ABSTRACT
The current study is related to the United Nations Sustainable Development Goal 13, “Climate action”. As a consequence of environmental variations caused by global climate change, turf-forming algae supposedly tend to replace dominant foundation species, resulting in loss of marine biodiversity. Hypnea brasiliensis, H. cervicornis and H. spinella are three turf-forming species widely distributed along the Brazilian coast with a problematic taxonomic history. Specimens from the warm temperate region are morphologically similar, and their correct identification is only possible based on molecular markers. In the present study, specimens- were cultivated in the laboratory, and temperature gradient experiments (20, 24, 28 and 32°C) were carried out for 21 days. At 28°C, the growth rate, branching ratio, and photosynthetic pigment content were higher in all species, however in vivo chlorophyll fluorescence parameters were at the lowest. Moreover, no species survived at the warmest temperature 32°C. The effects of temperature on the three Hypnea species revealed that useful morphological characters for species identification were only observed in specimens cultured at 28°C, and the morphology of the three species was basically identical in low temperature treatments of 20 and 24°C. The annual average temperature of the sea surface in the Brazilian warm temperate region is 24°C, which may explain the current difficulty of distinguishing the three Hypnea species from this region using only morphology. Furthermore, our findings show that these turf-forming Hypnea species are negatively impacted by rising temperatures, emphasizing the importance of species-specific research to better understand the effects of global climate change. Consequently, predictions of the replacement of dominant foundation species by turf-forming seaweed in an elevated sea surface temperature scenario are less certain. The negative impact of temperature increases raises awareness of the importance of public policies to mitigate the future degradation of marine ecosystems.
INTRODUCTION
The geographic distribution of benthic marine algae is constrained by variations in several environmental factors. One of the most important factors is the sea surface temperature, where they are able to grow and reproduce under a variation of several degree Celsius (Becklin et al., Citation2016; Wernberg, Kendrick, & Phillips, Citation2003). One of the factors that enables algae to withstand this temperature variation is the adjustment of photosynthesis and respiration rates to seasonal and/or latitudinal temperature conditions (Staehr & Wernberg, Citation2009). For this purpose, physiological responses include changes in the ratio of photosynthetic pigments relative to photoprotective pigments (Falkowski & Laroche, Citation1991), cellular carboxylation activity (Davison, Greene, & Podolak, Citation1991), and membrane fluidity and electron chain transfer (Raison, Berry, Armond, & Pike, Citation1980). However, there is a limit to the temperature increase that seaweeds can withstand, which when reached or exceeded leads to the death of the organism and a decrease in wild populations. Thus, understanding the species-specific responses to increase in ocean temperature is essential to elucidating the shifts in seaweed biogeography under a global warming scenario (Becklin et al., Citation2016; Leliaert, Anderson, Bolton, & Coppejans, Citation2000; Staehr & Wernberg, Citation2009; Wernberg et al., Citation2003).
In marine ecosystems, more specifically in coastal habitats, spatially dominant foundation species, such as coral reefs and canopy-forming macrophytes, are being replaced by turf algae (Harvey, Kon, Agostini, Wada, & Hall-Spencer, Citation2021; Wernberg, Bettignies, Joy, & Finnegan, Citation2016). Turf-forming algae are a multispecies assemblage of short algae, with high growth rates and high tolerance rates to stressful conditions (Connell, Foster, & Airoldi, Citation2014), therefore anthropogenic stressors that negatively affect dominant foundation species can favour the proliferation of turf algae (Connell et al., Citation2014; Harvey et al., Citation2021; Hughes & Connell, Citation1999). Due to local and global driver of climate change, these shifts are projected to increase in frequency and distribution, and the ability of management to mitigate or reverse an ecological regime shift depends upon an understanding of how species interactions stabilize marine ecosystems (Harvey et al., Citation2021).
The genus Hypnea J.V.Lamouroux is shrouded in a past of taxonomic confusion, with countless species being synonymous and others being dismembered into a complex of closely related species (Jesus et al., Citation2019, Citation2016; Masuda, Yamagishi, Chiang, Lewmanomont, & Xia, Citation1997; Nauer, Deluqui Gurgel, Ayres-Ostrock, Plastino, & Oliveira, Nauer, et al., Citation2019a; Price, John, & Lawson, Citation1992). Several factors contribute to the difficulty in taxonomically separating Hypnea species: the absence of useful morphological characters (Nauer, et al., Citation2019b), aggravated by high morphological plasticity that is still poorly understood (Nauer, Cassano, & Oliveira, Citation2015), and the presence of some species with similar morphologies that occupy the same ecological niche (Nauer, Cassano, & Oliveira, Citation2016).
Hypnea brasiliensis, H. cervicornis and H. spinella generally grow in the intertidal region of sheltered rocky shores, but they can also be found in the subtidal to a depth of 10 m (Nauer et al., Nauer, et al., Citation2019b) or as an epiphyte on other algae (Jesus et al., Citation2016). In both cases, these Hypnea species are usually found forming turf communities, which can be composed of just one or several species of algae (). In terms of morphology, H. brasiliensis is similar to H. spinella in having a prostrate habit, but it can be distinguished by a soft texture and the presence of branchlets that are smaller and narrower than branches and principal axes (Jesus et al., Citation2016). H. cervicornis, in turn, has a delicate thalli with soft to cartilaginous texture, and decumbent branches with many secondary accessory holdfasts (Jesus et al., Citation2016). Spine-like branchlets irregularly scattered throughout the thalli are useful for differentiating H. cervicornis from H. brasiliensis. Lastly, H. spinella forms dense entangled cushion-like turfs with creeping branches, attached to the substratum by several discoid holdfasts formed throughout the thallus (Jesus et al., Citation2016). It can also be differentiated from other species by an indistinguishable main axis. However, in all three species, the presence of secondary fixation discs and anastomoses between branches are common, which help to give the appearance of small turfs (Jesus et al., Citation2016; Nauer, et al., Citation2019b), and they are often found growing side by side. In this way, it is only with the help of molecular markers that it is possible to be certain of the species identification.
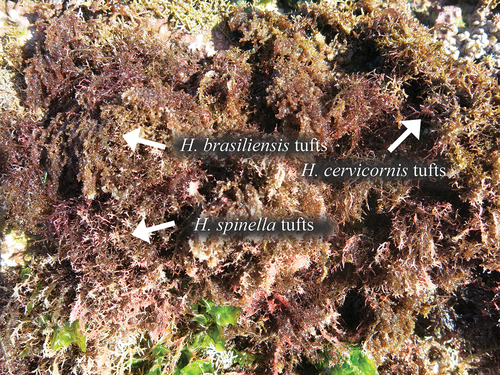
Figure 1. Intertidal region of Vermelha Beach, Ubatuba, São Paulo, warm temperate region of Brazil showing the species Hypnea brasiliensis, H. cervicornis and H. spinella growing as tufts in the intertidal region of the rocky shore.
Studies on the effects of temperature on the physiology and morphology of Hypnea spp. are scarce (Yokoya, Nauer, & Oliveira, Citation2020). Previous studies concentrated on the species H. musciformis and H. pseudomusciformis, which present an eurythermal response, tolerating temperature variations between 18 –30°C (Yokoya & Oliveira, Citation1992), with an optimum temperature for growth between 25–30°C (Yokoya, Plastino, & Artel, Citation2003). Physiological studies on Hypnea species are needed, because in addition to the genus economic importance as a source of carrageenan, H. cervicornis and H. spinella have been used as human food in Asian countries, like China and the Philippines (Yokoya, Nauer, & Oliveira, Citation2020).
Here, we report for the first time the temperature tolerance of H. brasiliensis, H. cervicornis and H. spinella under four different treatments (20, 24, 28 and 32°C) to test the hypothesis that temperature affects both morphology and physiology, which could explain the difficulty in identifying these Hypnea species. This study provides information on how these turf-forming seaweeds will be affected by global climate change stressors, specifically sea surface temperature in relation to the UN Sustainable Development Goal 13, “Climate action”.
Materials and Methods
Collection and sampling procedures
On 21 February 2021, four tetrasporophytic specimens of three Hypnea species (H. brasiliensis, H. cervicornis, and H. spinella) growing on rocky substratum were collected at the intertidal zone at low tide in Vermelha Beach, São Paulo, Brazil (−23.512215°, −45.7171752°). This collecting site is located in the warm temperate province of the Brazilian coast, where the maximum sea surface temperature is 28°C and the annual average is 24°C (Guimaraens & Coutinho, Citation1996).
Apical branches (~1 cm length) were cut from each field-collecting specimen, cleaned and transported alive in seawater containers to the laboratory for culture studies. For molecular analyses, apical regions were separated from the rest of the thallus, cleaned and stored in silica gel. The remainder of the samples were stored in a 4% formalin solution for morphological studies. Samples were sectioned by hand with a razor blade and stained with 1% aniline blue acidified with 1 N HCl. Habit and diagnostic characters (following Masuda et al., Citation1997) of each species were obtained with digital capture and image analysis, using a compound microscope with a Leica DM4000 digital camera (Solms, Germany). Voucher specimens were deposited in the herbarium of the Institute of Environmental Research (SP513951 for H. brasiliensis, SP513952 for H. cervicornis and SP513950 for H. spinella).
DNA extraction, PCR and sequencing
COI-5P was amplified using the same protocol described in Nauer et al. (Citation2015) using primers GazF1 and GazR1 and PCR cycles described by Saunders (Citation2005). A total of 23 COI-5P sequences (12 news and 11 from Genbank) with 465 nucleotides (nt) of Brazilian species of Hypnea were aligned using Clustal-W within BioEdit 7.0 sequence editor and checked visually. Neighbour-Joining (NJ) analysis was performed under heuristic search with 2000 bootstrap replicates using the MEGA 7 software. Caliblepharis ciliata (KJ960365) was used as an outgroup for COI-5P.
General culture conditions
Unialgal cultures were established for a total of 12 collected specimens by the isolation of a single apical branch (1 cm length). Unialgal isolates were cultured under the following conditions for one month: 400 ml of sterilized seawater (32 psu) enriched with von Stosch’s solution prepared as described by Edwards (Citation1976) and modified by Yokoya (Citation2000) with reduction of 50% in the vitamin concentrations (VSES medium), a light: dark cycle of 14 h:10 h, 24 ± 1°C and 100 ± 5 µmol photons.m2.s−1 (provided by Osram 40 W cool-withe fluorescent tubes and measured with a Li-COR quantameter model L1-185 and spherical sensor, model LI-192 SA, USA. The VSES medium was renewed weekly.
Experimental design
Three apical branches (~1 cm length) were used for each individual. A total of 48 apical branches were cut off from 12 individual tetrasporophytes (4 samples x 3 species) and incubated under the experimental culture conditions for one week, as an acclimation period. The experiment design consisted of 4 treatments (20°C, 24°C, 28°C and 32°C) provided by culture chambers (EL141/3, Eletrolab). Each treatment was tested with 4 replicates (n = 4), and each replicate was composed of three apical branches (2 mg of fresh mass and 1 cm in length each) of the same isolate and cultured in flasks with 400 ml of VSES medium in a 500 ml Erlenmeyer flask. We chose to use only three apices per replicate to avoid nutritional limitation and shading. Apical branches with one apical cell were used in the experiments, since Hypnea species have uniaxial thallus organization and apical growth. The fresh mass and chlorophyll fluorescence measurements were measured at the end of the experimental period (21 days). After that, samples were frozen in liquid nitrogen for posterior extraction for pigment analysis.
Growth rates and morphology
Growth rates (GRs) were estimated using wet weight (briefly dried on absorbent paper before measurement) and the following formulae from Yong, Yong, & Anton (Citation2013):
GR = [(Wt/W0)1/t – 1] x 100%
where Wt is the final wet weight, W0 is the initial wet weight, and t is the time. The total number was calculated from the total number of apices present in all replicates under the same conditions (n = 4) on the first day and the 21st day. The branching ratio (Br) was calculated among the number of differentiated branches at 21 days (Faria, Barufi, & Plastino, Citation2017). The mean and standard deviation were calculated from three replicates per condition.
In vivo chlorophyll fluorescence
Fluorescence measurements were performed using a pulse amplitude-modulated (PAM) fluorometer (Walz, Effeltrich, Germany). Prior to PAM measurements, light-exposed samples were dark-acclimated for 30 minutes. With the samples at a very low irradiance of blue light (0.3 μmol photons. m–2.s–1), the basal fluorescence Fo was measured; followed by a saturation pulse of 0.8 seconds (s) and approximately 6000 μmol photons.m–2.s–1 to obtain the maximum fluorescence yield of dark-adapted specimen (Fm), thus allowing the calculation of maximum quantum yield (Fv/Fm), were Fv = Fm-Fo (Schreiber, Citation2004). Subsequently, the specimens were subject to Rapid Light Curves (RLCs) where cells were exposed to a series of increasing actinic light intensities, each of 120 seconds duration before application of a saturation pulse (F), followed by a saturation pulse of 0.8 seconds (s) and approximately 6000 μmol photons m–2s–1 to obtain the maximum fluorescence yield of illuminated specimens (Fm’). The values of the effective quantum yield of photosystem II (Y(II), Kromkamp & Forster, Citation2003), the quantum yield of regulated non-photochemical energy loss of PS II (Y(NPQ)) and the quantum yield of non-regulated non-photochemical energy loss of PS II (YNO) (Kramer, Johson, Kiirats, & Edwards, Citation2004a; Hendrickson, Furnbank, & Chow, Citation2004) were calculated following the formulae:
Y(II) = (Fm’ – F)/Fm’
Y(NO) = F/Fm
Y(NPQ) = (F/Fm’) – Y(NO)
Pigment extraction and concentration determination
Pigment contents were extracted from 70 mg of fresh mass (FM) of each sample (n = 4, for each treatment, and for each species). Extraction of pigments was based on Kursar, Van Der Meer, & Alberte (Citation1983), modified by Plastino & Guimarães (Citation2001). Absorbance readings (400 nm to 700 nm) were measured in a 96-well microplate with a final volume of 400 µl in a UV–visible spectrophotometer (Epoch 2-BioTek, USA). The phycobiliprotein contents (µg g FM–1) were calculated from absorbances at 495.5, 614 and 651 nm according to Kursar et al. (Citation1983), while the chlorophyll a content (µg g FM–1) was calculated from the absorbances at 630, 647, and 664 nm according to Ritchie (Citation2006). The phycobiliprotein concentrations were estimated by the following equations: phycoerythrin (PE) = 155.8 × A498.5 – 40.0 × A614 – 10.5 × A651; phycocyanin (PC) = 151.1 × A614 – 99.1 × A651, allophycocyanin (APC) = 181.3 × A651 – 22.3 × A614 and Chlorophyll a (Chl-a) = 11.85 × A664 – 1.54 × A647 – 0.08 × A630.
Data analysis
Data was tested for normality (Kolmogorov–Smirnov) and homoscedasticity (Bartlett’s test). For each species, one-way ANOVA with independent variables [temperature (20, 24, 28, and 32°C)] and quantitative dependent variables (growth, Fv/Fm, Y(II), Y(NO), Y(NPQ), pigment contents, and branching ratios) was performed using the software STATISTICA 10.0 (StatSoft, Inc.). In ANOVA’s significant results, the a posteriori Newman-Keuls test (p < 0.05) was used to establish statistical differences.
Results
Molecular and morphological data
Based on the 5P-COI marker (Supplementary figure S1), we confirmed the identity of specimens collected in the field and used in temperature experiments. The sequences of specimens identified as H. brasiliensis (OL661332, OL661333, OL661334, OL661335) formed a cluster with the sequence from the holotype (KU905078). Sequences from H. cervicornis (OL661336, OL661337, OL661338, OL661339) specimens formed a cluster with a sequence from the type locality (KU905109). Finally, H. spinella sequences (OL661340, OL661341, OL661342, OL661343) formed a cluster with a sequence from the Brazilian coast (KU905111).
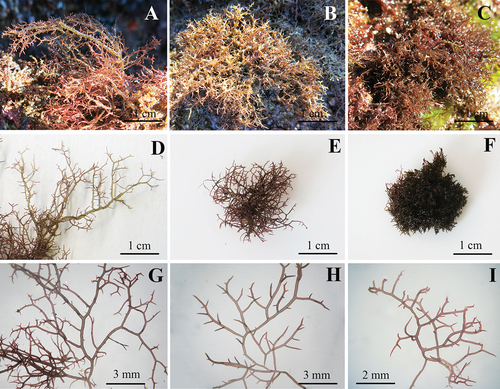
The morphology of each species is shown in , including the habitat of specimens collected in the field. Field-specimens of H. brasiliensis () were identified by the presence of branchlets that were smaller and narrower than branches and principal axes, and by the highest thallus length (up to 7 cm). H. cervicornis () and H. spinella () were very similar in terms of habit and thallus length (average of 3 cm). However, H. cervicornis had a cartilaginous texture while H. spinella formed a dense entangled turf. In the field specimens, secondary fixation discs were found, but anastomoses were observed only in H. spinella.
Figure 2. Habit of Hypnea species analysed in this study. A. H. brasiliensis on the field. B. H. cervicornis on the field. C. H. spinella on the field. D. Habit of H. brasiliensis. E. Habit of H. cervicornis. F. Habit of H. spinella. G. Detail of H. brasiliensis branches and branchlets. H. Detail of H. cervicornis branches and branchlets. I. Detail of H. spinella branches and branchlets.
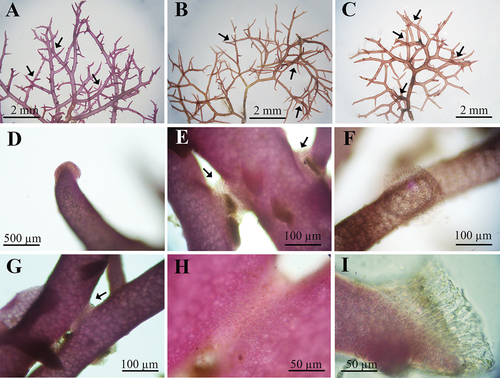
When comparing the morphology of Hypnea species cultivated at 20 and 24°C, no differences were observed (). At 20°C, the three species had a single principal axis, while at 24°C, the principal axis was indistinguishable. For these two temperatures, no differences were observed in branching ratio. The three species could be morphologically distinguished in specimens cultured at a temperature of 28°C (). For H. brasiliensis (), branchlets smaller and narrower than branches were formed only at this temperature. H. cervicornis () and H. spinella () presented a cartilaginous texture. The formation of secondary fixation discs was observed for the three species at 28°C (). The presence of anastomoses was only observed in specimens of H. spinella cultured at 28°C (). It is important to emphasize that although the experiment lasted 21 days, specimens of H. brasiliensis, H. cervicornis and H. spinella cultivated continuously under light in the culture chambers, under standard and 24°C culture conditions, did not show morphological differences independently of the culture time.
Figure 3. Morphology of Hypnea brasiliensis, H. cervicornis, and H. spinella cultured under different temperatures after 21 days of experiment. Scale bars = 1 cm.
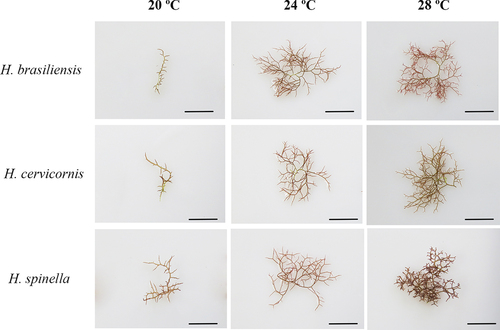
Figure 4. Morphological details of Hypnea species analysed in this study. A. Secondary fixing discs in H. brasiliensis (arrows). B. Secondary fixing discs in H. cervicornis (arrows). C. Secondary fixing discs in H. spinella (arrows). D. Detail of secondary fixing in H. brasiliensis. E and F. Detail of secondary fixing discs in H. cervicornis. G. Anastomoses in H. spinella. H. Detail of anastomoses in H. spinella. I. Detail of of secondary fixing discs in H. spinella.
Growth rates and branching ratio
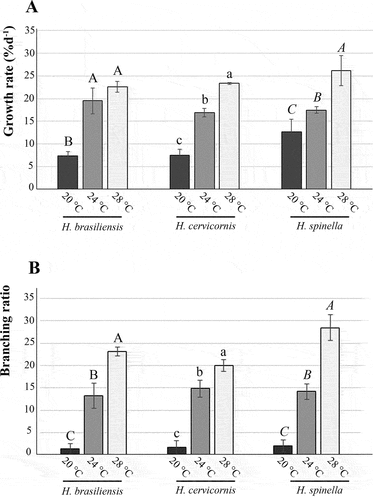
Temperature had significant effects on growth rates (GR) for the three Hypnea species (, ). No differences were observed in growth rates between 24 and 28°C (p = 0.57) for H. brasiliensis, while for both H. cervicornis and H. spinella the highest GRs were found at 28°C (p < 0.01). The lowest growth rate was observed at 20°C for the three species, while none of the species survived at 32°C for 21 days. Temperature also had significant effects on branching ratio (Br) for all species (, ). For the three species, the lowest values were found at 20°C (p < 0.01), while the highest were found at 28°C (p < 0.01).
Table 1. Summary of one-way analysis of variance of Hypnea brasiliensis, H. cervicornis and H. spinella from warm temperate region of Brazil, cultivated at 20, 24 and 28°C, considering growth rates (GR), branching ratio (Br), maximum quantum yield (Fv/Fm), at 21 days; and pigment content (PE, phycoerythrin; PC, phycocyanin; APC, allophycocyanin; Chl-a, chlorophyll a).
Figure 5. Temperature effects on tetrasporophytes of Hypnea brasiliensis, H. cervicornis, and H. spinella cultured under different temperatures for 21 days. A. Growth rates. B. Branching ratio. Bars indicate standard deviation (n = 4). Different letters indicate significant differences according to one-way ANOVA and Newman-Keuls test (p < 0.05).
In vivo chlorophyll fluorescence
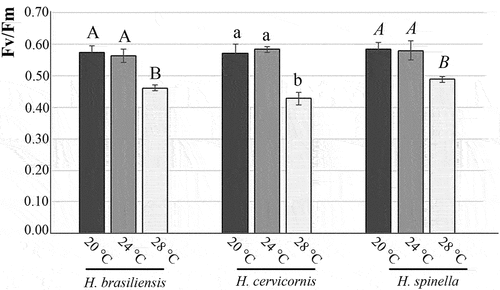
The Fv/Fm values were affected by the temperature for all species (, ). Fv/Fm values between 20 and 24°C did not differ between the three species (p = 0.32 for H. brasiliensis, p = 0.42 for H. cervicornis, and p = 0.48 for H. spinella). However, the three species’ lowest values were found at 28°C (p < 0.01).
Figure 6. Values are mean of maximum quantum yield (Fv/Fm), and bars indicate standard deviation (n = 4). Different letters indicate significant differences according to one-way ANOVA and Newman-Keuls test (p < 0.05).
In the case of H. brasiliensis, the highest Y (II) was found at 24°C (p < 0.01) and the lowest at 28°C (p < 0.01). The highest Y(NO) was discovered at 28°C (p < 0.01), and the highest Y(NPQ) was discovered at 20°C (p < 0.01) (). The highest Y(II) for H. cervicornis was found at 24°C (p 0.01), with no differences observed between 20 and 28°C (p = 0.37). The highest Y(NO) was discovered at 28°C (p < 0.01), and the highest Y(NPQ) was discovered at 20°C (p < 0.01) (). Finally, the highest Y(II) for H. spinella was found at 24°C (p < 0.01), while the lowest was found at 28°C (p < 0.01).The highest Y(NO) was discovered at 28°C (p < 0.01), and the highest Y(NPQ) was discovered at 20°C (p < 0.01) ().
Table 2. Chlorophyll a fluorescence parameters of Hypnea spp. cultured in different temperatures for 21 days. The effective quantum yield of photosystem II (Y(II), the quantum yield of regulated non-photochemical energy loss of PS II (Y(NPQ)) and the quantum yield of non-regulated non-photochemical energy loss of PS II (YNO) were obtained from the light-saturated region of rapid light curves. Mean ± SD (n = 4). Distinct letters indicate significant differences according to the a posteriori Newman-Keuls test (p < 0.05).
Pigment content
We observed the interaction of temperature on pigment content (, ) for all species. In the case of H. brasiliensis, all pigments have higher content at 28°C (p < 0.01), except for chlorophyll a, which has no difference between 24 and 28°C (p = 0.56). The lowest content of PE and chlorophyll a was observed at 20°C (p < 0.01), while no differences in PC and APC were observed between 20 and 24°C (p = 0.51 and p = 0.40, respectively).
Figure 7. Concentrations of Phycobiliproteins (phycoerythrin, PE; phycocyanin, PC; and allophycocyanin, APC) and Chlorophyll a (Chl a). A. Hypnea brasiliensis. B. H. cervicornis, C. H. spinella; cultured under different temperatures for 21 days. Values are mean and bars indicate standard deviation (n = 4). Different letters indicate significant differences according to one-way ANOVA and Newman-Keuls test (p < 0.05).
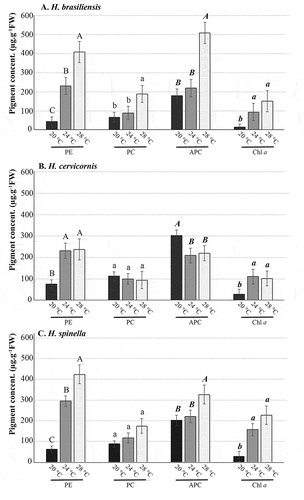
Considering H. cervicornis, each pigment showed different patterns between the analysed temperatures (, ). There were no differences in PE between 24 and 28°C (p = 0.39). For PC, no differences were observed between all three temperatures (p = 0.40). Higher APC content at 20°C (p < 0.01), but no differences between 24 and 28°C (p = 0.11). Finally, no differences in chlorophyll a were found between 24 and 28°C (p = 0.23).
Considering H. spinella, higher concentrations of all pigments were observed in specimens cultured at 28°C (p < 0.01), except for PC and chlorophyll a content (, ), where no differences were observed among all temperatures (p = 0.26) for PC, and no differences between 24 and 28°C (p = 0.16) for chlorophyll a. Furthermore, no differences in APC concentrations were observed between 20 and 24°C (p = 0.31).
Discussion
The turf-forming species H. brasiliensis, H. cervicornis and H. spinella were negatively affected by the increase in temperature in laboratory conditions, which highlights the importance of species-specific studies addressing general predictions that turf-forming algae will benefit from global climate change and replace foundation species in coastal ecosystems. The three Hypnea spp. in this study did not survive at 32°C for 21 days, which could explain the absence of H. spinella in the most parts of the tropical region of Brazil during the Hypnea DNA barcode survey, where the geographical distribution limit of the species was the state of Bahia (latitude-12°) (Nauer, et al. Citation2019b). In the Brazilian tropical region, the annual seawater surface temperature can reach 32°C in summer months. However, H. brasiliensis and H. cervicornis were widely found along the entire coast during the same study (Nauer et al. Citation2019b). Thus, other environmental factors should be acting on these species to offset the negative effect of high sea surface temperature. Faveri, Schmidt, and Simioni et al. (Citation2015) suggested that H. pseudomusciformis (as H. musciformis) survives temperatures of 35°C. However, the experiments lasted only four days, and specimens presented low growth rates.
Macroalgae’s ability to adjust photosynthesis to the temperature conditions includes the regulation of pigment content (Lopéz-Figueroa & Niell, Citation1990). The growth rates of the three Hypnea species were high at 28°C, as well as the contents of most phycobiliproteins and chlorophyll a, which could indicate an optimal range of growth at this temperature if it were not for the photosynthesis parameter data. The Fv/Fm values, as well as the Y (II) values, decreased by 28°C for all three species. Moreover, the Y(NO) values increased by 28°C, for the three species, indicating a large loss of light energy. The inefficiency of the PSII’s protection against damage is demonstrated by the low Y(NPQ) values, which declined in relation to the increase in temperature, mainly to H. brasiliensis and H. cervicornis. Low values of Y(NPQ) can lead to reduced plant growth and fitness, since indicate a reduction in the fraction of energy utilized in photosynthesis caused by saturation of the PSII reaction centre (Niyogi & Truong, Citation2013). The decrease in values of Fv/Fm at higher temperatures might be considered an inhibition of the photosynthetic apparatus, which induces an imbalance between energy intake and utilization (Maxwell & Johnson, Citation2000). These findings, combined with mortality at 32°C, suggest that temperatures in the 28°C range are already the physiological limit of these species. Thus, we believe that these species would have a warm temperate climate rather than a tropical climate, with the range between 24–28°C more favourable to their development. These data are relevant when we consider that these species form turfs and that these algal turfs are organisms that benefit from global climate changes, such as heat wave events and acidification (Cetz-Navarro, Quan-Young, & Espinoza-Avalos, Citation2015; Harvey et al., Citation2021).
Considering the mortality of H. spinella at 32°C and its absence in the tropical region of Brazil, we believe that this species will be negatively affected by the increase in sea water surface temperature, and it would be interesting to evaluate how populations in the temperate region would respond to heat wave events. On the other hand, H. brasiliensis and H. cervicornis are widely distributed in the tropical region of Brazil, despite the opposite result presented in the physiological response to temperature experiment. These results are similar to those found for H. pseudomusciformis, which is another species of Hypnea widely distributed along the entire Brazilian coast. Yokoya & Oliveira (Citation1992) demonstrated that specimens of H. pseudomusciformis (as H. musciformis) from the tropical province of Brazil survived in temperatures ranging from 18 to 30°C, and maximum growth rates were observed at 24°C and 28°C. Warm temperate zone macroalgae showing similar photosynthetic performances at 24°C and 28°C have been reported in the literature for Chondracanthus chamissoi (C.Agardh) Kützing (Bulboa & Macchiavello, Citation2001), Gracilaria domingensis (Kützing) Sonder ex Dickie (Ramlov et al., Citation2012; Yokoya & Oliveira, Citation1992), and even for freshwater red algae (Necchi, Citation2004).
The proliferation of turf forming algae results in the loss of ecosystem services, since these species generally possess less ecological, functional and human value compared to the species they replaced (Harvey et al., Citation2021; Rogers, Blanchard, & Mumby, Citation2014; Suding, Gross, & Houseman, Citation2004). In addition to temperature, another stressor factor related to global climate change that favours the proliferation of these turf algae is ocean acidification (Harvey et al., Citation2021), where the increase in the availability of bicarbonate and CO2 is a resource to primary producers (Cornwall, Citation2017). Nauer et al. (Citation2021) demonstrated that the growth rate of H. pseudomusciformis decreased significantly with decreased pH levels, which raises the hypothesis that Hypnea species may be negatively affected by both temperature increases and ocean acidification.
Our data on the effects of temperature on the morphology of H. brasiliensis, H. cervicornis and H. spinella explain the environmental drivers behind taxonomic difficulty in identifying field specimens on the coast of the warm temperate province of Brazil, where the annual average seawater surface temperature is 24°C, with a maximum of 28°C in extreme events during the summer months, and below 20°C in extreme events during the winter months (Guimaraens & Coutinho, Citation1996). In culture, these three Hypnea species were undistinguishable based on the morphology of specimens cultured lower than 24°C – the typical morphological characteristics of each species were only observed at 28°C. These data reveal why it is that common specimens in the warm temperate region of the Brazilian coast do not often present the morphological characters useful for their diagnosis, in addition to their reduced size compared to specimens from the tropical region. Furthermore, these data highlight the importance of carrying out collections both in the winter and in the summer months, especially in the warm temperate region.
Based on morphological similarities between Hypnea spp. (in addition to low DNA barcode divergence) of warm temperate Brazilian specimens, Nauer, Cassano, and Oliveira (Citation2014) considered the occurrence of H. aspera Kützing in the Atlantic Ocean. However, Jesus et al. (Citation2016) demonstrated that Brazilian tropical specimens were genetically identical to warm temperate specimens but morphologically similar to H. cervicornis, which led the authors to propose the synonymy of H. aspera to H. cervicornis, following the International Code of Nomenclature for Algae, Fungi, and Plants.
Morphological differences between specimens from the tropical region compared to the warm temperate region have already been documented for H. pseudomusciformis before, where specimens from the tropical region showed up to triple the size of the thallus and higher branching ratio (Nauer, Naves, & Plastino, Citation2020). The morphological differences between specimens of H. cervicornis from the tropical region compared to the warm temperate region can be explained by the influence of temperature on morphology, as demonstrated in this study.
In Brazil, for a long time, specimens with entangled habit have been misidentified as H. spinella based on morphology (Guimarães, Citation2006; Jesus, Schnadelbach, & Nunes, Citation2013). Jesus et al. (Citation2016) proposed that “the habit should be the main feature to be analyzed to assist in the circumscription of these species: H. brasiliensis forms loose tufts with percurrent axis evident, and broader than branches and branchlets; H. cervicornis usually presents a percurrent axis with the same diameter of the branches; and H. spinella has no percurrent axis and forms intricate tufts”. Our data show that this is not true for specimens collected in Brazil’s warm temperate region, where the seawater surface temperature is frequently below 24°C and, as a result, the morphology of these species becomes indistinct. However, the distinction by habit remains valid for specimens from the tropical region, where the average sea-water surface temperature is 28°C. H. brasiliensis presented an axis broader than branchlets; H. cervicornis still presented an axis with the same diameter as the branches; and H. spinella formed an intricate turf. Future studies would be relevant to analyse the phenology of species in the field during the seasons of the year and verify how annual temperature variations influence the morphology and distribution of populations in nature.
Conclusions
Not all tuft-forming non-calcareous algae species benefit from the increase in temperature, which illustrates the importance of species-specific studies to get a better understanding of how marine biodiversity will react to extreme climate events caused by global climate change, raising awareness of the importance of public policies to mitigate the future degradation of coastal ecosystems. Furthermore, the effects of temperature on the morphology of H. brasiliensis, H. cervicornis and H. spinella explain the difficulty in identifying these species based on morphology, which consequently led to this problematic history of taxonomy, not only of these species but of the genus Hypnea as a whole.
Supplemental Material
Download PNG Image (206 KB)Acknowledgments
We thank Valdilene Maria dos Santos for technical support. We thank Profs. Drs. Estela Maria Plastino and Mariana Cabral de Oliveira from the Marine Algae Laboratory of the Institute of Biosciences of the University of São Paulo for the authorization to use the Diving-PAM and spectrophotometer.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Supplementary Material
Supplemental data for this article can be accessed here.
Additional information
Funding
References
- Becklin, K. M., Anderson, J. T., Gerhart, L. M., Wadgymar, S. M., Wessinger, C. A., & Ward, J. K., et al. (2016). Examining plant physiological responses to climate change through an evolutionary lens. Plant. Physiol, 172, 635–649.
- Bulboa, C. R., & Macchiavello, J. E. (2001). The effects of light and temperature on different phases of the life cycle in the carrageenan producing alga Chondracanthus chamissoi (rhodophyta, gigartinales). Bot. Mar, 44, 371–374.
- Cetz-Navarro, N., Quan-Young, L., & Espinoza-Avalos, J. (2015). Morphological and community changes of turf algae in competition with corals. Sci. Rep, 5, 12814.
- Connell, S. D., Foster, M. S., & Airoldi, L. (2014). What are algal turfs? towards a better description of turfs. Marine Ecology Progress Series, 495, 299–307.
- Cornwall, C. E. (2017). Inorganic carbon physiology underpins macroalgal responses to elevated CO2. Sci. Rep, 7, 46297.
- Davison, I. R., Greene, R. M., & Podolak, E. J. (1991). Temperature acclimation of respiration and photosynthesis in the brown alga Laminaria saccharina. Mar. Biol, 110, 449–454.
- Edwards, P. (1976). Illustrated guide to the seaweeds and seagrass in the vicinity of Porto Aransas, Texas. In Contributions to Marine Science. Austin: University of Texas.
- Falkowski, P. G., & Laroche, J. (1991). Acclimation to spectral irradiance in algae. Journal of Phycology, 27, 8–14.
- Faria, A. V. F., Barufi, J. B., & Plastino, E. M. (2017). Ecotypes of Gracilaria caudata (gracilariales, rhodophyta): Physiological and morphological approaches considering life history phases. Journal of Applied Phycology, 29, 707–719.
- Faveri, C., Schmidt, É. C., Simioni, C., Martins, C.D.L., Bonomi-Barufi, J., Horta, P.A., Bouzon, Z.L. (2015). Effects of eutrophic seawater and temperature on the physiology and morphology of Hypnea musciformis J.V. Lamouroux (gigartinales, rhodophyta). Ecotoxicology, 24, 1040–1052.
- Guimaraens, M. A., & Coutinho, R. (1996). Spatial and temporal variation of benthic marine algae at the Cabo Frio upwelling region, Rio de Janeiro, Brazil. Aquatic. Bot, 52, 283–299.
- Guimarães, S. M. P. B. (2006). Checklist of rhodophyta from the State of Espírito Santo. Bull. Inst. Bot, 17, 143–194.
- Harvey, B. P., Kon, K., Agostini, S., Wada, S., & Hall-Spencer, J. M. (2021). Ocean acidification locks algal communities in a species-poor early successional stage. Glob Change Biol, 27, 2174–2187.
- Hendrickson, L., Furnbank, R. T., & Chow, W. S. (2004). A simple alternative approach to assessing the fate of absorbed light energy using chlorophyll fluorescence. Photosynth. Res, 82, 73–81.
- Hughes, T. P., & Connell, J. H. (1999). Multiple stressors on coral reefs: A long-term perspective. Limnology and Oceanography, 44, 932–940.
- Jesus, P. B., Costa, A. L., Nunes, J. M. C., Manghisi, A., Genovese, G., Morabito, M., & Schnadelbach, A. S., et al. (2019). Species delimitation methods reveal cryptic diversity in the Hypnea cornuta complex (cystocloniaceae, rhodophyta). European Journal of Phycology, 54, 135–153.
- Jesus, P. B., Nauer, F., Lyra, G. M., Cassano, V., Oliveira, M. C., Nunes, J. M. C., & Schnadelbach, A. S., et al. (2016). Species delimitation and phylogenetic analyses of some cosmopolitan species of Hypnea (rhodophyta) reveal synonyms and misapplied names to H. cervicornis, including a new species from Brazil. Journal of Phycology, 52, 1–44.
- Jesus, P. B., Schnadelbach, A. S., & Nunes, J. M. C. (2013). O gênero Hypnea (cystocloniaceae, rhodophyta) no litoral do estado da Bahia, Brasil. Sitientibus Série Ciências Biológicas, 13, 1–21.
- Kramer, D. M., Johson, G., Kiirats, O., & Edwards, G. E. (2004). New fluorescence parameters for the determination of QA redox state and excitation energy fluxes. Photosynth. Res, 79, 209–218.
- Kromkamp, J. C., & Forster, R. M. (2003). The use of variable fluorescence measurements in aquatic ecosystems: Differences between multiple and single turnover measuring protocols and suggested terminology. Eur. J. Phycol, 38, 103–112.
- Kursar, T. A., Van Der Meer, J., & Alberte, R. S. (1983). Light-harvesting system of the red alga Gracilaria tikvahiae. I. Biochemical analyses of pigment mutations. Plant. Physiol, 73, 353–360.
- Leliaert, F., Anderson, R. J., Bolton, J. J., & Coppejans, E. (2000). Subtidal understorey algal community structure in kelp beds around the Cape Peninsula (Western Cape, South Africa). Bot. Mar, 43, 359–366.
- Lopéz-Figueroa, F., & Niell, F. X. (1990). Effects of light quality on chlorophyll and biliprotein accumulation in seaweeds. Mar. Biol, 104, 321–327.
- Masuda, M., Yamagishi, Y., Chiang, Y. M., Lewmanomont, K., & Xia, B. M. (1997). Overview of Hypnea (Rhodophyta, Hypneaceae). In I. A. Abbott (Ed.), Taxonomy of Economic Seaweeds, Vol. 6. California Sea Grant College (pp. 127–133). La Jolla: University of California.
- Maxwell, K., & Johnson, G. N. (2000). Chlorophyll fluorescence - a practical guide. Journal of Experimental Botany, 51, 659–668.
- Nauer, F., Borburema, H. D. S., & Yokoya, N. S. (2021). Effects of ocean acidification on growth, pigment contents and antioxidant potential of the subtropical Atlantic red alga Hypnea pseudomusciformis Nauer, Cassano & M.C. Oliveira (Gigartinales) in laboratory. Brazil Journal Botany 44, 69–77.
- Nauer, F., Cassano, V., & Oliveira, M. C. (2014). Hypnea species (gigartinales, rhodophyta) from the southeastern coast of Brazil based on molecular studies complemented with morphological analyses, including descriptions of hypnea edeniana sp. nov. and H. flava sp. nov. European Journal Of Phycology, 49, 550–575.
- Nauer, F., Cassano, V., & Oliveira, M. C. (2015). Description of Hypnea pseudomusciformis sp. nov., a new species based on molecular and morphological analyses, in the context of the H. musciformis complex (gigartinales, rhodophyta). Journal of Applied Phycology, 27, 2405–2417.
- Nauer, F., Cassano, V., & Oliveira, M. C. (2016). Hypnea wynnei and Hypnea yokoyana (cystocloniaceae rhodophyta), two new species revealed by a DNA barcoding survey on the Brazilian coast. Phytotaxa, 268, 123–134.
- Nauer, F., Deluqui Gurgel, C. F., Ayres-Ostrock, L. M., Plastino, E. M., & Oliveira, M. C. (2019a). Phylogeography of the Hypnea musciformis species complex (gigartinales, rhodophyta) with the recognition of cryptic species in the western Atlantic Ocean. Journal of Phycology, 55, 676–687.
- Nauer, F., Jesus, P. B., Cassano, V., Nunes, J. M. C., Schnadelbach, A. S., & Oliveira, M. C. (2019b). A taxonomic review of the genus Hypnea (gigartinales, rhodophyta) in Brazil based on DNA barcode and morphology. Braz. J. Bot, 42, 561–574.
- Nauer, F., Naves, M., Plastino, E. M., Oliveira, M.C., & Fujii, M.T. (2020). Ecotypes of Hypnea pseudomusciformis (cystocloniaceae, rhodophyta) revealed by physiological, morphological, and molecular data. Journal of Applied Phycology, 32, 4399–4409.
- Necchi, O., Jr. (2004). Photosynthetic responses to temperature in tropical lotic macroalgae. Phycol. Res, 51, 140–148.
- Niyogi, K. K., & Truong, T. B. (2013). Evolution of flexible non-photochemical quenching mechanisms that regulate light harvesting in oxygenic photosynthesis. Curr. Opin. Plant. Biol, 16, 307–314.
- Plastino, E. M., & Guimarães, M. (2001). Diversidade intraespecífica. In K. Alveal, and T. Antezana (Eds.), Sustentabilidad de la biodiversidad, un problema actual. Bases cientifico-tecnicas, teorizaciones y proyecciones (pp. 19–27). Concepción, Chile: Universidad de Concepción.
- Price, J. H., John, D. M., & Lawson, G. W. (1992). Seaweeds of the western coast of tropical Africa and adjacent Islands: A critical assessment. IV. Rhodophyta (florideae) 3. Genera H-K. Bull. Br. Mus. Nat. Hist. Bot, 22, 123–146.
- Raison, J. K., Berry, J. A., Armond, P. A., & Pike, C. S. (1980). Membrane properties in relation to the adaptation of plants to temperature stress. In N. C. Turner & P. J. Kramer (Eds.), Adaptation of Plants to Water and Temperature Stress (pp. 261–273). New York: John Wiley and Sons.
- Ramlov, F., de Souza, J. C., Farias, A., Maraschin, M., Horta, P. A., & Yokoya, N. S., et al. (2012). Effects of temperature, salinity, irradiance, and nutrients on the development of carposporelings and tetrasporophytes in gracilaria domingensis (Kütz.) Sonder ex Dickie (rhodophyta, gracilariales). Bot. Mar, 55, 253–259.
- Ritchie, R. J. (2006). Consistent sets of spectrophotometric chlorophyll equations for acetone, methanol and ethanol solvents. Photosynth. Res, 89, 27–41.
- Rogers, A., Blanchard, J. L., & Mumby, P. J. (2014). Vulnerability of coral reef fisheries to a loss of structural complexity. Current Biology: CB, 24, 1000–1005.
- Saunders, G. W. (2005). Applying DNA barcoding to red macroalgae: A preliminary appraisal holds promise for future applications. Philos. Trans. Royal. Soc. B, 360, 1879–1888.
- Schreiber, U. (2004). Pulse-amplitude-modulation (PAM) fluorometry and saturation pulse method: An overview. In G. C. Papageorgiou,& Govindjee (Ed.), Chlorophyll a fluorescence: A signature of photosynthesis (pp. 279–319). Dordrecht: Springer.
- Staehr, P. A., & Wernberg, T. (2009). Physiological responses of ecklonia radiata (laminariales) to a latitudinal gradient in ocean temperature. Journal of Phycology, 45, 91–99.
- Suding, K. N., Gross, K. L., & Houseman, G. R. (2004). Alternative states and positive feedbacks in restoration ecology. Trends Ecol. Evol, 19, 46–53.
- Wernberg, T., Bettignies, T., Joy, B. A., & Finnegan, P. M. (2016). Physiological responses of habitat-forming seaweeds to increasing temperatures. Limnology and Oceanography, 61, 1–11.
- Wernberg, T., Kendrick, G. A., & Phillips, J. C. (2003). Regional differences in kelp-associated algal assemblages on temperate limestone reefs in south-western Australia. Divers Distrib, 9, 427–441.
- Yokoya, N. S., Nauer, F., & Oliveira, M. C. (2020). A concise review of the genus Hypnea J.V. Lamoroux. J. App. Phycol, 32, 3585–3603.
- Yokoya, N. S., & Oliveira, E. C. (1992). Geographic distribution and growth responses to temperature variation of some South American red algae of economic importance. Journal of Applied Phycology, 4, 339–345.
- Yokoya, N. S., Plastino, E. M., & Artel, R. (2003). Physiological responses and pigment characterization of two colour strains of the carrageenophyte Hypnea musciformis (rhodophyta). In: A. R. O. Chapman, R. J. Anderson, V. J. Vreeland, and I. R. Davison (eds), Proc 17th International Seaweed Symposium (pp. 425–433). New York: Oxford University Press.
- Yokoya, N. S. (2000). Apical callus formation and plant regeneration controlled by plant growth regulators on axenic culture of the red alga Gracilariopsis tenuifrons (gracilariales, rhodophyta). Phycol. Res, 48, 133–142.
- Yong, Y. S., Yong, W. T. L., & Anton, A. (2013). Analysis of formulae for determination of seaweed growth rate. Journal of Applied Phycology, 25, 1831–1834.
