?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Anthropogenic activities leads to increase in toxic metals in the environment. The toxicity of Cd, Cr and Pb to P. juliflora were tested in agar media; Seeds germinated in 25, 50 and 100 mg/L Pb; 5, 10 and 20 mg/ 10 L Cd; and 10 mg/L Cr were mostly unaffected. At 20 and 40 mg/L, Cr inhibited germination. Similarly, Cd and Cr treatments but not Pb disturbed seedlings development. Up to 3366.3 mg/kg and 1228.6 mg/kg of Pb accumulates in the root and shoot. The bioconcentration factor for both tissues ranged from 27.8 to 115.4 and 11.4 to 45.7, respectively. The translocation factor ranged from approximately 0.4 to 0.5, suggesting that it preferentially accumulates Pb in the root. Fourier Transformed Infrared Spectroscopy confirms Pb ions complexation with functional groups on the plant dry tissue biomass. These findings therefore, suggest that P. juliflora is suitable for Pb phytostabilization in metalliferous soil.
Abbreviations: ICP-OES: Inductively Coupled Plasma Optical Emission Spectrometry; FTIR: Fourier Transform Infrared Spectroscopy; BCF: Bioconcentration Factor; TF: Translocation Factor.
1. Introduction
The desert plant, Prosopis juliflora, commonly known as honey mesquite is widely distributed in arid and semi-arid environments. It is an important fuel and timbers source. A native of South and Central America, P. juliflora was introduced to many desert environments including the Gulf countries mainly to mitigate the effect of desertification. However, in many countries where the mesquite was introduced, it is fast becoming the most invasive plant and commonly found in managed environments including agricultural fields. It is becoming a threat to native and exotic plant species due to its aggressive growth nature and coppices formation [Citation1]. However, it offers many advantages over these local species [Citation2]. Many invasive plant species are known to be bioindicators of environmental contamination [Citation3].
Like many other countries, honey mesquite (locally known as Ghwaif) is an invasive plant in the state of Qatar. Due to increasing anthropogenic activities, especially that of oil and gas as well as construction works in the state of Qatar, the accumulation of toxic metals such as Cadmium (Cd), Chromium (Cr) and lead (Pb) has been on the rise [Citation4]. Therefore, several baseline studies are being carried out to prospect for native and invasive plants species with the capacity to efficiently remediate these contaminants. In Qatari soil, the plant spreads across all disturbed environments, including agricultural and industrial areas, which suggest its toxitolerant character. Therefore, considering the extreme nature of the environment, it will be interesting to evaluate metal hyperaccumulation capacity of P. juliflora found in these areas for beneficial purposes such as phytoremediation of metal-contaminated areas.
During phytoremediation, the process by which contaminants are remediated differs; these may be in the form of removal, transfer, degradation and immobilization, from either soil or water. It is a unique approach capitalizing on plants roots ability for the initial uptake of pollutants and eventually accumulating them in the shoot tissue by translocation across the stem. Compared to other conventional treatment techniques, phytoremediation is new, with great potential to provide the much-needed green technology solution to our deteriorating environment. To date, hundreds of plant species were suggested to be potential phytoremediators [Citation5]. Strategies used in the clean-up of environmental pollutants, both organic and inorganic are either by physical, chemical and biological treatment [Citation6]. However, physical and chemical methods are recognized for some disadvantages or limitations such as high cost and labour intensiveness; additionally, chemical processes create further pollution and are especially costly [Citation7]. In this context, new and better approaches to treat metal contamination became imperative, hence the exploration of various bio-based techniques. The use of biological agents are considered cheap, safer and was proven to have limited or no negative impact to the environment [Citation8]. Biological remediation methods include bio-augmentation, bioremediation, bioventing, composting and phytoremediation. However, phytoremediation proves the most viable and useful alternative among all, thereby gaining increased attention in recent years [Citation9].
Seed phytotoxicity test can be used to evaluate plants response to various metal stress [Citation10]. Although seed germination and seedlings growth are inhibited by metals concentration, however, toxicity is subject to plant, metal types and concentration level [Citation11]. Compared to filter papers, metal phytotoxicity test is more sensitive in agar media [Citation12]. The effects of Cd and Cr on P. juliflora is well documented in the literature [Citation13]. Some studies reported Prosopis sp. Pb bioaccumulation, such as fungal and EDTA assisted Pb accumulation in hydroponic medium [Citation14,Citation15] or powdered seed biosorption potential [Citation16]. However, information on the effect of Pb toxicity on the desert inhabitant’s seed germination, seedlings growth and accumulation is not sufficiently established. In fact, to the best of our knowledge, this study is probably the first to report Pb toxicity on P. juliflora in agar media.
Several parameters, including Bioconcentration (BCF) and Translocation factors (TF) [Citation17] can be used to evaluate plants phytoremediation potential. BCF values higher than one (>1) and less than one (<1) indicate that a plant is a ‘hyperaccumulator’ and ‘excluder’ respectively [Citation18]. TFs are useful in the determination of plants efficiency in heavy metals translocation right from the root and on to the shooting part. According to [Citation19] the plant is considered useful in metal translocation from root to shoot when the TF is higher than one (>1) due to the efficient plants metal transport system [Citation20]. However, TF values less than one (<1) indicate ineffective metal transfer and hence suggest that plants accumulate the studied metals in roots/rhizomes more than in the shoot/leaf.
Metal properties such as charge, hydrated ion radius, electronegativity and covalent binding parameter contribute to the behaviour, strength of metal binding and hence influence adsorption mechanism in plant biomass. The affinities of different plant tissues towards specific metal ions depend on the available binding sites [Citation21]. The translocation of certain metals such as Cd to the shoot may be due to their soft cationic nature. They are more likely to form stable complexes with like donors, the soft ligands, such as the amino and sulfhydryl groups. Whereas metal elements like Cr and Pb have more affinity to root due to their more stable complex formation with hard ligands; hydroxyl, carboxylate, carbonate and phosphate groups [Citation22]. Biological samples are composed of macromolecules including carbohydrates, lipids, and nucleic acids. These biomolecules bear distinct functional groups with unique fingerprints corresponding to specified infrared light frequencies [Citation23]. When present in a given biological sample, transition metals interact with the functional groups of these biomolecules, the composition of which can be determined by analyzing their infrared light adsorption [Citation24]. Therefore, Fourier Transformed Infrared Spectroscopy (FTIR) spectra can be used to study metal cation binding in biological samples [Citation16], and hence, useful in phytoremediation studies.
2. Materials and method
2.1. Seed collection and storage
Seeds were taken from mature plants in Qatar University campus (25°22ʹ21.60″N 51°29ʹ45.28″E) Doha-Qatar, during March and April 2018. Following cleaning and drying, seeds were then stored in the dark at 4°C for subsequent germination experiment.
2.2. Seed treatment
The seeds were prepared by washing with tap water, and later soaked in concentrated sulfuric acid (H2SO4) for 15 minutes with occasional stirring. Afterwards, soaked in 6% sodium hypochlorite for 5mins, and subsequently rinsed with deionised water for ten minutes.
2.3. Germination condition
Metal phytotoxicity tests are more sensitive in agar media than filter paper [Citation12]. Metal treatment solutions in this study were solidified in 0.8% (w/v) nutrient based Bacto agar (DIFCO) in 500 mL mason jars containing about 250 ml each. The nutrient medium was made up of modified Hoagland solution according to Peralta et al. [Citation25]. Since agar solidification is pH dependent and said to be optimal between 5.4 and 5.8 [Citation10], all metal treatment solutions pH were adjusted before autoclaving. Three jars were spiked with the metal solutions 0, 5, 10 and 20 mg/L of Cd, 0, 20, 40 and 80 mg/L of Cr or 0, 25, 50 and 100 mg/L of Pb obtained from CdCl2, K2Cr2O7 and PbCl2.
2.4. Metal treatments
Three different concentrations of Cd, Cr and Pb, were prepared and used for the phytotoxicity test. Treatments were 5, 10 and 50 mg/L for Cd. 20, 40 and 80 mg/L for Cr. 25, 50 and 100 mg/L for Pb. All metal treatments were chosen based on a preliminary study on the background concentrations in select industrial areas of Qatar (Unpublished data), and hyperaccumulation threshold [Citation26]. Metal salts dissolved in deionised water further diluted to prepare treatment solutions, while deionised water only was the control.
2.5. Seed germination and seedlings growth
Five seeds were aseptically planted into each of the jars (3 replicates per treatment) in a completely randomised design and transferred to a growth chamber at a temperature of 25°C, 12 h photoperiod and photon irradiance of 39 µmol m−2 s−1 (3000 lux). Seed germination experiment ran for seven (7) days. Seed germination was recorded every 24 h. Radicle emergence of at least 2 mm long was considered for seed germination [Citation10]. Subsequently, germination variables; final germination percentage (TG), germination rate (T50), which is the time taken to reach 50% germination and germination index (seed vigor) were computed as described by Farooq et al. [Citation27]. At the end of the germination experiment, Cd and Cr treated P. juliflora showed severe signs of physiological stress, including wilting and chlorosis. Hence, only Pb treatments were extended to seedlings growth stage for another twenty-five (25) days, after which plant biomass (Root and shoot) morphological, Inductively Coupled Plasma Optical Emission Spectrometry (ICP-OES) and Fourier Transformed Infrared Spectroscopy (FTIR) analysis was performed to evaluate P. juliflora Pb tolerance and hyperaccumulation capacity. Before biomass preparation for metal analysis, growth parameters such as fresh weight, shoot and root length as well as chlorophyll contents were all computed using weighing balance (Intell-Lab PBW-3200), electronic digital calliper (Titan 23175) and chlorophyll meter (SPAD 502 Plus), respectively. For this purpose, three plants per replicate were chosen at random from each treatment. Measurements of shoot and root lengths were from the crown to main shoot apex and from root apex to the crown, respectively. Total chlorophyll measurement taken from three leaves per replicate, a total of nine per treatment and average values reported.
2.6. Metal quantitation by inductively coupled plasma optical emission spectroscopy (ICP-OES)
All Pb treated samples washed with tap water, followed by acid treatment in 0.01% HCl and further rinsed with deionised water. Following sterilisation, individual plants were then separated into two parts; shoot (above the ground biomass) and root (below the ground biomass). Both tissues were air and oven dried for 24 h and 48 h at room and 65°C temperature, respectively. Dried shoot and root tissues were later ground to a powder using a mechanical stainless steel grinder, and a wooden mortar and pestle, respectively. A fine powdered sample starting materials were then obtained by sieving through a 0.25 µm diameter mesh utensil.
Samples were digested in nitric acid (HNO3) and hydrogen peroxide (H2O2). A large capacity Environmental Express SC154 HotBlock® digestion system was used at alternating temperature until clear solutions were obtained by the Environmental Protection Agency (EPA) method 3050. All digests analysed by direct injection into Inductively Coupled Plasma Optical Emission Spectrometry (ICP-OES). The accumulation of Pb in P. juliflora root and shoot were determined against National Institute of Standards and Technology (NIST) multi-element Standard Reference Material (SRM’s); Apple leaves 1515. Before analysis, standard linear curves obtained using five different concentrations of 1.0 µg/L to 500 µg/L. One standard and quality check analysis performed and good correlation coefficient >0.999 obtained. All samples were analyzed in triplicates.
2.7. Bio-concentration (BC F) and translocation (TF) factor
Bio-concentration (BCF) and Translocation (TF) factors were determined as described by Bose and Bhattacharyya [Citation28], as follows.
Bioconcentration factor (BCF) computed as heavy metal accumulated in each plant tissue to that dissolved in the agar medium as shown below.
Where C is the metal concentration
Translocation factor (TF) of examined heavy metals were computed using the above equations as follows
2.8.. Fourier transformed infrared spectroscopy (FTIR)
Before FTIR analysis, samples were prepared by Naumann et al. [Citation29]. Summarily, about 1 mg of dry plant tissue samples were mixed with approximately 2.5 mg of potassium bromide (KBr) using agate mortar and pestle. Diffuse reflectance infrared spectra data obtained by filling in the powdered sample into the 2 mm micro cup. All samples were analyzed in triplicates using Fourier Transformed Infrared Spectrometer (FTIR-8201PC). IR spectra data recorded at room temperature and infrared range of 4000–400 cm−1
2.9. Statistical analysis
All data were statistically analysed using one-way ANOVA with the statistical package Sigma Plot, Systat Software Inc., and treatment means compared by Tukey test [Citation30]. Statistical significance was considered at P ˂ 0.05.
3. Result and discussion
3.1. Germination response
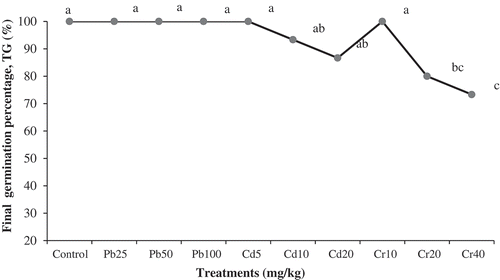
Plant seeds imbibition and softening increase its permeability to various stress conditions. At this stage, the first exchange with the immediate environment occurs and is responsive to variable changes [Citation31]. In the present study, all-metal treatments recorded a TG of 80% and above, except for 40 mg/L Cr (). When compared to the control, which recorded a TG of 100%, 40 mg/L Cr treated P. juliflora TG fell by approximately 17%. In contrast, all Pb and 5 mg/L Cd treatments recorded 100% TG. Generally, heavy metals exert varying degree of toxicity to plants; however, much of these is a function of metal types and plant species. Similarly, while plants exposure to certain metal concentration may result in the manifestations of its toxicity, the same concentration may be tolerated by some species [Citation11]. Germination rate (T50) and index (GI), which together demonstrates the speed of germination and seedlings vigour are important parameters in the assessment of heavy metal toxicity to plants [Citation32]. Accordingly, considering T50 and GI, we found P. juliflora to be more tolerant of Pb than Cd and Cr. The T50 and GI demonstrated an inverse relationship between increasing concentration of all metals. Compared to the control and other treatments, the highest T50 and lowest GI recorded by Cr treatments from this study suggest that it has the greatest inhibitory effect on P. juliflora (). A regular trend of increasing inhibitory effects of Cd and Cr toxicity can be observed with increase in respective metal concentrations as 20 mg/L ˃ 10 mg/L ˃ 5 mg/L Cd and 40 mg/L ˃ 20 mg/L ˃ 10 mg/L Cr, respectively (). At 25 mg/kg concentration, Pb demonstrates a more interesting scenario by stimulating P. juliflora germination compared to control with a T50 of 0.3 days and GI of 13.9, compared to the control’s 0.4 days and 12.8, respectively (). Together, the results of germination experiment suggest that the effects of metal treatments were in a dose-dependent manner. Although 100% TG were recorded by all Pb treatments, higher T50 and lower GI for 50 and 100 mg/L compared to the control (), suggest that, at higher concentration, Pb negatively affects P. juliflora germination. Accordingly, Mishra and Choudhuri [Citation33],reported that at 36 mg/kg, Pb negatively impacted on rice seed germination by up to one third, and inhibited further seedlings development by approximately 50% [Citation34]. Similarly, it reduce seed germination and caused stunted growth in roots of another South American native plant Lupinus, belonging to the same family, Fabaceae.
Table 1. P. juliflora germination index (GI) and time (T50) after 7 days treatment in agar media.
However, of all the metal treatments, Cr was the most toxic to P. juliflora, whereas Pb even promotes seed germination at 25 mg/kg concentration. This is supported by Jamal et al. [Citation35],work, where Cr demonstrated high toxicity to P. juliflora germination and seedlings development. Meanwhile, compared to the control and except for Pb at 25 mg/L, the germination response variables for Cd at 5 mg/kg concentration ( and ) suggest that at this concentration, Cd is the most tolerated by P. juliflora. Indeed, some studies proved Cd tolerance by P. juliflora at a higher concentration than reported in this study. Khan [Citation36],noted that at 400 mg/L, Cd showed no detrimental effects to the plant’s seed germination. Similarly, Michel-López et al. [Citation37],found no effects on its photosynthetic activities when exposed to 1000 µM CdCl2 for 48 h. Other studies Ling et al. [Citation38], Senthilkumar et al. [Citation39], also corroborate our findings.
3.2. Seedlings growth
It is known fact that plants are more vulnerable to metal toxicity at seedlings stage [Citation40]. Therefore, the assessment of seedlings growth is an important stage upon which metal toxicity can be further evaluated. To this effect, several parameters can be tested. However, this study determines fresh weight, chlorophyll content as well as root and shoot length for Pb only, as noted above. On physical inspection at harvest, though all treatments generally appear healthy with no major physical damage, such as wilting, chlorosis or root darkening, partial leaf chlorosis began to manifest for 100 mg/L Pb treatment. Additionally, compared to the control and other treatments, root length also significantly differ ()), suggesting that at this concentration, Pb caused root stunted growth in P juliflora. In sharp agreement, Arias et al. [Citation15],noted that root elongation was negatively affected in arbuscular mycorrhizal fungi associated Prosopis sp. treated with 100 mg/L of Pb. Indeed, Pb toxicity is implicated in various physiological, biochemical and morphological stress symptom in plants, including chlorosis, root darkening, disturbed photosynthesis and general growth inhibition. Several workers noted root growth inhibition by Pb at a concentration below 1 mg/L in other plant species [Citation41]. Additionally, at higher concentration, not only does Pb inhibit root development but also that of other tissues, and subsequently affecting the general plant biomass accumulation [Citation42]. However, we observed no significant differences in fresh weight and shoot length on Pb treated P. juliflora compared to control (,b)). It is probably due to low Pb accumulation in the plant shoot relative compared to the root. The root has an important role in plant growth and development, which directly affects how other tissues respond to growth conditions [Citation43]). Under heavy metal stress, it suffers the first exposure. However, its cell wall has a mechanism of exchange that fixes heavy metal ions, thereby limiting transport to other plant tissues [Citation41], which may in part also be responsible for the significant effect observed in P. juliflora treated with 100 mg/kg of Pb, but not in fresh weight and shoot length.
Figure 2. Pb treated Prosopis juliflora (a) Root and shoot length. Mean length are averages of nine plants from three replicates (n = 9) ± SEM. *Mean difference between treatments in root length statistically significant at P ≤ 0.05 (ANOVA-TUKEY). (b) Fresh weight. Mean fresh weight are averages of nine plants from three replicates (n = 9) ± SEM at P ≤ 0.05 (ANOVA). (c) Leaf chlorophyll contents based on SPAD Values. Mean values are averages of nine plants from three replicates (n = 9) .
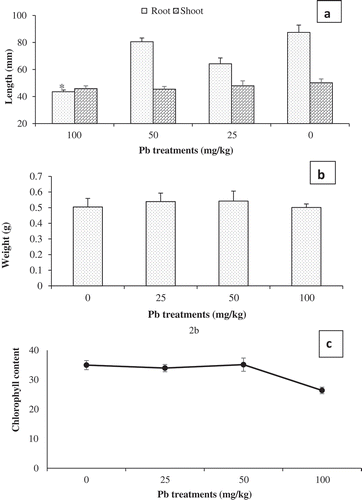
The chlorophyll contents is an important parameter used to assess plants health [Citation44], in the present study, SPAD values indicate a significant reduction in chlorophyll contents in P. juliflora treated with 100 mg/L Pb compared to control ()). Accordingly, Pb increases the activities of chlorophyllase enzymes in plants, which facilitates the degradation of chlorophyll [Citation45] Strong evidence also suggests a direct relationship between reduced photosynthetic activity and high Pb presence in plants. At high concentration, it also inhibits cell division and partly impacts on metabolic activities at the cellular level, all of which are responsible for the decreased growth of plants. Therefore, the behaviour of mesquite growth under Pb toxicity can be likened to that of other crop plants such as Triticum aestivum [Citation15].
3.3. Pb bioaccumulation in the root and shoot
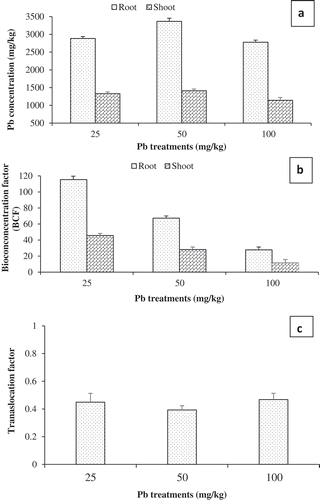
The amounts of Pb accumulated in the root and shoot tissues of P. juliflora cultivated in agar media containing 25, 50 and 100 mg/L of Pb are as shown in ). For all treatments, Pb accumulation is generally higher in the root than shoot. The accumulation of Pb by P. juliflora were (a) 25 mg/L of Pb: 2884 and 1142 mg/kg. (b) 50 mg/L of Pb: 3366.3 and 1228.6 mg/kg and (c) 100 mg/L of Pb: 2778 and 1410.4 mg/kg for root and shoot, respectively. Although Pb does translocate to the aerial part of plants, similar studies found that it preferentially accumulates in the root [Citation46]. Other similar studies are reviewed by Pourrut et al. [Citation41],in a critical review of Pb toxicity to plants. Understandably, translocation of metal to the aerial parts in plants is restricted in some species including Pb courtesy of many factors. It is largely regulated when the metal species is entering the root using apoplast via water streams and into the inner endodermis region [Citation41]. In the course of transport, negatively charged molecules in the cell wall such as the pectin can immobilize Pb ions; others are plasma membrane accumulation or precipitation of Pb insoluble salts [Citation47,Citation48]. Even more convincing is the fact that, since Pb may be trapped in the endodermis by the Casparian strip, it can resort to symplastic transport by which most of the isolated Pb can be excreted out of the plant [Citation41]. Our results suggest that for the root, P. juliflora absorbed the most Pb when treated with 50 mg/L of Pb. However, shoot recorded the highest Pb accumulation under 100 mg/L treatment ()). Supporting our findings, other workers report similar Pb accumulation pattern in P. juliflora tissues. For instance, Arias et al. [Citation15],observed that P. juliflora root accumulated higher Pb concentration when treated with 50 mg/L of Pb. The same observation was made by Aldrich et al. [Citation14], where at 50 mg/L of Pb treatment, the plant root tend to accumulate up to 63,396 mg/kg of the metal concentration in a hydroponic media amended with ethylenediaminetetraacetic acid (EDTA).
Figure 3. Prosopis juliflora Pb accumulation. (a) Root and shoot Pb concentration. (b) Root and shoot bioconcentration factor (BCF) (c) Translocation factor (TF). Mean values are averages from three replicates (n = 3) ± SEM. Mean difference between treatments of the same tissue and between tissues of the same treatment in (a) are statistically significant at P ≤ 0.05 level (ANOVA-TUKEY) .
3.4. Bioconcentration and translocation factors
Other important parameters considered in the evaluation of metal bioaccumulation and plants phytoremediation potentials are bio-concentration (BCF) and translocation factor (TF) [Citation17]. While BCF estimate metal accumulation in the tissues relative to that of the treatment medium, TF is useful in the determination of plants efficiency in heavy metals translocation right from the root and on to the shooting part. A BCF value greater than one (>1) and less than one (<1) indicates that a plant is a ‘hyperaccumulator’ and ‘excluder’ respectively [Citation49]. According to [Citation19] a plant is considered effective in metal translocation from root to shoot when the TF is greater than one (>1), which is due to the efficient plants metal transport system. However, TF value less than one (<1) indicate ineffective metal transfer and therefore suggest that the plant species accumulate more metals in roots than the shoot.
The BCF and TF of P. juliflora treated with 25, 50 and 100 mg/kg of Pb are as shown in ,c) respectively. As noted above, while both root and shoot accumulated Pb in varying concentrations, the root tissue recorded the most, and so is the case with the bioconcentration factor. The root BCF were 115.4, 67.3 and 27.8 for 25, 50 and 100 mg/L of Pb treatments, respectively. While that of the shoot are as follows: 45.7, 28.2 and 11.4 for 25, 50 and 100 mg/L of Pb treatments, respectively. As a factor of metal concentration in the medium, it can be seen that the BCF decreases with increasing Pb concentration ()). Despite the relative higher root BCF across all treatments, the shoot BCF also indicates the capacity of P. juliflora to translocate reasonable concentration of Pb onto the shoot part, especially at 25 mg/L concentration, where the BCF is highest for the shoot at 45.7 ()). Indeed, hyperaccumulator plants such as Brassica pekinensis and Pelargonium were reported to accumulate higher Pb concentration to their aerial parts [Citation50]. Regarding the TF, all treatments were less than one, indicating that P. juliflora does not translocate Pb to its aerial parts ()). However, a different trend to that of BCF is evident in that the least TF value was under 50 mg/L, whereas the highest is that of 100 mg/L of Pb treatment. Pb ability to break the Casparian crisp barrier in P. juliflora endoderm at 100 mg/L concentration, to enable further cationic transport across tissues and onto the aerial part may in part be responsible. Numerous plants are known for reduced metal uptake to aerial parts, which preferentially bioconcentrate in the root [Citation41]. Plant species including Nerium oleander L. and Brassica juncea are known to accumulate higher Pb accumulation in the root with no visible signs of stress [Citation51]. Notwithstanding, however, here, P. juliflora accumulates more than 1000 mg/kg Pb in both root and shoot under all treatments ()). A comparison of highest reported BCF and TF of some terrestrial plants is shown in .
Table 2. Comparison of bioconcentration (BCF) and translocation factor (TF) in some Pb accumulating terrestrial plants.
Phytoextraction, a form of metal phytoremediation, where plants translocate metals to other parts can be demonstrated by BCF and TF. In this context, alongside TF, above the ground metal BCF in plants tissues (leaf, shoot or stem) are considered. shows the shoot BCF in this study to be higher at all treatment levels with 45.7, 28.2 and 11.4. Further, as found in this study, except for Arabis paniculata Franch, Bidens frondosa, Setaria plicata, Phytolacca acinosa, Oryza sativa and Zea mays, majority of the plants reported as good candidates for the remediation of Pb polluted environment had TF ˂ 1. However, a TF > 1 in the above species could in part be due to the occurrence of Pb alongside other metals in the studied areas, which influences uptake behavior across the plant tissues [Citation52]. Together, our results suggest that P. juliflora is a hyperaccumulator of Pb [Citation53], and therefore a suitable candidate for the phytostabilisation of Pb contaminated areas.
3.5. FTIR analysis
In the recent past, FTIR has become a useful tool in the study of metal cation binding in biological samples [Citation16]. Transition metals found in biological samples interact with the functional groups of biomolecules, the composition of which can be determined by analysing their infrared light adsorption [Citation24]. P. Juliflora recorded the most Pb accumulation under 50 mg/L of Pb treatment. Therefore, it was chosen alongside the control for FTIR analysis. The FTIR spectra of 50 mg/L of Pb treated and untreated P. juliflora tissues are as shown in . In the present study, FTIR results showed that the plant’s dry biomass has different functional groups available for binding of heavy metal ions, such as carboxyl, phosphate, amide, and hydroxide. Spectral data for the root were, treatment (RoT): 3282.92, 2919.03, 1624.16, 1388.13, 1234.81 and 1031.98 cm−1, control (RoC): 3283.72, 2919.44, 1632.85, 1360.60, 1235.69 and 1034.35 cm−1 ()). While that of the shoot were, treatment (ShT): 3279.89, 2918.57, 1625.72, 1394.90, 1234.78 and 1050.10 cm−1, control (ShC): 3281.01, 2918.59, 1626.39, 1395.49, 1236.38 and 1051.35 cm−1 ()).
Figure 4. FTIR spectra of Prosopis juliflora (a) FTIR spectra of 50 mg/L Pb treatment in the root (RoT) and untreated root (RoC); (1) 3282.92, 3283.72 (2) 2919.03, 2919.44 cm−1 (3) 1624.16, 1632.85 cm−1 (4) 1388.13, 360.60 cm−1 (5) 1234.81, 1235.69 cm−1 and (6) 1031.98, 1034.35 cm−1. (b) FTIR spectra of 50 mg/L Pb (ShT) and untreated shoot (ShC); (1) 3279.89, 3281.03 cm−1 (2) 2918.57, 2918.59 cm−1 (3) 1625.72, 626.39 cm−1 (4) 1394.90, 1395.49 cm−1 (5) 1234.78, 1236.38 cm−1 and (6) 1050.10, 1051.35 cm−1.
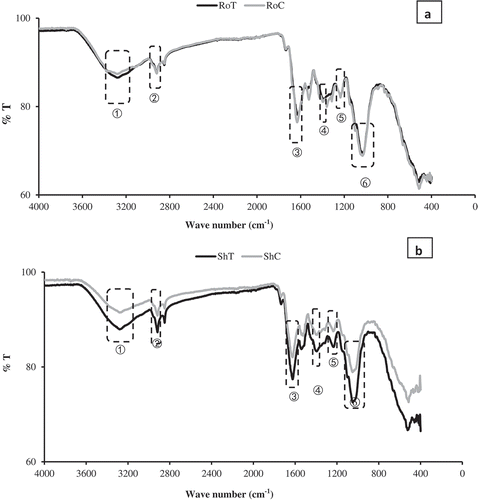
The results showed that P. juliflora tissues dry biomass have different functional groups available for the binding of Pb ions, such as carboxyl, phosphate, amide, and hydroxide. According to Panda et al. [Citation24], broad and robust IR spectra regions spanning 3600–3200 cm̵ 1 characterize O-H and N-H stretch. The peaks at 2919.03 and 2919.44 cm−1 ()), and 2918.57 and 2918.59 cm−1 ()) can be assigned to CH2 groups [Citation54]. All bands at 1624.16 and 1632.85 cm̵ 1 ()); 1625.72 and 1626.39 cm̵ 1 ()) corresponds to specific amide groups due to C = O stretch [Citation55]. Whereas O-H bending is also responsible for the peaks at 1388.13 and 1360.60 cm−1 ()); and 1394.90 and 1395.49 cm−1 ()) [Citation56]. The regions from 1200 to 900 Cm̵ 1 within which 1234.81, 1235.69, 1031.98, 1034.35 cm−1 ()); and 1234.78, 1236.38, 1050.10 and 1051.35 ()) falls, signify C-C, C-O and C-O-P stretch overlaps [Citation57], occurring mainly in cellular polysaccharides. Changes in the FTIR spectra confirmed that Pb ions complexes with the various functional groups found in P. juliflora tissues [Citation16].
4. Conclusion
Like many other countries, mesquite (locally known as Ghwaif) is an invasive plant in the state of Qatar. It is fast becoming problematic to other native and beneficial plant species. Therefore, the need to explore means to utilise P. juliflora for socioeconomic and or environmental benefits becomes imperative. Increased anthropogenic activities are associated with the prevalence of toxic metal species including Cd, Cr and Pb in the environment. In the present study, Cd, Cr and Pb toxicity to P. juliflora were tested in agar media, the desert plant’s Pb tissue accumulation capacity was also investigated, and the following conclusion was to be drawn.
The plant seeds germinate under all metal treatments, but Cd and Cr negatively affected seedlings growth in a dose-dependent manner. At low concentration, Pb stimulated P. juliflora growth. High Pb accumulation was shown by P. juliflora root and shoot. However, it demonstrates preferential bioaccumulation in the root. FTIR demonstrate the adsorption of Pb ions onto the plant tissue biomass, and different functional groups associated with the adsorption process were identified. The BCF excellently reveal the capacity of the plant to be a hyperaccumulator, and hence suitable for Pb phytostabilisation in the metalliferous soil.
Future work
Most certainly, P. Juliflora exposure to Pb would have stimulated the accumulation of protein transporter molecules, which assisted in the metal uptake onto the plant tissues. However, their accumulation and performance may be optimal at a certain concentration of Pb than others. Therefore, as a future work, our group will study the effect of the different Pb concentrations on the accumulation of stress response proteins such as proline, and evaluate the activities of antioxidant enzymes such as superoxide dismutase (SOD), glutathione reductase (GR) and catalase.
Competing interests
The authors declare no potential competing interests.
Acknowledgments
The authors wish to acknowledge the Environmental Studies Center (ESC) and Central Laboratory Unit at Qatar University for providing support to ICP-OES and FTIR analysis, respectively. We would like to thank the office of research support at Qatar University’s for the student grant QUST-CAS-SPR-2017-33. We also wish to acknowledge Ms. Abeer Al-Muhannadi and Ms. Muneera Al-Mesaifri for their technical support in the Laboratory, as well as identification and collection of plants seeds. The publication of this article was funded by the Qatar National Library.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- El-Keblawy A. Causes and consequences of the invasion of the exotic Prosopis juliflora in the environment of the UAE. Third Annual Conference for Research Funded by UAE University, Al-Ain: UAE University Press; 2002. p. B5–B8.
- Deans J, Diagne O, Nizinski J, et al. Comparative growth, biomass production, nutrient use and soil amelioration by nitrogen-fixing tree species in semi-arid Senegal. For Ecol Manage. 2003;176:253–264.
- Al-Qahtani KM. Assessment of heavy metals accumulation in native plant species from soils contaminated in Riyadh City, Saudi Arabia. Life Sci J. 2012;9:384–392.
- Yasseen BT, Al-Thani RF. Ecophysiology of wild plants and conservation perspectives in the state of Qatar. In: Stoytcheva M, Zlatev R, editors. Agricultural Chemistry. USA: InTech; 2013. p. 37-70
- Da Conceição Gomes MA, Hauser-Davis RA, de Souza AN, et al. Metal phytoremediation: general strategies, genetically modified plants and applications in metal nanoparticle contamination. Ecotoxicol Environ Saf. 2016;134:133–147.
- Hasegawa H, Rahman IMM, Rahman MA. Environmental remediation technologies for metal-contaminated soils. Japan: Springer; 2016.
- Usman K, Al-Ghouti MA, Abu-Dieyeh MH. Phytoremediation: halophytes as promising heavy metal hyperaccumulators; 2018.
- Doble M, Kumar A. Biotreatment of industrial effluents. Oxford (UK): Butterworth-Heinemann; 2005.
- Ullah A, Heng S, Munis MFH, et al. Phytoremediation of heavy metals assisted by plant growth promoting (PGP) bacteria: a review. Environ Exp Bot. 2015;117:28–40.
- Bae J, Benoit DL, Watson AK. Effect of heavy metals on seed germination and seedling growth of common ragweed and roadside ground cover legumes. Environ Pollut. 2016;213:112–118.
- Kranner I, Colville L. Metals and seeds: biochemical and molecular implications and their significance for seed germination. Environ Exp Bot. 2011;72:93–105.
- Di Salvatore M, Carafa A, Carratù G. Assessment of heavy metals phytotoxicity using seed germination and root elongation tests: a comparison of two growth substrates. Chemosphere. 2008;73:1461–1464.
- Nivethitha P, Thangavel P, Prince S, et al. Identification of heavy metal accumulating plants and their use in reclamation of soil contaminated with heavy metals. Ecol EnvironConserv. 2002;8:249–251.
- Aldrich MV, Ellzey J, Peralta-Videa J, et al. Lead uptake and the effects of EDTA on lead-tissue concentrations in the desert species mesquite (Prosopis spp.). Int J Phytoremediation. 2004;6:195–207.
- Arias JA, Peralta-Videa JR, Ellzey JT, et al. Effects of Glomus deserticola inoculation on Prosopis: enhancing chromium and lead uptake and translocation as confirmed by X-ray mapping, ICP-OES and TEM techniques. Environ Exp Bot. 2010;68:139–148.
- Jayaram K, Prasad M. Removal of Pb (II) from aqueous solution by seed powder of Prosopis juliflora DC. J Hazard Mater. 2009;169:991–997.
- Yoon J, Cao X, Zhou Q, et al. Accumulation of Pb, Cu, and Zn in native plants growing on a contaminated Florida site. Sci Total Environ. 2006;368:456–464.
- Baker AJ. Accumulators and excluders‐strategies in the response of plants to heavy metals. J Plant Nutr. 1981;3:643–654.
- Srivastava M, Ma LQ, Singh N, et al. Antioxidant responses of hyper-accumulator and sensitive fern species to arsenic. J Exp Bot. 2005;56:1335–1342.
- Zhao FJ, Hamon RE, Lombi E, et al. Characteristics of cadmium uptake in two contrasting ecotypes of the hyperaccumulator Thlaspi caerulescens. J Exp Bot. 2002;53:535–543.
- Hawari AH, Mulligan CN. Effect of the presence of lead on the biosorption of copper, cadmium and nickel by anaerobic biomass. Process Biochem. 2007;42:1546–1552.
- Sengupta AK. Environmental separation of heavy metals: engineering processes. Boca Raton: CRC Press; 2001.
- Griffiths PR, De Haseth JA. Fourier transform infrared spectrometry. Hoboken (NJ): John Wiley & Sons; 2007.
- Panda G, Das S, Bandopadhyay T, et al. Adsorption of nickel on husk of Lathyrus sativus: behavior and binding mechanism. Colloids Surf B Biointerfaces. 2007;57:135–142.
- Peralta JR, Gardea-Torresdey JL, Tiemann KJ, et al. Uptake and effects of five heavy metals on seed germination and plant growth in alfalfa (medicago sativa l.). Bullet Environ Contamina Toxico. 2001 Jun 24;66(6):727-34
- Van der Ent A, Baker AJ, Reeves RD, et al. Hyperaccumulators of metal and metalloid trace elements: facts and fiction. Plant and Soil. 2013 Jan 1; 362 (1–2): 319–34.
- Farooq M, Basra S, Ahmad N, et al. Thermal hardening: a new seed vigor enhancement tool in rice. J Integr Plant Biol. 2005;47:187–193.
- Bose S, Bhattacharyya A. Heavy metal accumulation in wheat plant grown in soil amended with industrial sludge. Chemosphere. 2008;70:1264–1272.
- Naumann D, Helm D, Labischinski H, et al. The characterization of microorganisms by Fourier-transform infrared spectroscopy (FT-IR). In: Griffiths PR, de Haseth JA, editors. Modern techniques for rapid microbiological analysis. New York: VCH-Publishers; 1991. p. 43–96.
- Steel RG, Torrie JH, Dickey DA. Principles and procedures of statistics: A biological approach. New York: McGraw-Hill; 1997.
- Solanki R, Dhankhar R. Biochemical changes and adaptive strategies of plants under heavy metal stress. Biologia. 2011;66:195–204.
- Ranal MA, Santana DGD. How and why to measure the germination process? Braz J Bot. 2006;29:1–11.
- Mishra A, Choudhuri M. Amelioration of lead and mercury effects on germination and rice seedling growth by antioxidants. Biol Plant. 1998;41:469–473.
- Verma S, Dubey R. Lead toxicity induces lipid peroxidation and alters the activities of antioxidant enzymes in growing rice plants. Plant Sci. 2003;164:645–655.
- Jamal SN, Iqbal M, Athar M. Effect of aluminum and chromium on the growth and germination of mesquite (Prosopis juliflora swartz.) DC. Int J Environ Sci Technol Tehran. 2006;3:173–176.
- Khan D. Effects of cadmium on germination and seedling growth of Prosopis juliflora (Swartz) DC.–a potential metallophyte. Int J Biol Biotechnol. 2007;4:133–147.
- Michel-López CY, Espadas Y Gil F, Fuentes Ortíz G, et al. Bioaccumulation and effect of cadmium in the photosynthetic apparatus of Prosopis juliflora. Chem Speciation Bioavailability. 2016;28:1–6.
- Ling T, Gao Q, Du H, et al. Growing, physiological responses and Cd uptake of Corn (Zea mays L.) under different Cd supply. Chem Speciation Bioavailability. 2017;29:216–221.
- Senthilkumar P, Prince WS, Sivakumar S, et al. Prosopis juliflora—a green solution to decontaminate heavy metal (Cu and Cd) contaminated soils. Chemosphere. 2005;60:1493–1496.
- Lefèvre I, Marchal G, Corréal E, et al. Variation in response to heavy metals during vegetative growth in Dorycnium pentaphyllum Scop. Plant Growth Regul. 2009;59:1–11.
- Pourrut B, Shahid M, Dumat C, et al. Lead uptake, toxicity, and detoxification in plants. In: Reviews of environmental contamination and toxicology. Vol. 213. Berlin: Springer-Verlag; 2011. p. 113–136.
- Gupta D, Nicoloso F, Schetinger M, et al. Antioxidant defense mechanism in hydroponically grown Zea mays seedlings under moderate lead stress. J Hazard Mater. 2009;172:479–484.
- BiernackiM, Lovett‐Doust J. Developmental shifts in watermelon growth and reproduction caused by the squash bug, anasa tristis. New Phytolo. 2002;155(2):265–273.
- Chaerle L, Van Der Straeten D. Seeing is believing: imaging techniques to monitor plant health. Biochim Biophys Acta. 2001;1519:153–166.
- Hu R, Sun K, Su X, et al. Physiological responses and tolerance mechanisms to Pb in two xerophils: salsola passerina Bunge and Chenopodium album L. J Hazard Mater. 2012;205:131–138.
- Langley-Turnbaugh S, Belanger L. Phytoremediation of lead in urban residential soils of Portland, Maine. Soil Horiz. 2010;51:95–101.
- Nie M, Wang Y, Yu J, et al. Understanding plant-microbe interactions for phytoremediation of petroleum-polluted soil. PLoS One. 2011;6:e17961.
- Islam E, Yang X, Li T, et al. Effect of Pb toxicity on root morphology, physiology and ultrastructure in the two ecotypes of Elsholtzia argyi. J Hazard Mater. 2007;147:806–816.
- Maiti SK, Jaiswal S. Bioaccumulation and translocation of metals in the natural vegetation growing on fly ash lagoons: a field study from Santaldih thermal power plant, West Bengal, India. Environ Monit Assess. 2008;136:355–370.
- Arshad M, Silvestre J, Pinelli E, et al. A field study of lead phytoextraction by various scented Pelargonium cultivars. Chemosphere. 2008;71:2187–2192.
- Manousaki E, Kalogerakis N. Phytoextraction of Pb and Cd by the Mediterranean saltbush (Atriplex halimus L.): metal uptake in relation to salinity. Environ Sci Pollut Res. 2009;16:844–854.
- Kumar A, Maiti SK. Translocation and bioaccumulation of metals in Oryza sativa and Zea mays growing in chromite-asbestos contaminated agricultural fields, Jharkhand, India. Bull Environ Contam Toxicol. 2014;93:434–441.
- Maestri E, Marmiroli M, Visioli G, et al. Metal tolerance and hyperaccumulation: costs and trade-offs between traits and environment. Environ Exp Bot. 2010;68:1–13.
- Li Z, Tang X, Chen Y, et al. Activation of Firmiana simplex leaf and the enhanced Pb (II) adsorption performance: equilibrium and kinetic studies. J Hazard Mater. 2009;169:386–394.
- Dumas P, Miller L. The use of synchrotron infrared microspectroscopy in biological and biomedical investigations. Vib Spectrosc. 2003;32:3–21.
- Hanafiah M, Zakaria H, Ngah WW. Preparation, characterization, and adsorption behavior of Cu (II) ions onto alkali-treated weed (Imperata cylindrica) leaf powder. Water Air Soil Pollut. 2009;201:43–53.
- Wolkers WF, Oliver AE, Tablin F, et al. A Fourier-transform infrared spectroscopy study of sugar glasses. Carbohydr Res. 2004;339:1077–1085.
- Ng CC, Boyce AN, Rahman MM, et al. Phyto-evaluation of Cd-Pb using tropical plants in soil-leachate conditions. Air Soil Water Res. 2018;11:1178622118777763.
- Tang Y-T, Qiu R-L, Zeng X-W, et al. Lead, zinc, cadmium hyperaccumulation and growth stimulation in Arabis paniculata Franch. Environ Exp Bot. 2009;66:126–134.
- Yang S, Liang S, Yi L, et al. Heavy metal accumulation and phytostabilization potential of dominant plant species growing on manganese mine tailings. Front Environ Sci Eng. 2014;8:394–404.
- Hladun KR, Parker DR, Trumble JT. Cadmium, copper, and lead accumulation and bioconcentration in the vegetative and reproductive organs of Raphanus sativus: implications for plant performance and pollination. J Chem Ecol. 2015;41:386–395.