Abstract
Background: The relationship between anti-Ro52/SS-A antibody (anti-Ro52) and the clinical manifestations of Sjögren's syndrome (SS) has not been fully clarified. We determined the clinical factors relevant to SS patients with anti-Ro52.
Methods: We conducted a retrospective study of 149 subjects suspicious for SS and 50 healthy control subjects. We analyzed items of the American-European Consensus Group (AECG) criteria and the EULAR Sjögren's Syndrome Disease Activity Index (ESSDAI).
Results: SS was documented in 115 subjects. Anti-Ro52 was observed in 70 SS patients. Anti-Ro52 positivity showed a significantly higher association with anti-Ro60 positivity than with anti-centromere antibody (ACA) positivity (p < 0.05). Regarding the difference in the anti-Ro52 concentration, we observed six significantly relevant components: two AECG components and four non-AECG components. The anti-Ro52 concentration well-discriminated three clinical factors (ROC AUC >0.75), i.e., ACA seropositivity, ESSDAI score ≥1, and RF, and it moderately discriminated high serum IgG, focus score ≥1, and anti-La/SS-B antibody seropositivity (ROC AUC >0.7). A linear relationship between the ESSDAI score and the anti-Ro52 concentration was observed.
Conclusion: A significant association between clinical factors (including the ESSDAI) and the anti-Ro52 concentration were revealed. Anti-Ro52 was more highly associated with anti-Ro60 positivity than with ACA positivity.
Background
Sjögren's syndrome (SS) is a systemic autoimmune disease that has unique clinical manifestations, including xerostomia, xerophthalmia, and extraglandular clinical manifestations such as interstitial pneumonia and tubulointerstitial nephritis [Citation1,Citation2]. SS also has characteristic autoantibodies, including anti-Ro/SS-A and La/SS-B autoantibodies [Citation3]. The presence of anti-Ro/SS-A antibody is a critical item in the 2002 American-European Consensus Group (AECG) [Citation4] classification criteria, the 2016 American College of Rheumatology (ACR)/European League Against Rheumatism (EULAR) classification criteria [Citation5], and the 1999 revised Japanese Ministry of Health criteria [Citation6] for primary SS. Anti-Ro/SS-A antibody (anti-Ro) is not specific for SS [it is also detected in sera from patients with systemic lupus erythematosus (SLE)], and Ro/SS-A antigen has two distinct subtypes: Ro52 (which was identified as TRIM21) and Ro60 [Citation7]. Robbins et al. examined the frequency of anti-Ro52 and anti-Ro60 in SS and reported that 32.6% of anti-Ro52-positive patients were both anti-Ro60 and anti-Ro52 positive [Citation8]. Regarding the clinical relationship between anti-Ro60 and SS, a high prevalence of lymphadenopathy, cytopenia, hypergammaglobulinemia, and vasculitis has been reported in SS patients with anti-Ro60 [Citation9]. Regarding the association between the titer of anti-Ro52 and clinical activity in patients with SS, a single study from 2012 (by the Sjögren's Syndrome Research Group, in a Spanish cohort [Citation10]) reported that age, the presence of RP, the serum IgG level, and the presence of anti-La/SS-B antibody were associated with the titer of anti-Ro52. There has been no subsequent publication regarding the anti-Ro52 titer and clinical manifestations. In particular, there has been no report establishing a relationship between the EULAR Sjögren's Syndrome Disease Activity Index (ESSDAI) and anti-Ro52.
It is widely known that anti-La coexists with anti-Ro, but the frequency of coexistence of anti-Ro/La and ACA has not been established. The presence of anti-La/SS-B antibody has been reported to be associated with lymphoma [Citation11], and SS patients with isolated anti-La/SS-B antibody showed increased systemic activity, although these patients accounted for only 2.3% of a series of SS patients [Citation12]. The clinical manifestations of SS patients with positivity for anti-centromere antibody (ACA) were recently described [Citation13]. The ACA-seropositive SS subgroup (ACA-positive SS) accounts for approx. 10% of patients with SS. Because it was reported that ACA-positive SS patients had low percentages of anti-Ro, the subgroup of ACA-positive SS patients is recognized as a distinct group that has a high prevalence of Raynaud's phenomenon (RP) and normal serum IgG levels [Citation14,Citation15].
The clinical manifestations of SS vary by race. Our 2020 international multicenter study including 10,007 SS patients [Citation16] demonstrated that Asian patients with SS had hematological, pulmonary, and renal involvement, whereas Caucasian SS patients dominantly showed glandular, cutaneous, and muscular involvement. In addition, the ESSDAI score tended to be higher in southern parts of the world. A French study reported that anti-Ro52 was closely associated with interstitial lung disease [Citation17], but the relationship between clinical manifestations and anti-Ro52 in Japanese patients with SS has not been determined. The main aim of the present study was to determine the clinical factors relevant to anti-Ro52/SS-A antibodies in patients with SS. A secondary purpose was to clarify the clinical characteristics of positivity for anti-Ro, anti-La, ACA and SS having these autoantibodies.
Subjects and methods
Subjects
We enrolled 149 subjects suspected of having SS who visited Nagasaki University Hospital between 2009 and 2018. The classification of primary (p) SS was determined based on the 2002 AECG classification criteria [Citation4]. Among the 149 subjects with suspected SS, 115 subjects were classified as having SS based on the AECG criteria. The 115 patients with SS were classified as 87 cases of primary SS and 28 cases of secondary SS (). Among the 149 subjects, there were 34 non-SS subjects who did not fulfill the 2002 AECG criteria, although some of these subjects had one or more of the AECG components: seven of the non-SS subjects were positive for anti-Ro52. Among these seven anti-Ro52-positive, non-SS subjects, five were also positive for anti-Ro60, and two were positive for ACA without a salivary gland biopsy. The reasons why these seven subjects were not classified as having SS by the AECG criteria might have been the coexistence of anti-Ro antibodies and/or the lack of information on pathological findings.
Table 1. The subjects' background information.
As a disease control group, we examined the cases of 62 patients with rheumatoid arthritis (RA) who had been classified based on the 2010 ACR/EULAR classification criteria or the 1987 American Rheumatism Association criteria for RA [Citation18,Citation19]. For the exclusion of latent SS, we excluded RA patients who were positive for anti-Ro60 or who had sicca symptoms, including xerostomia and/or xerophthalmia, as determined by the Mesacup SS-A/Ro test and SS-B/La Test. Blood samples from 50 healthy subjects who showed participation intention without ophthalmic examinations were used as normal controls. The healthy subjects were tested only for anti-Ro52 by EliA Anti-Ro52. This study was conducted with the approval of the Clinical Studies Ethics Committee of Nagasaki University Hospital (approval no. 18121007).
Evaluation of clinical parameters
The degree of mononuclear cell infiltration in the subjects’ labial salivary glands (LSGs) was evaluated based on the Chisholm & Mason grading or the focus score (FS) determined by Greenspan [Citation20,Citation21]. We retrospectively evaluated the disease activity of SS by using the ESSDAI [Citation22,Citation23], which is composed of 12 items used to determine the systemic involvement of SS. The present FS calculation was subjected to the standardization method recommended by the EULAR SS Study Group [Citation24]. For the calculation of the subjects' FSs, images of salivary glands were captured by a microscope (BZ-X-710; Keyence, Osaka, Japan), and whole areas of salivary glands were measured by the hybrid cell count system; the FS was then manually calculated as the number of foci per 4 mm2 as described by Fisher et al. [Citation24]. We also examined the subjects' treatment for sicca symptoms and the use of immunosuppressants.
Kits and reagents
Anti-Ro (Ro60) and anti-La/SS-B antibody were detected by the Mesacup SS-A/Ro test and SS-B/La Test, respectively (Medical & Biological Laboratories, Nagoya, Japan). An enzyme-linked immunosorbent assay (ELISA) was used for the Mesacup SS-A/Ro and SS-B/La tests. The immobilized native antigens, including SS-A/Ro and SS-B/La, were subjected to antigen-antibody reaction. The reactants were incubated with peroxidase-conjugated anti-human immunoglobulin polyclonal goat antibody. ACA was quantified by an ELISA kit (Mesacup-2 test CENP-B; Medical & Biological Laboratories). Anti-Ro52 was measured by an EliA Anti-Ro52 assay (ThermoFisher Scientific, Waltham, MA); this product is based on fluorescence enzyme immunoassay (FEIA) technology. Anti-SS-A/Ro52 antibody was also measured by an ELISA-based method.
Statistical analyses
For the comparison of age and sex among the patients with SS, the patients with RA, and the healthy subjects, the Wilcoxon rank-sum test and Fisher exact test were used, respectively. We included the following items in the analyses as clinical factors of interest: AECG criteria components including xerostomia, xerophthalmia, salivary secretion by Saxon test, lacrimal secretion by Schirmer's test, the FS, labial salivary gland biopsy (LSGB) grading (0–2/3/4), anti-Ro/SS-A antibody, anti-La/SS-B antibody, and relevant items including RP, ACA, serum IgG, rheumatoid factor (RF), and the ESSDAI score.
The concentrations of anti-Ro52 and anti-Ro60 were transformed to logarithm with base 10. The association of clinical factors with relevance between anti-Ro52 and anti-Ro60 was inferred as the 95% confidence interval (95% CI) of the regression coefficient of a term of interaction between anti-Ro52 and the clinical factor in a robust linear regression model [Citation25], which regressed anti-Ro60 onto the interaction term with covariates of anti-Ro52 and the clinical factor. The model did not include other covariates to control for confounders. We used the multiple imputations by the chained equation (MICE) method [Citation26] to estimate the regression coefficients. The patterns of missingness are shown in (labeled ‘NA’ on the x-axis) and (the right column of each plot). The contour of two-dimensional probability density was drawn based on a kernel density estimate with the Gaussian kernel with bandwidth selected by the ‘solve-the equation’ estimator [Citation27].
We compared the odds of anti-Ro60 positivity between the positivity of ACA and that of anti-Ro52 by using the ratio of the two odds ratios (the ratio of ORs). The null hypothesis that the ratio is 1 was tested via a permutation test of anti-Ro60 positivity. We evaluated the associations between the respective clinical factors and the concentration of antibodies against the two respective subtypes of Ro/SS-A antigen by determining the maximal information coefficients [Citation28]. The null hypothesis of independence between respective clinical factors and the concentration was tested by a permutation test. The odds of positivity in the respective parameters given the concentration of anti-Ro52 were evaluated with the area under the curve (AUC) of a receiver operator characteristic (ROC) curve. The confidence intervals of sensitivity and specificity were obtained from 2,000 bootstrap samplings. Hypothesis testing was conducted with a significance level of 0.05 without adjustment for multiple comparisons. All statistical analyses were conducted under the R environment [Citation29] ver. 3.6.0, especially the robustbase package [Citation30] ver. 0.95-5 in the robust regression, the mice package [Citation26] ver. 3.5.0 in the multiple imputation, and the pROC package [Citation31] ver. 1.15.3.
Results
Subject backgrounds
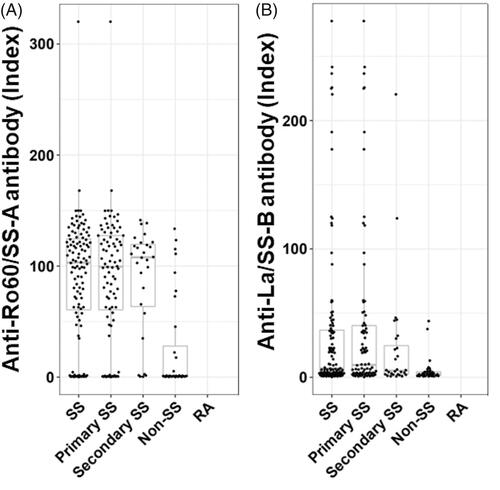
The groups of the enrolled subjects and their age and gender distributions are shown in . The 115 patients with SS were classified as 87 cases of primary SS and 28 cases of secondary SS. There were significant differences between the ages of SS patients and those of the RA patients and healthy controls. The frequencies of anti-Ro/SS-A antibodies and La/SS-B antibodies in the non-SS subjects were significantly lower than those of the SS patients, although there was no difference regarding the frequency of xerophthalmia or xerostomia. There was no significant difference in the frequency of ACA between the SS patients and non-SS subjects. In addition, there were no significant differences in positivity for anti-Ro60, anti-La/SS-B antibody and ACA between primary and secondary SS. Among the 115 patients with SS, there were 27 patients with ACA + SS. The numbers of patients with positivity for anti-Ro60 and ACA among the 87 patients with primary SS were 72 (82.8%) and 22 (25.3%), respectively. The number of patients with positivity for anti-Ro52 among the 87 patients with primary SS was 51 (72.9%). Among the 28 patients with secondary SS, the numbers of patients with positivity for anti-Ro60 and ACA were 23 (82.1%) and 5 (17.9%), respectively. Among them, the number of patients with positivity for anti-Ro52 was 19 (67.9%). The 2 secondary SS patients with ACA + systemic sclerosis (SSc) fulfilled the 2013 ACR/EULAR classification criteria for SSc and had neither anti-Ro52 nor anti-Ro60. The distribution of concentrations of anti-Ro antibody and anti-La/SS-B antibody in the SS and non-SS groups is illustrated in .
Figure 1. The distribution of anti-Ro60 and anti-La/SS-B antibodies concentrations in SS and non-SS subjects. The distributions are presented using box-whisker plots (boxes: interquartile ranges; whiskers extend from the upper/lower hinges to the highest/lowest value no further than 1.5 times the ranges of the 1st and 3rd quartile points). The horizontal line in each box indicates the median. Subjects are grouped according to disease group (SS, n = 115; primary SS, n = 87; secondary SS, n = 28; non-SS, n = 34, rheumatoid arthritis, n = 62). (A) The distribution of anti-Ro60 antibody. (B) The distribution of anti-La/SS-B antibody.
Prevalence of anti-Ro52 in the SS patients and other conditions
Among these 115 patients with SS, 70 patients (60.9%) were anti-Ro52 positive (). The group of 115 SS patients included 95 patients with positivity for anti-Ro60 (82.6%). Among the non-SS subjects, seven subjects were positive for anti-Ro52 (). There were 2 cases (3.2%) of positive anti-Ro52 among the 62 patients with RA, and there were no positive cases among the 50 normal control subjects (). The background characteristics of the patients with SS according to the presence or absence of anti-Ro52 are summarized in . Five items were significantly higher in the SS patients who were positive for anti-Ro52: the frequencies of anti-La and SS-B antibodies, the RF positivity, the level of serum IgG, and the ESSDAI score. There was no case of congenital heart block (CHB).
Figure 2. The relevance and distribution of anti-Ro52-seropositive SS patients. The boxes and whiskers in the respective plots represent the interquartile ranges and the ranges of 1.5 times the 1st and 3rd quartile points. Horizontal lines: the median values. (A) All subjects according to disease group (SS, n = 115; primary SS, n = 87; secondary SS, n = 28; non-SS, n = 34; RA, n = 62; and healthy subjects, n = 50). (B) SS subjects according to anti-Ro60 seropositivity. (C) SS subjects according to ACA seropositivity. (D) SS subjects with (+)/without (−) ACA seropositivity according to anti-Ro60 seropositivity. ACA: anti-centromere antibody; HC: healthy control; RA: rheumatoid arthritis.
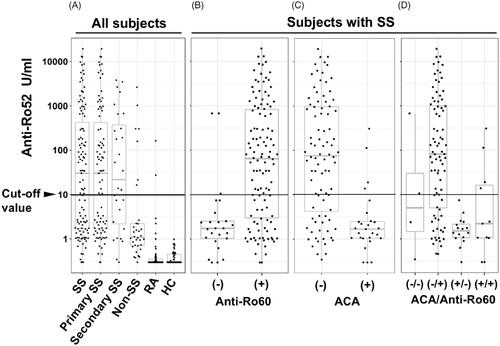
Table 2. Clinical parameters of the SS patients.
The relationship between anti-Ro52 and anti-Ro60, ACA, or anti-La in the SS patients
When we divided the SS patients into anti-Ro60-negative and -positive groups (), we observed that 67 of the 95 anti-Ro60+ patients with SS (70.5%) were positive for anti-Ro52 (). A shown in , anti-Ro52 positivity was observed in 73.9% (65/88) of the anti-ACA − patients with SS. Although we identified anti-Ro52 positivity in 75% (63/84) of the anti-Ro60 + ACA − patients with SS, there was only 1 anti-Ro52-positive case among the 16 anti-Ro60 − ACA + patients with SS (); this case showed an equivocal anti-Ro52 concentration (7.44 U/ml). The odds of anti-Ro60 being positive were significantly higher in the anti-Ro52-positive patients than in the ACA-positive patients (p < 0.05, permutation test for odds ratio).
In addition, 19 of the 28 secondary SS patients (67.9%) were positive for anti-Ro52. Only 3 of the anti-Ro60 negative SS patients had anti-Ro52 positivity (). We observed that the anti-Ro60+ SS patients included 67 anti-Ro60 + anti-Ro52+ patients and 28 anti-Ro60 + anti-Ro52− patients. In contrast, the anti-Ro60− SS patients included 3 anti-Ro60 − anti-Ro52+ patients and 17 anti-Ro60 − anti-Ro52− patients.
The association between anti-Ro60/Ro52 and clinical parameters of SS
We compared the concentration of anti-Ro52 and anti-Ro60 between the subgroups of subjects classified by components in the AECG criteria and by other items including RP, ACA, RF, and serum IgG (). Three of these parameters (RP, ACA, and IgG) were significantly different according to the titer for both antibodies (p < 0.05 for each parameter).
Figure 3. The difference in anti-Ro52/60 concentration by AECG criteria components and other clinical parameters. SS subjects according to the AECG criteria components and other items (RP, ACA, RF, and serum IgG). The results are presented as box-whisker plots (boxes: interquartile ranges; whiskers extend from the upper/lower hinges to the highest/lowest value no further than 1.5 times the ranges of the 1st and 3rd quartile points). Horizontal line in each box: the median. NA group: subjects who had missing data in each group. ACA: anti-centromere antibody; NA: not available; RF: rheumatoid factor; RP: Raynaud’s phenomenon.
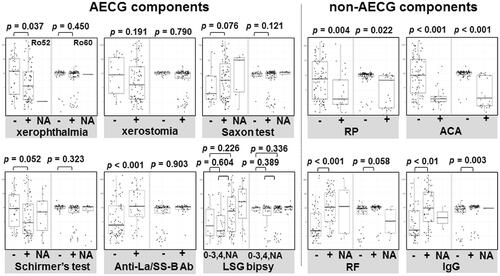
Regarding the anti-Ro52 concentration, the differences were significant in xerophthalmia, anti-La/SS-B antibody, and RF. However, the anti-Ro52 levels in the anti-La/SS-B antibody and RF-positive group were higher than those in the group of patients negative for these factors. In contrast, the anti-Ro52 level in the xerophthalmia-negative group was higher than that in the xerophthalmia-positive group. In regard to the anti-Ro60 concentration, there were no additional parameters for which the association was significant aside from RP, ACA, and IgG ().
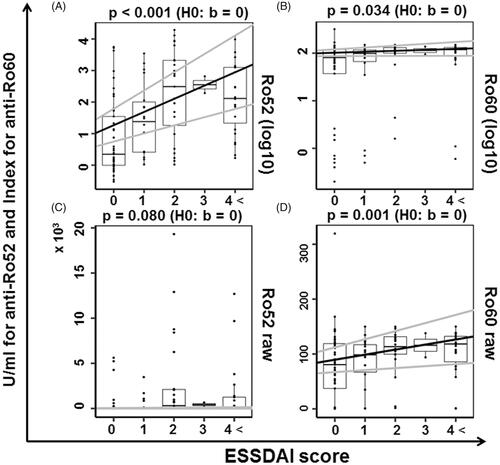
The association between anti-La/SS-B and both anti-Ro52 and anti-Ro60 was significant (p < 0.05 for each antibody) (). The parameters in which positivity was highly discriminated by the concentration of anti-Ro52 were as follows: ACA, ESSDAI ≥1, and RF (AUC >0.75, respectively) (). Moderately discriminated parameters were serum IgG, FS ≥1, and anti-La/SS-B antibody (AUC >0.70, respectively) (). The parameters in which positivity was highly discriminated by the concentration of anti-Ro60 were RP and ACA. The patients with an ESSDAI score >4 were classified together in the subgroup with ESSDAI ≥4, and in this analysis, significant linearity between the ESSDAI scores and anti-Ro52 was confirmed (), as anticipated from the correlation with anti-Ro60. The ESSDAI scores in the anti-Ro52-positive SS patients are shown in Supplementary Figure S1, indicating a high frequency of the biological item and a low frequency of the articular item. Our analyses also revealed that the anti-Ro60 + Ro52 + SS patients had significantly higher ESSDAI scores (2.1 ± 2.0) compared to the anti-Ro60 + Ro52-SS patients (0.5 ± 1.1) (p-value: 2.7 × 10−6 by Welch's t-test). We classified the levels of anti-Ro52 and anti-Ro60 according to the positive ESSDAI domain (Supplementary Figures S2 and S3) and observed that the level of anti-Ro52 in all domains that had a positive number was greater than the cutoff value, 10 U/ml. As shown in Supplementary Figure S2, the presence of hematological and biological items was associated with a high anti-Ro52 titer.
Figure 4. The relationship between the concentration of anti-Ro52/60 and the anti-La/SS-B antibody/FS. The concentration of anti-Ro52 (A, C) or anti-Ro60 (B, D) is plotted on the x-axis as the measured concentration of anti-La/SS-B antibody (A, B) or the focus score (FS) (C, D). The distribution of the titers of the subjects with missing x-axis values is shown as a box-whisker plot beside each scatterplot.
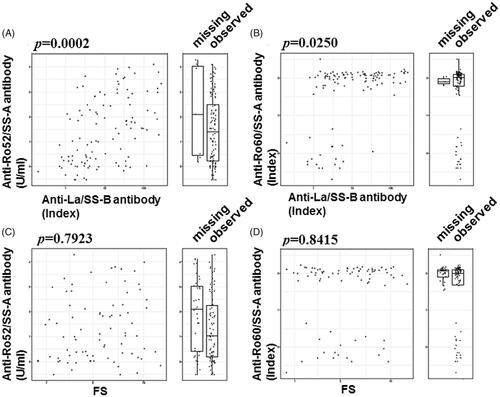
Figure 5. The clinical parameters relevant to the transition of the anti-Ro52 concentration. The ROC curves of the levels of anti-Ro52 and anti-Ro60 for each clinical parameter are shown. The clinical parameters were 10 AECG components and 5 other factors. The p-value in each panel is the result of a test of the null hypothesis that the AUC of the anti-Ro52 ROC curve was 0.5. Ab: antibody; ACA: anti-centromere antibody; ESSDAI: EULAR Sjögren's Syndrome Disease Activity Index; FS: focus score; LSGB: labial salivary gland biopsy; RF: rheumatoid factor; RP: Raynaud’s phenomenon.
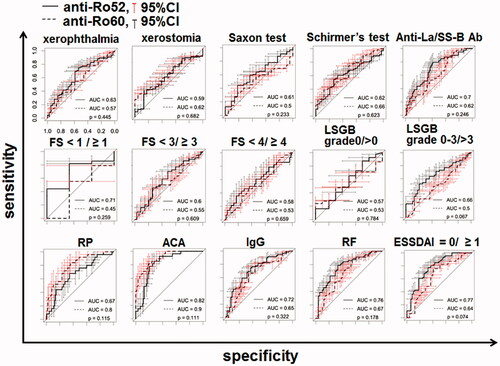
Figure 6. The relevance of anti-Ro52/60 antibodies according to the ESSDAI score. The lines with the intercept and slope were obtained from the linear regression (gray line: 95%CI) and box-whisker plots (boxes: interquartile ranges; whiskers extend from the upper/lower hinges to the highest/lowest value no further than 1.5 times the ranges of the 1st and 3rd quartile points). The y-axis data were scaled on a logarithmic scale with base 10 in panels A and B and not scaled in (C) and (D). ESSDAI: EULAR Sjögren's Syndrome Disease Activity Index.
Discussion
Our results demonstrated that anti-Ro52 was more frequently present in the sera from patients with SS compared to the sera from RA patients and healthy controls. The group of anti-Ro52-positive SS patients had significantly high prevalences of anti-Ro60, anti-La/SS-B antibody, and RF, plus high serum IgG levels and high ESSDAI scores. Our present findings thus replicate the previously reported association between the positivity of anti-Ro52 and that of anti-Ro60 [Citation8,Citation32]. The anti-Ro52 concentration showed significant differences in two AECG components and all four non-AECG components, and ROC AUC was >0.7 in six items including the ESSDAI score. The ESSDAI score was linearly correlated with the anti-Ro52 concentration. In addition, the ESSDAI scores of the Ro60 + Ro52 + SS patients were higher than those of the Ro60 + Ro52-SS patients. In addition, in this study, the relationship between anti-Ro52 and ACA was not proved, and the relationship between anti-Ro52 and anti-Ro60 was strong as previously reported.
Because the Ro52 protein has a motif with three regions (RING, B-box, and CC) and thus belongs to the family of tripartite motif proteins (TRIMs), Ro52 is also referred to as TRIM21 [Citation7,Citation33]. With regard to its function, Ro52 has been shown to mediate ubiquitination of intranuclear target molecules as well as E3 ligase activity [Citation34]. Type 1 interferon is known to induce the translocation of Ro52 to the nucleus [Citation35]. Since it is possible that the change of ubiquitination by anti-Ro52 may increase inflammation in various organs in patients with SS, the serum IgG level, RF, FS, and ESSDAI score (which showed an AUC >0.7 with respect to anti-Ro52) might be explained by the effect of anti-Ro52 on ubiquitination. In addition, this biochemical action of anti-Ro52 and the wide distribution of Ro52 antigen in various organs [Citation36, Citation37] might be related to the relevance of the ESSDAI among the items with an AUC >0.7 and the change in anti-Ro52 concentration.
Few studies have investigated the relevance of the association between the anti-Ro52 concentration and clinical manifestations in SS. In a 2006 study of pregnancies presenting a risk of CHB, decreased levels of anti-Ro52 IgG1 and IgG4 were observed [Citation38]. In another study, the anti-Ro concentration was observed to fluctuate in SS patients with skin vasculitis, suggesting that a fluctuating concentration of anti-Ro was associated with the disease activity of SS [Citation39]. In our present analyses, the anti-Ro52 concentration showed moderate or high discriminability for six clinical factors. The significant linear relationship between the ESSDAI scores and anti-Ro52 illustrated in indicates that the anti-Ro52 level was significantly associated with clinical parameters in SS.
Ramos-Casals et al. [Citation40] examined a cohort of 921 Spanish patients with SS, and they reported that the most frequently involved organs were the joints, skin, and peripheral nerves. Although a direct correlation between anti-Ro52 positivity and the ESSDAI score was not noted in that study, the reason why the details of our patients' ESSDAI scores (Supplementary Figure S1) differed from the findings reported by Ramos-Casals et al. may be that our ESSDAI results revealed a low frequency of articular involvement and no peripheral nerve symptoms. Ethnic differences should be taken into account, since the components of the ESSDAI might not be the same among different geographic regions. Indeed, our recent data supported the notion that the frequency of specific domains in ESSDAI varied according to ethnicity [Citation16]. In our present study, we also examined non-ESSDAI cardiovascular items, including the recently reported 14.8% (17/115) of SS patients with RP [Citation41] plus one case of pulmonary arterial hypertension. In addition, three cases of autonomic dystonia were observed among our 115 present patients with SS.
Regarding ACA, we observed 25 patients with RP among the 115 SS patients in this study. Interestingly, 17 of the 25 RP + SS patients (68.0%) were positive for ACA only, while eight patients were anti-Ro-positive but ACA-negative, suggesting that positivity for ACA alone might be associated with RP in SS. In addition, our examination of ACA in the ROC analysis () showed that anti-Ro60 had a higher AUC (0.9) than anti-Ro52 (0.82), suggesting the novel finding of a relationship between ACA and anti-Ro antibodies.
Although CENP-A and C were demonstrated to be involved in the assembly of the kinetochore [Citation42], the function of CENP-B remains unknown. Regarding the coexistence of ACA and anti-Ro52 in SSc, the Canadian Scleroderma Research Group reported that CENP-B was present in approx. 43% of 194 patients with anti-Ro52-positive SSc [Citation43]. In contrast, we observed significantly low odds of anti-Ro52 seropositivity in our ACA + SS patients. This paradoxical result suggests a potential difference in the coexistence of anti-Ro52 in ACA + between SS and SSc.
Our study has some limitations. We did not use the 2016 ACR/EULAR criteria because the ocular staining test, which is one of the main items in the 2016 ACR/EULAR criteria, was not performed in many of our patients. In addition, the use of the Saxon test (one of the stimulated salivary secretion tests used instead of an unstimulated salivary secretion test) might influence the classification of SS. The low execution rate of the fluorescein staining test might also have affected the classification of SS. A fluorescein test is essential for the diagnosis of dry eye. Because this test is required in the 2016 ACR/EULAR criteria as a high-score item, rheumatologists should consult ophthalmologists for the accurate classification of SS. When a sufficient number of enrolled subjects with a complete data set for statistical analyses is obtained, an analysis using the 2016 ACR/EULAR criteria will be necessary.
Our present findings confirmed the linearity between the levels of antibodies against two respective subtypes of Ro/SSA antigen and the ESSDAI scores (0, 1, 2, 3, and >4); however, as shown in Supplemental Figure S1A, this relationship remains unclear in the subjects with ESSDAI scores >4. In addition, the clustering tendency of anti-Ro60 determined by the Mesacup SS-A/Ro test with a blank range was not determined biochemically.
Conclusion
Taken together, our results demonstrate a close association between the anti-Ro52 concentration and SS-related clinical items including the ESSDAI, all of which should be tested to determine therapeutic strategies in daily practice. Large-scale studies of other populations are also necessary, since the present study included only Japanese subjects.
Ethics approval and consent to participate
This study was performed with the disclosure of information according to the approval of the Clinical Studies Ethics Committee of Nagasaki University Hospital (approval no. 18121007).
Consent for publication
All co-authors approved the publication of this manuscript.
Author contributions
Study conception and design: H.N.; Salivary gland biopsy: T.S., A.T., S.N.; Acquisition of data: H.N., S.M.; Statistical analysis: S.M.; Interpretation of data: T.S., A.T., H.N., A.K.
Supplemental Material
Download TIFF Image (2.5 MB)Supplemental Material
Download TIFF Image (913.9 KB)Supplemental Material
Download TIFF Image (10 MB)Acknowledgements
The authors thank Ms. Rika Hirayama for the sample collection.
Disclosure statement
The authors declare no conflict of interest.
Data availability statement
The datasets used and/or analyzed in this study are available from the corresponding author on reasonable request.
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
Additional information
Funding
References
- Mariette X, Criswell LA. Primary Sjögren's syndrome. N Engl J Med. 2018;378(10):931–939.
- Nakamura H, Kawakami A, Eguchi K. Mechanisms of autoantibody production and the relationship between autoantibodies and the clinical manifestations in Sjögren's syndrome. Transl Res. 2006;148(6):281–288.
- Psianou K, Panagoulias I, Papanastasiou AD, et al. Clinical and immunological parameters of Sjögren's syndrome. Autoimmun Rev. 2018;17(10):1053–1064.
- Vitali C, Bombardieri S, Jonsson R, et al. Classification criteria for Sjögren’s syndrome: a revised version of the European criteria proposed by the American-European Consensus Group. Ann Rheum Dis. 2002;61(6):554–558.
- Shiboski CH, Shiboski SC, Seror R, the International Sjögren's Syndrome Criteria Working Group, et al. 2016 American College of Rheumatology/European League Against Rheumatism classification criteria for primary Sjögren's syndrome: a consensus and data-driven methodology involving three international patient cohorts. Ann Rheum Dis. 2017;76(1):9–16.
- Tsuboi H, Hagiwara S, Asashima H, et al. Validation of different sets of criteria for the diagnosis of Sjögren's syndrome in Japanese patients. Mod Rheumatol. 2013;23(2):219–225.
- Brauner S, Ivanchenko M, Thorlacius GE, et al. The Sjögren's syndrome-associated autoantigen Ro52/TRIM21 modulates follicular B cell homeostasis and immunoglobulin production. Clin Exp Immunol. 2018;194(3):315–326.
- Robbins A, Hentzien M, Toquet S, et al. Diagnostic utility of separate anti-Ro60 and anti-Ro52/TRIM21 antibody detection in autoimmune diseases. Front Immunol. 2019;10:444.
- Ben-Chetrit E, Fox RI, Tan EM. Dissociation of immune responses to the SS. A (Ro) 52-kd and 60-kd Polypeptides in Systemic Lupus Erythematosus and Sjögren's Syndrome. 1990;33(3):349–355.
- Retamozo S, Akasbi M, Brito-Zerón P, et al. Anti-Ro52 antibody testing influences the classification and clinical characterisation of primary Sjögren's syndrome. Clin Exp Rheumatol. 2012;30:686–692.
- Quartuccio L, Isola M, Baldini C, et al. Biomarkers of lymphoma in Sjogren's syndrome and evaluation of the lymphoma risk in prelymphomatous conditions: results of a multicenter study. J Autoimm. 2014;51:75–80.
- Acar-Denizli N, Horváth IF, Mandl T, et al. Systemic phenotype related to primary Sjögren's syndrome in 279 patients carrying isolated anti-La/SSB antibodies. Clin Exp Rheumatol. 2020;38:85–94.
- Takeshita M, Suzuki K, Kaneda Y, et al. Antigen-driven selection of antibodies against SSA, SSB and the centromere 'complex', including a novel antigen, MIS12 complex, in human salivary glands. Ann Rheum Dis. 2020;79(1):150–158.
- Katano K, Kawano M, Koni I, et al. Clinical and laboratory features of anticentromere antibody positive primary Sjögren's syndrome. J Rheumatol. 2001;28:2238–2244.
- Nakamura H, Kawakami A, Hayashi T, et al. Anti-centromere antibody-seropositive Sjögren's syndrome differs from conventional subgroup in clinical and pathological study. BMC Musculoskelet Disord. 2010;11(1):140.
- Brito-Zerón P, Acar-Denizli N, Ng WF, for the Sjögren Big Data Consortium, et al. Epidemiological profile and north-south gradient driving baseline systemic involvement of primary Sjögren's syndrome. Rheumatology (Oxford). 2020;59(9):2350–2359.
- Mekinian A, Nicaise-Roland P, Chollet-Martin S, et al. Anti-SSA Ro52/Ro60 antibody testing by immunodot could help the diagnosis of Sjogren's syndrome in the absence of anti-SSA/SSB antibodies by ELISA. Rheumatology (Oxford). 2013;52(12):2223–2228.
- Arnett FC, Edworthy SM, Bloch DA, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31(3):315–324.
- Aletaha D, Neogi T, Silman AJ, et al. 2010 rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Ann Rheum Dis. 2010;69(9):1580–1588.
- Chisholm DM, Mason DK. Labial salivary gland biopsy in Sjögren's disease. J Clin Pathol. 1968;21(5):656–660.
- Greenspan JS, Daniels TE, Talal N, et al. The histopathology of Sjögren's syndrome in labial salivary gland biopsies. Oral Surg Oral Med Oral Pathol. 1974;37(2):217–229.
- Seror R, Ravaud P, Bowman SJ, et al. EULAR Sjogren's syndrome disease activity index: Development of a consensus systemic disease activity index for primary Sjogren's syndrome. Ann Rheum Dis. 2010;69(6):1103–1109.
- Seror R, Bowman SJ, Brito-Zeron P, on behalf of the EULAR Sjogren's Task Force, el al. EULAR Sjögren's syndrome disease activity index (ESSDAI): a user guide. RMD Open. 2015;1(1):e000022–e000022.
- Fisher BA, Jonsson R, Daniels T, et al. Standardisation of labial salivary gland histopathology in clinical trials in primary Sjögren's syndrome. Ann Rheum Dis. 2017;76(7):1161–1168.
- Koller M, Stahel WA. Stahel. Sharpening Wald-type inference in robust regression for small samples. Comput. Stat. Data Anal. 2011;55(8):2504–2515.
- Buuren S, van Groothuis-Oudshoorn K. mice: multivariate imputation by chained equations in R. J. Stat. Softw. 2010;45:1–68.
- Venables WN, Ripley BD. Modern applied statistics with S (Statistics and Computing). New York (NY): Springer; 2002.
- Reshef DN, Reshef YA, Finucane HK, et al. Detecting novel associations in large data sets. Science. 2011;334(6062):1518–1524.
- R Core Team. R: a language and environment for statistical computing. Vienna, Austria; 2018.
- Maechler M, Rousseeuw P, Croux C, et al. Robustbase: basic robust statistics. R package version 0.93-7. 2019.
- Robin X, Turck N, Hainard A, et al. pROC: An open-source package for R and S + to analyze and compare ROC curves. BMC Bioinf . 2011;12(1):77.
- Menéndez A, Gómez J, Escanlar E, et al. Clinical associations of anti-SSA/Ro60 and anti-Ro52/TRIM21 antibodies: Diagnostic utility of their separate detection. Autoimmunity. 2013;46(1):32–39.
- Hennig J, Bresell A, Sandberg M, et al. The fellowship of the RING: The RING-B-box linker region interacts with the RING in TRIM21/Ro52, contains a native autoantigenic epitope in Sjögren syndrome, and is an integral and conserved region in TRIM proteins. J Mol Biol. 2008;377(2):431–449.
- Espinosa A, Zhou W, Ek M, et al. The Sjogren's syndrome-associated autoantigen Ro52 is an E3 ligase that regulates proliferation and cell death. J Immunol. 2006;176(10):6277–6285.
- Strandberg L, Ambrosi A, Espinosa A, et al. Interferon-alpha induces up-regulation and nuclear translocation of the Ro52 autoantigen as detected by a panel of novel Ro52-specific monoclonal antibodies. J Clin Immunol. 2008;28(3):220–231.
- Itoh Y, Kriet JD, Reichlin M. Organ distribution of the Ro (SS-A) antigen in the guinea pig. Arthritis Rheum. 1990;33(12):1815–1821.
- Itoh Y, Reichlin M. Ro/SS-A antigen in human platelets. Different distributions of the isoforms of Ro/SS-A protein and the Ro/SS-A-binding RNA. Arthritis Rheum. 1991;34(7):888–893.
- Strandberg L, Salomonsson S, Bremme K, et al. Ro52, Ro60 and La IgG autoantibody levels and Ro52 IgG subclass profiles longitudinally throughout pregnancy in congenital heart block risk pregnancies. Lupus. 2006;15(6):346–353.
- Praprotnik S, Bozic B, Kveder T, et al. Fluctuation of anti-Ro/SS-A antibody levels in patients with systemic lupus erythematosus and Sjögren's syndrome: A prospective study. Clin Exp Rheumatol. 1999;17:63–68.
- Ramos-Casals M, Brito-Zerón P, Solans R, on behalf of the SS Study Group, et al. Systemic involvement in primary Sjogren's syndrome evaluated by the EULAR-SS disease activity index: Analysis of 921 Spanish patients (GEAS-SS Registry). Rheumatology (Oxford). 2014;53(2):321–331.,
- Retamozo S, Acar-Denizli N, Rasmussen A, et al. Systemic manifestations of primary Sjögren's syndrome out of the ESSDAI classification: Prevalence and clinical relevance in a large international, multi-ethnic cohort of patients. Clin Exp Rheumatol. 2019;37 (Suppl 118):97–106.
- Moree B, Meyer CB, Fuller CJ, et al. CENP-C recruits M18BP1 to centromeres to promote CENP-A chromatin assembly. J Cell Biol. 2011;194(6):855–871.
- Hudson M, Pope J, Mahler M, Canadian Scleroderma Research Group (CSRG), et al. Clinical significance of antibodies to Ro52/TRIM21 in systemic sclerosis. Arthritis Res Ther. 2012;14(2):R50.
