. S. M., Azizi, J., & Amdan, N. A. N. (2021). Potential applications of conducting polymers to reduce secondary bacterial infections among COVID-19 patients: A review. Emergent Materials, 4(1), 279–292. doi:10.1007/s42247-021-00188-4
- Mankoci, S., Kaiser, R. L., Sahai, N., Barton, H. A., & Joy, A. (2017). Bactericidal peptidomimetic polyurethanes with remarkable selectivity against Escherichia coli. ACS Biomaterials Science & Engineering, 3(10), 2588–2597. doi:10.1021/acsbiomaterials.7b00309
- Maowa, J., Alam, A., Rana, K. M., Dey, S., Hosen, A., Fujii, Y., … Kaws, S. M. A. (2021). Synthesis, characterization, synergistic antimicrobial properties and molecular docking of sugar modified uridine derivatives. Ovidius University Annals of Chemistry, 32(1), 6–21. doi:10.2478/auoc-2021-0002
- Martinez, L. R., Mihu, M. R., Han, G., Frases, S., Cordero, R. J. B., Casadevall, A., … Nosanchuk, J. D. (2010). The use of chitosan to damage Cryptococcus neoformans biofilms. Biomaterials, 31(4), 669–679. doi:10.1016/j.biomaterials.2009.09.087
- Másson, M. (2024). The quantitative molecular weight-antimicrobial activity relationship for chitosan polymers, oligomers, and derivatives. Carbohydrate Polymers, 337(1), 122159. doi:10.1016/j.carbpol.2024.122159
- Mignani, S., Rodrigues, J., Tomas, H., Roy, R., Shi, X., & Majoral, J. P. (2018). Bench-to-bedside translation of dendrimers: Reality or utopia? A concise analysis. Advanced Drug Delivery Reviews, 136-137, 73–81. doi:10.1016/j.addr.2017.11.007
- Milewska, A., Chi, Y., Szczepanski, A., Barreto-Duran, E., Liu, K., Liu, D., … Pyrc, K. (2020). HTCC as a highly effective polymeric inhibitor of SARS-CoV-2 and MERS-CoV. BioRxiv, 3 2020.03.29.014183.
- Milewska, A., Ciejka, J., Kaminski, K., Karewicz, A., Bielska, D., Zeglen, S., … Szczubialka, K. (2013). Novel polymeric inhibitors of HCoV-NL63. Antiviral Research, 97(2), 112–121. doi:10.1016/j.antiviral.2012.11.006
- Milović, N. M., Wang, J., Lewis, K., & Klibanov, A. M. (2005). Immobilized N-alkylated polyethylenimine avidly kills bacteria by rupturing cell membranes with no resistance developed. Biotechnology and Bioengineering, 90(6), 715–722. doi:10.1002/bit.20454
- Morris, H., & Murray, R. (2021). Medical Textiles. LTD. doi:10.1201/9781003170570
- Mubaraki, M. A., Ali, J., Khattak, B., Fozia, F., Khan, T. A., Hussain, M., … Ahmad, I. (2024). Characterization and antibacterial potential of iron oxide nanoparticles in eradicating uropathogenic E. coli. ACS Omega, 9(1), 166–177. doi:10.1021/acsomega.3c03078
- Munia, N. S., Hosen, M. A., Azzam, K. M. A., Al-Ghorbani, M., Baashen, M., Hossain, M. K., … Kawsar, S. M. A. (2022). Synthesis, antimicrobial, SAR, PASS, molecular docking, molecular dynamics and pharmacokinetics studies of 5′-O-uridine derivatives bearing acyl moieties: POM study and identification of the pharmacophore sites. Nucleosides, Nucleotides & Nucleic Acids, 41(10), 1036–1083. doi:10.1080/15257770.2022.2096898
- Murata, H., Koepsel, R. R., Matyjaszewski, K., & Russell, A. J. (2007). Permanent, non-leaching antibacterial surfaces-2: How high density cationic surfaces kill bacterial cells. Biomaterials, 28(32), 4870–4879. doi:10.1016/j.biomaterials.2007.06.012
- Musa, N. H., Chang, Z. H., Teow, Y. H., Rosli, N. A., & Mohammad, A. W. (2022). A review on polymer based antimicrobial coating. Journal of Biochemistry, Microbiology and Biotechnology, 10(SP2), 1–8. doi:10.54987/jobimb.v10iSP2.720
- Nagaraja, A., Jalageri, M. D., Puttaiahgowda, Y. M., Raghava Reddy, K., & Raghu, A. V. (2019). A review on various maleic anhydride antimicrobial polymers. Journal of Microbiological Methods, 163(February), 105650. doi:10.1016/j.mimet.2019.105650
- Nasri, N., Rusli, A., Teramoto, N., Jaafar, M., Ishak, K. M. K., Shafiq, M. D., & Hamid, Z. A. A. (2021). Past and current progress in the development of antiviral/antimicrobial polymer coating towards covid-19 prevention: A review. Polymers, 13(23), 4234. doi:10.3390/polym13234234
- Nguyen, T. K., Lam, S. J., Ho, K. K. K., Kumar, N., Qiao, G. G., Egan, S., … Wong, E. H. H. (2017). Rational design of single-chain polymeric nanoparticles that kill planktonic and biofilm bacteria. ACS Infectious Diseases, 3(3), 237–248. doi:10.1021/acsinfecdis.6b00203
- Ornelas-Megiatto, C., Wich, P. R., & Fréchet, J. M. J. (2012). Polyphosphonium polymers for siRNA delivery: An efficient and nontoxic alternative to polyammonium carriers. Journal of the American Chemical Society, 134(4), 1902–1905. doi:10.1021/ja207366k
- Pachla, J., Kopiasz, R. J., Marek, G., Tomaszewski, W., Głogowska, A., Kowalczyk, S., … Ciach, T. (2023). Polytrimethylenimines: Highly potent antibacterial agents with activity and toxicity modulated by the polymer molecular weight. doi:10.1021/acs.biomac.3c00139
- Palermo, E. F., & Kuroda, K. (2009). Chemical structure of cationic groups in amphiphilic polymethacrylates modulates the antimicrobial and hemolytic activities. Biomacromolecules, 10(6), 1416–1428. doi:10.1021/bm900044x
- Palermo, E. F., & Kuroda, K. (2010). Structural determinants of antimicrobial activity in polymers which mimic host defense peptides. Applied Microbiology and Biotechnology, 87(5), 1605–1615. doi:10.1007/s00253-010-2687-z
- Palermo, E. F., Vemparala, S., & Kuroda, K. (2012). Cationic spacer arm design strategy for control of antimicrobial activity and conformation of amphiphilic methacrylate random copolymers. Biomacromolecules, 13(5), 1632–1641. doi:10.1021/bm300342u
- Parcheta, M., & Sobiesiak, M. (2023). Preparation and functionalization of polymers with antibacterial properties—Review of the recent developments. Materials, 16(12), 4411. doi:10.3390/ma16124411
- Paslay, L. C., Abel, B. A., Brown, T. D., Koul, V., Choudhary, V., McCormick, C. L., & Morgan, S. E. (2012). Antimicrobial poly(methacrylamide) derivatives prepared via aqueous RAFT polymerization exhibit biocidal efficiency dependent upon cation structure. Biomacromolecules, 13(8), 2472–2482. doi:10.1021/bm3007083
- Pauls, T. (2016). Chitosan as an antiviral. University of Arkansas. https://scholarworks.uark.edu/bmeguht/28/.
- Pham, P., Oliver, S., & Boyer, C. (2023). Design of antimicrobial polymers. Macromolecular Chemistry and Physics, 224(3), 1–28. doi:10.1002/macp.202200226
- Piskláková, L., Skuhrovcová, K., Bártová, T., Seidelmannová, J., Vondrovic, Š., & Velebný, V. (2024). Trends in the incorporation of antiseptics into natural polymer-based nanofibrous mats. Polymers, 16(5), 664. doi:10.3390/polym16050664
- Pu, Y., Hou, Z., Khin, M. M., Zamudio-Vázquez, R., Poon, K. L., Duan, H., & Chan-Park, M. B. (2017). Synthesis and antibacterial study of sulfobetaine/quaternary ammonium-modified star-shaped poly[2-(dimethylamino)ethyl methacrylate]-based copolymers with an inorganic core. Biomacromolecules, 18(1), 44–55. doi:10.1021/acs.biomac.6b01279
- Qiao, Z., Wang, Z., Zhang, C., Yuan, S., Zhu, Y., Wang, J., & Wang, S. (2012). PVAm–PIP/PS composite membrane with high performance for CO2/N2 separation. AIChE Journal, 59(1), 215–228. doi:10.1002/aic
- Qiu, H., Si, Z., Luo, Y., Feng, P., Wu, X., Hou, W., … Huang, D. (2020). The mechanisms and the applications of antibacterial polymers in surface modification on medical devices. Frontiers in Bioengineering and Biotechnology, 8(vember), 910. doi:10.3389/fbioe.2020.00910
- Raafat, D., & Sahl, H. G. (2009). Chitosan and its antimicrobial potential - A critical literature survey. Microbial Biotechnology, 2(2), 186–201. doi:10.1111/j.1751-7915.2008.00080.x
- Ren, W., Cheng, W., Wang, G., & Liu, Y. (2017). Developments in antimicrobial polymers. Journal of Polymer Science Part A: Polymer Chemistry, 55(4), 632–639. doi:10.1002/pola.28446
- Riga, E. K., Vöhringer, M., Widyaya, V. T., & Lienkamp, K. (2017). Polymer-based surfaces designed to reduce biofilm formation: From antimicrobial polymers to strategies for long-term applications. Macromolecular Rapid Communications, 38(20) doi:10.1002/marc.201700216
- Samal, S. K., Dash, M., Vlierberghe, S., Van, Kaplan, D. L., Chiellini, E., Blitterswijk, C., … Dubruel, P. (2012). Cationic polymers and their therapeutic potential. Chemical Society Reviews, 41(21), 7147–7194. doi:10.1039/c2cs35094g
- Santos, M. R. E., Fonseca, A. C., Mendonça, P. V., Branco, R., Serra, A. C., Morais, P. V., & Coelho, J. F. J. (2016). Recent developments in antimicrobial polymers: A review. Materials (Basel, Switzerland), 9(7), 599. doi:10.3390/MA9070599
- Sayed, S., & Jardine, M. A. (2015). Antimicrobial biopolymers. Advanced Functional Materials, 493–533 doi:10.1002/9781118998977.ch12
- Schandock, F., Riber, C. F., Röcker, A., Müller, J. A., Harms, M., Gajda, P., … Zelikin, A. N. (2017). Macromolecular antiviral agents against zika, ebola, SARS, and other pathogenic viruses. Advanced Healthcare Materials, 6(23) doi:10.1002/adhm.201700748
- Sellenet, P. H., Allison, B., Applegate, B. M., & Youngblood, J. P. (2007). Synergistic activity of hydrophilic modification in antibiotic polymers. Biomacromolecules, 8(1), 19–23. doi:10.1021/bm0605513
- Sharma, N., Modak, C., Singh, P. K., Kumar, R., Khatri, D., & Singh, S. B. (2021). Underscoring the immense potential of chitosan in fighting a wide spectrum of viruses: A plausible molecule against SARS-CoV-2? International Journal of Biological Macromolecules, 179, 33–44. doi:10.1016/j.ijbiomac.2021.02.090
- Siedenbiedel, F., & Tiller, J. C. (2012). Antimicrobial polymers in solution and on surfaces: Overview and functional principles. Polymers, 4(1), 46–71. doi:10.3390/polym4010046
- Sinclair, T. R., Robles, D., Raza, B., van den Hengel, S., Rutjes, S. A., de Roda Husman, A. M., … Roesink, H. D. W. (2018). Virus reduction through microfiltration membranes modified with a cationic polymer for drinking water applications. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 551(March), 33–41. doi:10.1016/j.colsurfa.2018.04.056
- Smola-Dmochowska, A., Lewicka, K., Macyk, A., Rychter, P., Pamuła, E., & Dobrzyński, P. (2023). Biodegradable polymers and polymer composites with antibacterial properties. International Journal of Molecular Sciences, 24(8), 7473. doi:10.3390/ijms24087473
- Srisa, A., Promhuad, K., San, H., Laorenza, Y., Wongphan, P., Wadaugsorn, K., … Harnkarnsujarit, N. (2022). Antibacterial, antifungal and antiviral polymeric food packaging in post-COVID-19 era. Polymers, 14(19), 4042. doi:10.3390/polym14194042
- Stelmakh, S. A., Grigor’eva, M. N., Garkusheva, N. M., Lebedeva, S. N., Ochirov, O. S., Mognonov, D. M., … Batoev, V. B. (2021). Studies of new biocidal polyguanidines: Antibacterial action and toxicity. Polymer Bulletin, 78(4), 1997–2008. doi:10.1007/s00289-020-03197-1
- Sukhareva, K., Chernetsov, V., & Burmistrov, I. (2024). A review of antimicrobial polymer coatings on steel for the food processing industry. Polymers, 16(6), 809. doi:10.3390/polym16060809
- Takahashi, H., Caputo, G. A., Vemparala, S., & Kuroda, K. (2017). Synthetic random copolymers as a molecular platform to mimic host-defense antimicrobial peptides. Bioconjugate Chemistry, 28(5), 1340–1350. doi:10.1021/acs.bioconjchem.7b00114
- Talu, M., Uzluk, E., & Yüksel, B. (2010). Synthesis, characterization and bactericidal properties of poly(N-vinyl-2-pyrrolidone-co-maleic anhydride-co-N-isopropyl acrylamide). Macromolecular Symposia, 297(1), 188–199. doi:10.1002/masy.200900140
- Teixeira-Santos, R., Lima, M., Gomes, L. C., & Mergulhão, F. J. (2021). Antimicrobial coatings based on chitosan to prevent implant-associated infections: A systematic review. iScience, 24(12), 103480. doi:10.1016/j.isci.2021.103480
- Tejero, R., López, D., López-Fabal, F., Gómez-Garcés, J. L., & Fernández-García, M. (2015). High efficiency antimicrobial thiazolium and triazolium side-chain polymethacrylates obtained by controlled alkylation of the corresponding azole derivatives. Biomacromolecules, 16(6), 1844–1854. doi:10.1021/acs.biomac.5b00427
- Teratanatorn, P., Hoskins, R., Swift, T., Douglas, C. W. I., Shepherd, J., & Rimmer, S. (2017). Binding of bacteria to poly(N-isopropylacrylamide) modified with vancomycin: comparison of behavior of linear and highly branched polymers. Biomacromolecules, 18(9), 2887–2899. doi:10.1021/acs.biomac.7b00800
- Tew, G. N., Scott, R. W., Klein, M. L., & Degrado, W. F. (2010). De novo design of antimicrobial polymers, foldamers, and small molecules: From discovery to practical applications. Accounts of Chemical Research, 43(1), 30–39. doi:10.1021/ar900036b
- Thoma, L. M., Boles, B. R., & Kuroda, K. (2014). Cationic methacrylate polymers as topical antimicrobial agents against Staphylococcus aureus nasal colonization. Biomacromolecules, 15(8), 2933–2943. doi:10.1021/bm500557d
- Tiller, J. C., Liao, C. J., Lewis, K., & Klibanov, A. M. (2001a). Designing surfaces that kill bacteria on contact. Proceedings of the National Academy of Sciences of the United States of America, 98(11), 5981–5985. doi:10.1073/pnas.111143098
- Tiller, J. C., Liao, C., Lewis, K., & Klibanov, A. M. (2001b). Designing surfaces that kill bacteria on contact. Proceedings of the National Academy of Sciences, 98(11), 5981–5985. doi:10.1073/pnas.111143098
- Vigliotta, G., Mella, M., Rega, D., & Izzo, L. (2012). Modulating antimicrobial activity by synthesis: Dendritic copolymers based on nonquaternized 2-(dimethylamino)ethyl methacrylate by Cu-mediated ATRP. Biomacromolecules, 13(3), 833–841. doi:10.1021/bm2017349
- Wahid, F., Wang, F.-P., Xie, Y.-Y., Chu, L.-Q., Jia, S.-R., Duan, Y.-X., … Zhong, C. (2019). Colloids and surfaces B : Biointerfaces reusable ternary PVA films containing bacterial cellulose fibers and ε - Polylysine with improved mechanical and antibacterial properties. Colloids and Surfaces. B, Biointerfaces, 183(July), 110486. doi:10.1016/j.colsurfb.2019.110486
- Wang, J., Chen, Y. P., Yao, K., Wilbon, P. A., Zhang, W., Ren, L., … Tang, C. (2012). Robust antimicrobial compounds and polymers derived from natural resin acids. Chemical Communications (Cambridge, England), 48(6), 916–918. doi:10.1039/c1cc16432e
- Wang, Y., Jett, S. D., Crum, J., Schanze, K. S., Chi, E. Y., & Whitten, D. G. (2013). Understanding the dark and light-enhanced bactericidal action of cationic conjugated polyelectrolytes and oligomers. Langmuir: The ACS Journal of Surfaces and Colloids, 29(2), 781–792. doi:10.1021/la3044889
- Wee, V., Ng, L., Pang, J., Tan, K., Leong, J., Voo, Z. X., & Hedrick, J. L. (2014). Antimicrobial polycarbonates: Investigating the impact of nitrogen- containing heterocycles as quaternizing agents. Macromolecules, 47, 1285–1291.
- Wiegand, C., Bauer, M., Hipler, U. C., & Fischer, D. (2013). Poly(ethyleneimines) in dermal applications: Biocompatibility and antimicrobial effects. International Journal of Pharmaceutics, 456(1), 165–174. doi:10.1016/j.ijpharm.2013.08.001
- Xue, Y., Pan, Y., Xiao, H., & Zhao, Y. (2014). Novel quaternary phosphonium-type cationic polyacrylamide and elucidation of dual-functional antibacterial/antiviral activity. RSC Adv, 4(87), 46887–46895. doi:10.1039/C4RA08634A
- Xue, Y., Xiao, H., & Zhang, Y. (2015). Antimicrobial polymeric materials with quaternary ammonium and phosphonium salts. International Journal of Molecular Sciences, 16(2), 3626–3655. doi:10.3390/ijms16023626
- Yan, D., Li, Y., Liu, Y., Li, N., Zhang, X., & Yan, C. (2021). Antimicrobial properties of chitosan and chitosan derivatives in the treatment of enteric infections. Molecules (Basel, Switzerland), 26(23), 7136. doi:10.3390/molecules26237136
- Yang, Y., Cai, Z., Huang, Z., Tang, X., & Zhang, X. (2018). Antimicrobial cationic polymers: From structural design to functional control. Polymer Journal, 50(1), 33–44. doi:10.1038/pj.2017.72
- Ye, R., Xu, H., Wan, C., Peng, S., Wang, L., Xu, H., … Wei, H. (2013). Antibacterial activity and mechanism of action of ε-poly-l-lysine. Biochemical and Biophysical Research Communications, 439(1), 148–153. doi:10.1016/j.bbrc.2013.08.001
- Zaltsman, N., Kesler-Shvero, D., Weiss, E. I., & Beyth, N. (2016). Synthesis variants of quaternary ammonium polyethyleneimine nanoparticles and their antibacterial efficacy in dental materials. Journal of Applied Biomaterials & Functional Materials, 14(2), 205–211. doi:10.5301/jabfm.5000269
- Zhang, M., Teo, J. J., Liu, S., Liang, Z. C., Ding, X., Ono, R. J., … Hedrick, J. L. (2016). Simple and cost-effective polycondensation routes to antimicrobial consumer products. Polymer Chemistry, 7(23), 3923–3932. doi:10.1039/C6PY00592F
- Zheng, Z., Xu, Q., Guo, J., Qin, J., Mao, H., Wang, B., & Yan, F. (2016). Structure-antibacterial activity relationships of imidazolium-type ionic liquid monomers, poly(ionic liquids) and poly(ionic liquid) membranes: Effect of alkyl chain length and cations. ACS Applied Materials & Interfaces, 8(20), 12684–12692. doi:10.1021/acsami.6b03391
- Zou, P., Laird, D., Riga, E. K., Deng, Z., Dorner, F., Guevara-Solarte, D. L., … Lienkamp, K. (2015). Antimicrobial and cell-compatible surfaceattached polymer networks – How the correlation of chemical structure to physical and biological data leads to a modified mechanism of action. †Journal of Materials Chemistry B, 3(30), 6224–6238. doi:10.1039/C5TB00906E
- Zuniga, J. M., & Cortes, A. (2020). The role of additive manufacturing and antimicrobial polymers in the COVID-19 pandemic. Expert Review of Medical Devices, 17(6), 477–481. doi:10.1080/17434440.2020.1756771