Abstract
Anti-histone antibodies (AHAs) make their appearance in a number of systemic autoimmune diseases including systemic lupus erythematosus (SLE) and drug-induced lupus erythematosus (DILE). Although being known for over 50 years, they are poorly studied and understood. There is emerging evidence for their use in predicting clinical features of SLE, diversifying their clinical use. AHAs, however, are probably less prevalent in DILE than once thought owing to a move away from older DILE drugs to modern biological agents which do not appear to elicit AHAs. This review examines the historical studies that have defined AHAs and looks at some of the recent work with these autoantibodies.
1. Basic histone biology
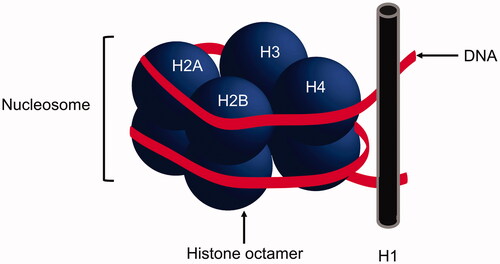
Histones are structural subunits that provide a core in which chromatin can be wrapped around. The core comprises of two H2A–H2B dimers and an (H3–H4)2 tetramer (histone octamer) with an external H1 histone, a conserved subunit that assists with maintaining this ‘beads on a string’ structure () [Citation1]. The histone octamer coupled to around 150 bp of DNA is known as the nucleosome and forms the basic fundamental unit of chromatin [Citation2]. Histones not only form scaffolds for chromatin, but they also play an important role in regulating gene expression [Citation3]. Histones themselves are encoded by multiple genes spanning a number of chromosomes including chromosomes 6, 11 and 12 in humans [Citation4].
Figure 1. Basic subunit of DNA. A nucleosome comprises a histone octamer core with DNA wrapped around, resembling ‘beads on a string’.
Histones may become ‘exposed’ through the formation of apoptotic blebs or neutrophil extracellular traps (NETs) [Citation5]. Either through excess formation, reduced clearance and/or post-translational modification, histones may become immunogenic [Citation6,Citation7]. Furthermore, recent attention has turned to the role of epigenetic modifications of these proteins in the rendering of immunogenicity and hence, the formation of anti-histone antibodies (AHAs) [Citation8,Citation9]. Deliberate post-translational modification of histones may be done therapeutically in inflammatory disorders. Murine systemic lupus erythematosus (SLE) models injected with an inhibitor of histone deacetylase attenuated renal pathology compared to a vehicle control [Citation10]. This may be through, in part, the reduction of autoreactive plasma cells and autoantibodies [Citation11].
Histone proteins are an example of a danger-associated molecular pattern (DAMPs) and possess the intrinsic ability to elicit inflammation. Notably, mice administered histones elicited systemic inflammation and suffer from multi-organ damage and eventually death, in a dose-dependent manner [Citation12]. In in vitro experiments, H1 and H2A histones were able to induce specific T cell proliferation in SLE patients. Moreover, they stimulated the production of interferon-γ, tumour necrosis factor and anti-double stranded DNA (dsDNA) autoantibodies [Citation13,Citation14].
2. Anti-histone antibodies (AHAs)
Amongst the antinuclear antibodies (ANAs), AHAs represent a clinically important subset [Citation15]. AHAs are found in a variety of immunological and infectious disorders. They may be directed against free histones or histones bound to DNA [Citation16]. Although AHAs may be of any isotype, IgG is considered the most clinically relevant and detected isotype. IgM AHAs are less specific and may be found in a wide range of other disorders such as rheumatoid arthritis and mixed connective tissue disease. These antibodies have been typed at the peptide level by microarray analyses to define their precise specificities [Citation17]. Although classically attributed to SLE and drug-induced lupus erythematosus (DILE), AHAs have been studied since the 1970s and are found in a large variety of pathologies including Sjögren’s syndrome, inflammatory myositides and rheumatoid arthritis [Citation18].[Citation16]
The functional activity of AHAs is largely unknown; although, they may possess proteolytic activity towards histone proteins [Citation19]. Whether this directly contributes to disease pathogenesis is unclear at this stage.
3. AHA detection in the laboratory
AHAs may refer to either total histones or histone subunits; although the latter is more often seen in the research laboratory. AHAs may be noted on indirect immunofluorescence that screens for ANAs. On the commonly-used HEp-2 substrate, AHAs commonly appear as a homogenous pattern; but is not specific for these autoantibodies [Citation20]. Some false positives have also been noted on the Crithidia luciliae immunofluorescence test (CLIFT) (which normally detects anti-dsDNA antibodies) in some patients that produce AHA that can bind Chrithidia luciliae kinetoplast [Citation21].
One of the now-outdated AHA tests is the complement fixation assay. These assays rely on the ability of complement to bind AHA and histone antigen immune complexes, and therefore, do not cause the haemolysis of added sensitised sheep red blood cells. In contrast, the absence of AHA will lead to unbound complement – due to lack of antibody-antigen immune complexes – and eventual haemolysis of sensitised SRBCs. Haemolysis is measured spectrophotometrically [Citation22].
A further historical test is the immunofluorescence assay on histone-reconstituted tissues [Citation23]. Briefly, fixed mouse kidney cryostat sections are treated with hydrochloric acid to elute histones. A proportion of these is then reconstituted with calf thymus histones. Comparison of acid-extracted and reconstituted tissues after incubation with diluted patient sera allows the specific detection of histone antibodies [Citation23]. The advantage of this assay is the specific detection of AHA; however, it is time-consuming and labour-intensive. The complement fixation test and histone reconstitution assays have good concordance [Citation24].
Modern-day assays, such as the chemiluminescence assay, are based on an ELISA format with various methods of antigen presentation and secondary antibody detection, which has the advantage of being able to detect whole histone or histone subunits. These may be single- or multi-plex and afford automaticity and quick turnaround times [Citation25]. The positivity may differ depending on assay use and if there is contaminating DNA which acts as an antigenic source for patient antibodies [Citation26].
Western blotting has been used to traditionally detect antibodies against acid-extracted histone subunits. Sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) is used to separate histones out into their constituents based on molecular weights: H1 is around 23 kDa whilst the core histones (H2A, H2B, H3 and H4) range from 10–15 kDa [Citation27]. Detection against these components using IgG-, IgA- or IgM-labelled secondary antibodies can be performed. The line immunoassay works in a similar manner. The major drawback to these assays is the histone antigens are denatured and therefore, reduced sensitivity in detecting antibodies against conformational epitopes [Citation1].
4. AHAs in drug-induced lupus erythematosus (DILE)
DILE is a syndrome that resembles some or most pathological and clinical features of idiopathic SLE but is temporally related to the commencement of a particular drug. On cessation of the offending drug, the syndrome abates [Citation28]. Numerous drugs and biological agents are implicated. Although hydralazine and procainamide are the most commonly implicated agents [Citation28]; infrequent drugs associated include interferon-α, sulfasalazine and captopril [Citation29]. The risk of DILE is generally increased in the older population and those that have been taking implicated medications for a longer period of time [Citation30]. It is estimated to affect up to 30,000 people each year in the United States [Citation30].
DILE is characterised by the emergence of autoantibodies, including AHAs which appear at a much higher frequency than idiopathic SLE [Citation28,Citation31]. In contrast, biologic agents such as anti-tumour necrosis factor (TNF) do not appear to induce AHAs as frequently, suggesting a different mechanism for the pathogenesis [Citation32,Citation33]. Anti-TNF DILE tends to produce more internal organ involvement and rashes compared to classic DILE [Citation34]. In addition, AHAs are generally absent in drug-induced ANA without symptoms [Citation35].
Overall, AHAs (mainly IgG isotype) are present in a substantial proportion of DILE patients (). In a cohort of patient samples submitted to a diagnostic laboratory, AHA had a sensitivity of 67% and specificity of 95% for the diagnosis of DILE [Citation18]. This is in contrast to the reported prevalence of 96% in one study [Citation36]; however, the latter was derived from a cohort of DILE patients rather than a general laboratory population. Furthermore, older studies in DILE excluded newer biological agents that cause DILE without AHA, so this may be somewhat biased.
Table 1. Prevalence of IgG antibodies to total histones and histone subunits in selected disorders.
IgG and IgM antibodies to the H2A–H2B and H2A–H2B–DNA histone complexes have been noted in patients with procainamide DILE [Citation37,Citation38]. In contrast, patients with hydralazine and chlorpromazine DILE had predominantly IgM directed against DNA-free histone complexes, namely to H2A–H2B, H1, (H3–H4)2 tetramers [Citation37]. These data reinforce the heterogeneous immune response in DILEs, yet the specific reasons for targeting these histone complexes are unclear.
5. AHAs in systemic lupus erythematosus
In contrast to DILE, SLE ANAs are more heterogeneous and include AHAs and non-histone antibodies, perhaps reflecting the complex pathogenesis. IgG, IgA and IgM to individual core histones are primarily directed against H1, H2A, H2B and H3; very little is directed against H2A–H2B complex or H4 [Citation39]. Antibodies to these histone subunits are generally more diverse and reflect epitope spreading than DILE AHAs [Citation38], and may also be directed to histone complexes (e.g., H2A–H2B) and histone coupled to DNA [Citation26].
Studies reveal the presence of antibodies against total histone in approximately half of the patients with SLE (). Compared to healthy controls and non-SLE ANA-positive patients, total histone autoantibody detection has a diagnostic sensitivity of 55–92% and specificity of 69–82% for SLE [Citation40,Citation41]. H4 antibodies are relatively rare in SLE but when present, they have excellent diagnostic sensitivity (95%) and specificity (90%) for SLE when compared to healthy controls [Citation25]. This is analogous to H1 and H3 specific antibodies which also demonstrate high specificity (94–96%) for the diagnosis [Citation42]. In general, AHA has lower sensitivity for SLE than anti-nucleosomes and lower specificity than anti-dsDNA for the diagnosis [Citation40].
AHAs often co-exist with anti-dsDNA and anti-nucleosome antibodies, particularly in lupus nephritis (LN), and may reflect more severe renal involvement than those LN patients negative for these autoantibodies [Citation43]. AHA quantitation appears to correlate with LN severity and may be predictive of flares [Citation43–45]. Although total IgG AHA may fluctuate with disease activity, levels do not reliably correlate with overall disease activity [Citation16]. Clinically, studies show that AHA is associated with oral ulceration, neuropsychiatric symptoms, lymphopaenia and fatigue [Citation39,Citation41,Citation46]. There is no association with arthritis or other cutaneous features [Citation46]. As of yet, there appears not to be clinical significance ascribed to the differential expression of antibodies to histone subunits [Citation47].
6. Conclusions
Although AHAs were classically attributed to DILE and SLE, there is an expanding spectrum of the association of these autoantibodies to other autoimmune and infectious disorders. The value of these may be to help predict and prognosticate clinical features in disorders such as SLE and systemic sclerosis [Citation48]. Subunit histone antibody analyses may also help distinguish SLE from certain cases of DILE but this is not routinely performed in the diagnostic laboratory and probably does not add significant diagnostic value. Moreover, the prevalence of AHAs in DILE likely is much less than reported in older literature since newer agents implicated in DILE do not elicit AHAs as frequently as older agents such as procainamide.
What can be done to further study AHAs? Firstly, the factors that make histones immunogenic are a matter of ongoing research, particularly in terms of epigenetics in recent times. In addition, there are only a handful of studies that examine isotype determination in various disorders and, apart from a research perspective, there appears to be little clinical relevance although further studies in this area are warranted. Certainly, isotypes other than IgG increase the sensitivity of picking up AHAs [Citation39]. Furthermore, emerging mass spectrometry technology to type these autoantibodies at the amino acid and peptide levels has afforded an innovative way to study these autoantibodies at high resolution [Citation49], increasing our understanding of their pathogenicity and the immunological epiphenomena of systemic autoimmunity. It is possible that with increasing observational studies, databases of disease associations may be established to enable clinicians and laboratorians to find value in the molecular typing of these fascinating autoantibodies.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Burlingame RW. The clinical utility of antihistone antibodies. Autoantibodies reactive with chromatin in systemic lupus erythematosus and drug-induced lupus. Clin Lab Med. 1997;17(3):367–378.
- McGinty RK, Tan S. Nucleosome structure and function. Chem Rev. 2015;115(6):2255–2273.
- Prado F, Jimeno-González S, Reyes JC. Histone availability as a strategy to control gene expression. RNA Biol. 2017;14(3):281–286.
- Marzluff WF, Gongidi P, Woods KR, et al. The human and mouse replication-dependent histone genes. Genomics. 2002;80(5):487–498.
- Liu CL, Tangsombatvisit S, Rosenberg JM, et al. Specific post-translational histone modifications of neutrophil extracellular traps as immunogens and potential targets of lupus autoantibodies. Arthritis Res Ther. 2012;14(1):R25.
- Janssen KMJ, de Smit MJ, Withaar C, et al. Autoantibodies against citrullinated histone H3 in rheumatoid arthritis and periodontitis patients. J Clin Periodontol. 2017;44(6):577–584.
- Muller S, Dieker J, Tincani A, et al. Pathogenic anti-nucleosome antibodies. Lupus. 2008;17(5):431–436.
- Hayashi-Takanaka Y, Maehara K, Harada A, et al. Distribution of histone H4 modifications as revealed by a panel of specific monoclonal antibodies. Chromosome Res. 2015;23(4):753–766.
- Khan MA, Alam K, Hassan SM, et al. Nitration of H2B histone elicits an immune response in experimental animals. Autoimmunity. 2017;50(4):232–240.
- Vieson MD, Gojmerac AM, Khan D, et al. Treatment with a selective histone deacetylase 6 inhibitor decreases lupus nephritis in NZB/W mice. Histol Histopathol. 2017;32(12):1317–1332.
- Waibel M, Christiansen AJ, Hibbs ML, et al. Manipulation of B-cell responses with histone deacetylase inhibitors. Nat Commun. 2015;6(1):6838.
- Kawai C, Kotani H, Miyao M, et al. Circulating extracellular histones are clinically relevant mediators of multiple organ injury. Am J Pathol. 2016;186(4):829–843.
- Stummvoll GH, Fritsch RD, Meyer B, et al. Characterisation of cellular and humoral autoimmune responses to histone H1 and core histones in human systemic lupus erythaematosus. Ann Rheum Dis. 2009;68(1):110–116.
- Voll RE, Roth EA, Girkontaite I, et al. Histone-specific Th0 and Th1 clones derived from systemic lupus erythematosus patients induce double-stranded DNA antibody production. Arthritis Rheum. 1997;40(12):2162–2171.
- Lee AYS, Ang EBH. A clinical overview of autoantibodies in general practice rheumatology. Br J Gen Pract. 2014;64(626):e599.
- Krippner H, Springer B, Merle S, et al. Antibodies to histones of the IgG and IgM class in systemic lupus erythematosus. Clin Exp Immunol. 1984;58(1):49–56.
- Cornett EM, Dickson BM, Rothbart SB. Analysis of histone antibody specificity with peptide microarrays. J Vis Exp. 2017;126:55912.
- Zirwas MJ, Kress DW, Deng J-S. The utility of antihistone antibody screening in the diagnosis of drug-induced lupus erythematosus. Arch Dermatol. 2004;140(4):494–495.
- Magorivska IB, Bilyy RO, Havrylyuk AM, et al. Anti-histone H1 IgGs from blood serum of systemic lupus erythematosus patients are capable of hydrolyzing histone H1 and myelin basic protein. J Mol Recognit. 2010;23(5):495–502.
- Kubo M, Ihn H, Yazawa N, et al. Prevalence and antigen specificity of anti-histone antibodies in patients with polymyositis/dermatomyositis. J Invest Dermatol. 1999;112(5):711–715.
- Deng JS, Sontheimer RD, Lipscomb MF, et al. The binding of antihistone antibodies to Crithidia luciliae kinetoplasts is growth cycle-dependent. Arthritis Rheum. 1985;28(2):163–168.
- Wasserman E, Levine L. Quantitative micro-complement fixation and its use in the study of antigenic structure by specific antigen-antibody inhibition. J Immunol. 1961;87(3):290–295.
- Tan EM, Robinson J, Robitaille P. Studies on antibodies to histones by immunofluorescence. Scand J Immunol. 1976;5(6–7):811–818.
- Fishbein E, Alarcon-Segovia D, Vega JM. Antibodies to histones in systemic lupus erythematosus. Clin Exp Immunol. 1979;36(1):145–150.
- Vordenbäumen S, Böhmer P, Brinks R, et al. High diagnostic accuracy of histone H4-IgG autoantibodies in systemic lupus erythematosus. Rheumatology. 2018;57(3):533–537.
- Wallace DJ, Lin H-C, Shen GQ, et al. Antibodies to histone (h2a–h2b)–DNA complexes in the absence of antibodies to double-stranded DNA or to (h2a–h2b) complexes are more sensitive and specific for scleroderma-related disorders than for lupus. Arthritis Rheum. 1994;37(12):1795–1797.
- Luzhetskaya OP, Sedykh SE, Nevinsky GA. How human H1 histone recognizes DNA. Molecules. 2020;25(19):4556.
- Araújo-Fernández S, Ahijón-Lana M, Isenberg DA. Drug-induced lupus: including anti-tumour necrosis factor and interferon induced. Lupus. 2014;23(6):545–553.
- Fritzler MJ. Drugs recently associated with lupus syndromes. Lupus. 1994;3(6):455–459.
- Borchers AT, Keen CL, Gershwin ME. Drug-induced lupus. Ann N Y Acad Sci. 2007;1108:166–182.
- Lee AYS, Gordon TP. Antinuclear antibody (ANA) monitoring in drug-induced lupus erythematosus (DILE). Rheumatology. 2021;60(4):2022–2023.
- De Rycke L, Baeten D, Kruithof E, et al. Infliximab, but not etanercept, induces IgM anti-double-stranded DNA autoantibodies as main antinuclear reactivity: biologic and clinical implications in autoimmune arthritis. Arthritis Rheum. 2005;52(7):2192–2201.
- Williams EL, Gadola S, Edwards CJ. Anti-TNF-induced lupus. Rheumatology. 2009;48(7):716–720.
- Vedove CD, Del Giglio M, Schena D, et al. Drug-induced lupus erythematosus. Arch Dermatol Res. 2009;301(1):99–105.
- Grossman L, Barland P. Histone reactivity of drug-induced antinuclear antibodies. A comparison of symptomatic and asymptomatic patients. Arthritis Rheum. 1981;24(7):927–931.
- Fritzler MJ, Tan EM. Antibodies to histones in drug-induced and idiopathic lupus erythematosus. J Clin Invest. 1978;62(3):560–567.
- Burlingame RW, Rubin RL. Drug-induced anti-histone autoantibodies display two patterns of reactivity with substructures of chromatin. J Clin Invest. 1991;88(2):680–690.
- Portanova JP, Arndt RE, Tan EM, et al. Anti-histone antibodies in idiopathic and drug-induced lupus recognize distinct intrahistone regions. J Immunol. 1987;138(2):446–451.
- Cohen MG, Pollard KM, Webb J. Antibodies to histones in systemic lupus erythematosus: prevalence, specificity, and relationship to clinical and laboratory features. Ann Rheum Dis. 1992;51(1):61–66.
- González C, Garcia-Berrocal B, Herráez O, et al. Anti-nucleosome, anti-chromatin, anti-dsDNA and anti-histone antibody reactivity in systemic lupus erythematosus. Clin Chem Lab Med. 2004;42(3):266–272.
- Shabana AA, El-Ghawet AE, Machaly SA, et al. Anti-chromatin and anti-histone antibodies in Egyptian patients with systemic lupus erythematosus. Clin Rheumatol. 2009;28(6):673–678.
- Sun X-Y, Shi J, Han L, et al. Anti-histones antibodies in systemic lupus erythematosus: prevalence and frequency in neuropsychiatric lupus. J Clin Lab Anal. 2008;22(4):271–277.
- Yang J, Xu Z, Sui M, et al. Co-positivity for anti-dsDNA, -nucleosome and -histone antibodies in lupus nephritis is indicative of high serum levels and severe nephropathy. PLoS One. 2015;10(10):e0140441.
- Cortés-Hernández J, Ordi-Ros J, Labrador M, et al. Antihistone and anti-double-stranded deoxyribonucleic acid antibodies are associated with renal disease in systemic lupus erythematosus. Am J Med. 2004;116(3):165–173.
- Sui M, Lin Q, Xu Z, et al. Simultaneous positivity for anti-DNA, anti-Nucleosome and anti-Histone antibodies is a marker for more severe lupus nephritis. J Clin Immunol. 2013;33(2):378–387.
- Yuan F, Wei F, Huang H, et al. The predictive value of autoantibody spectrum on organ damage in patients with systemic lupus erythematosus. Arch Rheumatol. 2019;34(2):157–165.
- Stemmer C, Tuaillon N, Prieur A-M, et al. Mapping of B-Cell epitopes recognized by antibodies to histones in subsets of juvenile chronic arthritis. Clin Immunol Immunopathol. 1995;76(1):82–89.
- Sato S, Ihn H, Kikuchi K, et al. Antihistone antibodies in systemic sclerosis. Association with pulmonary fibrosis. Arthritis Rheum. 1994;37(3):391–394.
- Lee AYS, Chataway T, Colella AD, et al. Quantitative mass spectrometric analysis of autoantibodies as a paradigm shift in autoimmune serology. Front Immunol. 2019;10:2845.
- Costa O, Monier JC. Antihistone antibodies detected by ELISA and immunoblotting in systemic lupus erythematosus and rheumatoid arthritis. J Rheumatol. 1986;13(4):722–725.
- Roy Chengappa KN, Betts Carpenter A, Yang ZW, et al. Elevated IgG anti-histone antibodies in a subgroup of medicated schizophrenic patients. Schizophr Res. 1992;7(1):49–54.
- Boey ML, Peebles CL, Tsay G, et al. Clinical and autoantibody correlations in orientals with systemic lupus erythematosus. Ann Rheum Dis. 1988;47(11):918–923.
- Shoenfeld Y, Segol G, Segol O, et al. Detection of antibodies to total histones and their subfractions in systemic lupus erythematosus patients and their asymptomatic relatives. Arthritis Rheum. 1987;30(2):169–175.
- Baranova SV, Dmitrienok PS, Ivanisenko NV, et al. Antibodies to H1 histone from the sera of HIV-infected patients recognize and catalyze site-specific degradation of this histone. J Mol Recognit. 2017;30(3):e2588.
- Wayaku T, Hasegawa M, Kaji K, et al. Antigen specificity of antihistone antibodies in connective tissue disease patients with anti-U1RNP antibodies. Rheumatol Int. 2007;28(2):113–119.
- Hasegawa M, Sato S, Kikuchi K, et al. Antigen specificity of antihistone antibodies in systemic sclerosis. Ann Rheum Dis. 1998;57(8):470–475.
- Rubin RL. Autoantibody specificity in drug-induced lupus and neutrophil-mediated metabolism of lupus-inducing drugs. Clin Biochem. 1992;25(3):223–234.
