ABSTRACT
Pesticides are a gift to agriculture for its flourish of crop production. For production of heavy grown crops, growing multiple crops in a season or enriching the soils for better productivity, pesticides are a mandate in modern world to meet the food requirement. However, controversy arises on the fact of overuse and the underlying problems associated with it. The problems include environmental contamination, public health compromisation, disturbance in the ecological niches, long-term persistence in soil and water bodies, etc. Pesticide residues present in the foods or crops may get circulated by bioamplification through the food chains. Therefore, it is imperative to detect the pesticides present in the environment and decontaminate the same by degradation to maintain the safety of environment. This review outlines the recent strategies of various pesticide detection techniques and discusses the overview of degradation strategies worked on for removal of pesticides from the environment.
1. Introduction
Pesticides started playing a pervasive role in modern agriculture evolution as early as 1950. The last century have observed a surge in pesticide utilization in benefit of modern agricultural practices, promoting improved crop production by managing the insects and diseases, which potentially caused harm to the crops [Citation1]. Escalation of human population has wiped away several hectares of cultivable lands and made it necessary for escalating the food production, which in turn lead to further surge in pesticide utilization. This is the point which raised the question about public health and environment safety and soon it emerged as a matter of concern. The pesticides not only degrade surface soil quality but also affect biodiversity. Pesticides covered soil organic matter is less mobile and is difficult for degradation. Pesticides can enter the food chain by bioamplification and hence disturbs the tropic levels. Pesticides may also percolate into the ground water table. Level of ground water, topographical outline and agriculture practices affect the pollution rate [Citation2]. Fatal dose of intoxication may also lead to cancers and death of individuals [Citation3]. There is often non-specific pesticide interference, which can lead to loss of biodiversity [Citation4]. Another aspect of negative impact of pesticide is air pollution, which is comparatively less noted [Citation2]. The weather conditions like humidity and temperature decide the degree of evaporation of pesticides. Some are short existing, whereas some are quite persistent in nature. These contaminants may intrude the environment by water cycle or may simply remain as air particulate matter. The risk associated with pesticides to human mostly depends on the extent of exposure [Citation5]. Based on that, pesticides may leave short-term effects like skin itching, eye irritation, dizziness, etc., or may lead to fatal long-term effects like cancer, breathing difficulties, ulcers, blood-related issues, neurological disabilities or even infertility [Citation6]. In connection to these, ‘cancer village’ terms became quite popular. Cancer villages are those villages, which are having high cancer morbidity rate than normal standard due to their unintentional occupational threat in exposure to harmful pesticide pollutants [Citation7].
The utilized pesticide residues or treated residual compounds are sometimes toxic too. The residues are mostly transformed products of the ‘Parent’ pesticides compounds, which are recalcitrant in nature for several decades [Citation8]. Pesticides may follow several pathways after their release into the environment, which may include transformation/degradation, sorption-desorption, volatilisation or is taken up by plants [Citation9]. These may also get carried to the water bodies or may get leached into ground water table leading to contamination [Citation10].
Thus, besides restricting the pesticide utilization, it is requisite to develop strategies to detect and quantify these necessary harmful compounds as well as lessen the toxic intensity or to remove the residual pesticide from the soil. This review portrays a cumulative report of recent researches and updates on various pesticide detection and degradation techniques. This discusses various approaches of quantification and detoxification of pesticide traces in the environment. It also gives a brief account of the detailed principles, detection efficiency, advantages and disadvantages of the advanced technologies used. The comprehensive compilation and multi-directional coverage of detection as well as degradation methods can support a quick overview of the current strategies blooming in this field.
2. Overview of pesticides in the environment
Pesticides are often toxic chemicals, mixtures of chemicals or bio-agents, which are purposely incorporated in the environment to curb the attack of pests, rodents and control plant diseases of microbial origin. Pesticides are available in several forms like powder, spray, granules, dust or cakes. However, agrochemical-based pesticides are the most widely used variety among all and its inadequate use is a major point of concern.
Classification of pesticides are quite complex. Pesticides can be a collective term which may include classes like insecticides, fungicides [Citation11], rodenticides, fumigants and insect repellents. All these groups broadly differ in their physical and chemical properties as well as mode of their activity [Citation12]. Besides this, it can also be classified into broader categories of organic and inorganic classes. Depending on functional group present in the parent structure of pesticides, it can be categorized as organophosphorous, organochlorine, carbamates, triazines and phenoxy subclasses. Although different pesticides have exclusively different action course, however, pesticides belonging to similar classes manifests similar activity, intoxication intensity, recalcitrance duration and responds to similar removal treatments [Citation13]. below lists the broad outline of pesticides classification.
Table 1. Classification of pesticides.
Different pesticides have different levels of persistence in nature. Soil adsorption coefficient and half-life of the pesticides determines whether it will be non-persistent, moderately persistent or long-term persistent. Pesticides with less soil adsorption coefficient adhere less with the soil organic particles and tends to leach. However, if the half-life is less, the toxic tendency can be considered minimal as it will probably degrade and mineralize within less time span. Thereby, remains in the environment for a very short time and has least capacity to become a biohazard. lists few commonly used pesticides stating their half-life and soil adsorption coefficient. According to Kerley et al., less than 30 days of half-life can be denoted as non-persistent and greater than 100 days can be categorized to be highly persistent [Citation16].
Table 2. Persistence and leaching ability of some common pesticides.
3. Detection of pesticides
Pesticides are necessary in development of agriculture. But there are certain limits that have been assigned for its usage by the authorized bodies. Beyond that limit, it is a threat to the nature and environmental components. The remediation process is at its bloom on this issue. However, it is equally necessary to detect the residual pesticide for proper detoxification of the targeted area. Some pesticides like atrazine, triazines are poorly absorbed by the soil due to their chemical structure and remains persistent leading to hazards [Citation18].
The traditional methods for pesticide determination generally follow the techniques like chromatography coupled with mass spectroscopy (MS), simple gas chromatography (GC) or high performance liquid chromatography (HPLC) [Citation19]. Although all these processes produce elaborate and nearly accurate result, however, conventional existing procedures of pesticide detection are often impractical in the field usage. Additionally, the constraint is also unavoidable as complex pre-treatments and cost-inducing instruments are required for the analysis including expertise for handling of the same [Citation20].
Currently, nanoparticle-mediated pesticide detection is a recent approach that is gaining stature. The nanoparticles have a wide spectrum of flexibility in stability, compatibility and sensitivity, which are easily modifiable. Nanoparticle-based pesticide detection is mostly annexed with electrochemical, antibody or enzyme immobilized sensors [Citation21] or grooved with fluorophores for fluorescence, chemiluminescence or surface-enhanced Raman spectroscopy based analysis. Modified silica nanoparticle (SiNP) is mentioned to be used as a mode of ‘direct detection’ of pesticide [Citation22]. Some of the recent advanced detection methods have been discussed in subsequent sections.
3.1. Electrochemical detection of pesticides
The conventional methods of field sampling or chromatographic detection procedures undoubtedly provides explicit results [Citation23]; however, outcome may confront dispute in cases of sources like water bodies which is dynamic.
Electrochemical sensing determines the target by measuring charge, current or potential, which occurs as an interaction between the targeted analyte and the sensing electrode. The three basic components of electrochemical sensing device are working electrode, counter electrode and reference electrode. The detection sensors can be based on direct-detection for electroactive analytes, enzymatic detection, immunosensors or microbial (biological) sensors depending on the suitability. Qiu et al. have detected organo-phosphate pesticide (OP) using the same method by targeting p-nitrophenol group using TiO2-amino acid conjugate. The range of detection was approximately 0.2 µM [Citation24]. Gold, copper, carbon and mercury electrode have been successfully used to detect glyphosate. In general, electrochemical techniques, bulk volume of samples and complex instrumentation contributed to the unsuitability for using the application in field samples. Development of screen printed electrodes (SPE) helped to overcome these limitations. Made of plastic or ceramic plates and imprinted with gold, silver, carbon or graphite ink, these SPEs are more advanced in terms of analytical value [Citation25]. Current upgradation is being scratched on immobilized enzyme or antibody with affinity towards particular pesticides [Citation26], selection of suitable electrode of different nanomaterials [Citation27]. Special emphasis is also focused on a specialized process called Molecular Imprinted Polymer (MIP), which creates a negative embossing on electrode surface enhancing the selectivity and specificity towards particular pesticide for more perfect detection [Citation28]. MIP is also used using to detect glyphosate using graphite electrode with gold nanoparticle imprinting.
Electrochemical sensors generally uses the change in current, electric potential or the resistance (electrical impedance) to detect the pesticides and the sensors are named accordingly. Electrochemical detection can be carried out by different voltammetric techniques like cyclic voltammetry, differential pulse voltammetry, square wave voltammetry, by amperometry or by electrochemical impedance spectroscopy [Citation25,Citation29]. The sample preparation for this technique is simple. Generally pesticide-spiked samples are applied on biosensors to detect the signals. Drechsel et al. designed an auto-dip biosensor to detect chlorpyrifos pesticide in food samples [Citation30]. Food sample like apple were cut fresh, homogenized with buffer and spiked with the selected pesticide. The signal was estimated by the current peaks. Some electrochemical detection techniques are listed in , which are drawn from different literature review.
Table 3. List of some successful electrochemical detection of pesticide.
However, the electrochemical sensors have some flaws like high maintenance and operating cost. Sometimes low electroactivity analytes like glyphosate may produce poor output [Citation40]. Moreover, sample pre-treatment in few cases, interference from co-existing compounds or pollutants in the targeted field sample often alters the desired result. Inspite of these drawbacks, electrochemical is one of the widely detection technique for pesticide even in field samples. It is efficiently fast, reliable, accurate and selective enough with minimal sample preparation compared to tedious traditional chromatographic techniques [Citation41]. Currently, improvisations with molecular imprinted electrodes, incorporation of nanotechnology are subjected to broader interest, which can be expected to overcome the flaws in terms of electrode fabrication, sample stability and continuous assessment of field samples.
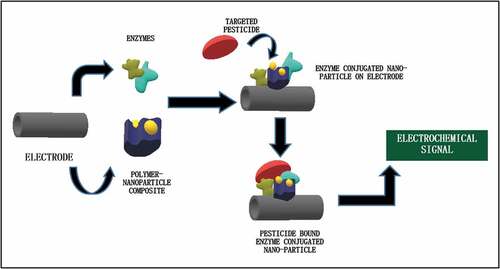
3.2. Enzyme conjugated nanoparticle based detection
Pesticides are developed for controlling pests by intending the inhibition of the functional enzymes present in the organism. The enzymes mostly include acetylcholinesterase, alkaline or acid phosphatases, organophosphate hydrolase, butyrylcholinesterase, tyrosinase, aldehyde dehydrogenases, etc [Citation41]. Thus, sensors are developed utilizing these enzymes. The enzyme-conjugated detectors mostly work by either inhibition mechanism or catalytic mechanism [Citation42]. Inhibition mechanism can be used for pesticide quantification on basis of the inhibited enzyme activity in presence or absence of the targeted analyte. However, the catalytic approach of enzyme-conjugated sensors uses the analyte directly as substrate. The result is interpreted on the fact that the extent of interaction is directly proportional to the concentration of pesticide in the sample [Citation43]. demonstrates the basic concept of enzyme conjugated nanoparticle-based pesticide detection.
Enzymatic sensors have promising potential in pesticide detection. However, fabrication of the micro-reactors is necessary for up-gradation of analytical efficiency. Enzyme immobilization plays a crucial part in this process. A summarized account of various enzyme immobilization processes is discussed in .
Table 4. List of some enzyme immobilization systems.
lists some of the successful works using this technique. The enzymes are often immobilized with different polymeric matrices by covalent bonding encapsulation, entrapment or by adsorption [Citation51]. As discussed earlier, nanoparticles are greatly modifiable. So enzyme substrates are coupled on nanoparticles to give stability for developing the sensor. In the presence of pesticide, the enzymes execute catalytic activity to generate hydrogen ion, which modulate the conductivity of the medium [Citation22,Citation52]. This strategy can correspond with the detection and quantification of the pesticide aided with different electrodes to facilitate the process. The conventional electrodes are not very appropriate for field utility. However, recent technology of screen-printed electrodes (SPE) expedites the detection method as well as aids to the field utility due to easy handling [Citation25].
Table 5. List of some enzyme conjugated nanoparticle-mediated pesticide detection.
In recent studies, microchip-based enzymatic sensors improvised with nanoparticles have opened a wide scope in sensor development[125]. These newly developed sensors are exclusively beneficial in terms of fast and accurate result, portability, field application and automation.
3.3. Fluorescence-driven pesticide detection
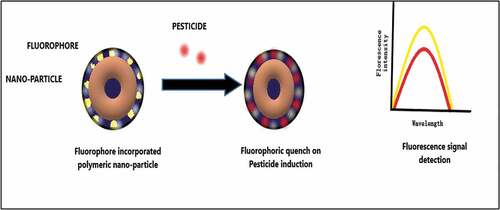
Fluorescence-driven detection of pesticide is an optical approach in identifying the presence of pesticide and has gained wide acceptance. Inert biosensors are designed for this purpose, where pesticide detection is determined by virtue of enhancement or quenching of fluorescence upon a specific pesticide interaction [Citation22]. The mode of action is shown in .
A good number of recent works have been reported on fluorescence-driven detection of pesticide. Enzyme-based sensors have been used to detect and study the interactions of different organophosphate pesticides. Esterase 2 from Alicyclobacillus acidocaldarius was used for the same. Additionally, fluorescence-based polarity-sensitive probe was also consolidated to study interactions [Citation56]. Wang et al. studied on a mixture of carbamate pesticides using 3D-fluorescence spectroscopy based on Genetic Algorithm optimized Back Propagation network model (GA-BP) [Citation13], indole-based biosensor for organophosphate detection [Citation57], alkaline-phosphatase triggered enzyme-based fluorescence detection method of organophosphorus pesticides [Citation58] are some of the recent works can be stated as examples of successful application of this technique.
3.4. Surface-enhanced Raman Spectroscopic analysis for pesticide detection
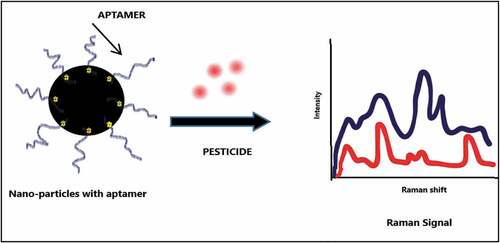
A recent mode developed for pesticide detection is Surface-Enhanced Raman Spectroscopic (SERS) method. SERS method is technically the amalgamation of nanotechnology and Raman spectroscopy [Citation59]. The detection method works on the basis of enhancement in the Raman signals. This is achieved by chemisorption of target pesticide on nano-metal substrate. When struck by laser light, the electronic state of the complex changes to new absorption frequency, thereby resonating with the frequency of laser excitation (surface plasmon), and there is a shift in Raman signal [Citation60]. The basic function mechanism is depicted in .
Surface-Enhanced Raman Spectroscopic analysis is a non-invasive detection tool and advantageous in terms of no unnecessary time consumption, in-situ sample preparation, field applicability, simple reagents usage and high sensitivity [Citation59]. Interference from other pesticides or complexes present in samples [Citation61], presence of similar compound like target analyte [Citation62], operating with a Raman instrument with disparate configuration of laser intensity, spectral properties, wavelength may lead to disputed result [Citation63]. Moreover, non-specific binding occurring due to non-target adsorption on SERS substrate surface generates false signals, background disturbance and reduced tagging-specificity [Citation64]. Amalgamating or functionalizing target molecule with aptamers, MIPs, antibodies facilitate the specificity and generates accuracy in analysis [Citation62].
Often some analytes like organophosphate pesticides show a very weak SERS spectrum due to their hydrophobic structure and have a very less affinity towards SERS substrates [Citation65]. This leads to feeble SERS signal. There are recent upgradations developed to detect these types of complex samples or compounds with low SERS substrate affinity. The quality of SERS substrates used may enhance the signal to a desired level. Metal nanoparticles of gold, silver and copper are considered as very good SERS substrate as they are easy to synthesize, stable and efficient in generating SERS signal. The shape, size and morphology of these nano-structures determine the extent of enhancement for the signal produced [Citation66]. Nanostructures with sharp morphological features serves as better substrate compared to the smooth featured nano-structures. The signal production is better when analyte and the substrate are at the maximum proximity. To obtain this, functionalization of the metal surface or metallic organic frameworks (Hybrid crystalline substance) is done, which brings the target analyte closer and generates enhanced SERS signal [Citation67]. Sometimes, trace or weakly-affined samples are pre-prepared by extracting and evaporating to gain high concentration, thus obtaining a prominent SERS signal [Citation65]. enlists some successful SERS enhanced strategies for pesticide detection.
Table 6. List of some enhanced SERS-based pesticide detection.
Interaction study of chlorpyrifos and thiabendazole using gold nano-finger [Citation74], 2,4-dichlorophenoxyacetic acid (2,4-D), pymetrozine and thiamethoxam were detected on food surface using mesoporous silica supported gold nanoparticles by Xu et al. [Citation75], cypermethrin and esfenvalerate were detected by Xiali et al. using this technique effectively [Citation66] are some of the recent works reported.
Different detection technologies that have been discussed so far have specific purpose, benefit and drawbacks. These are being constantly upgraded and studied to achieve more precise detection. Some common advantages and disadvantages for the detection techniques are compiled in .
Table 7. Advantages and disadvantages of common pesticide detection techniques.
4. Degradation strategies of pesticide elimination
Degradation of pesticides is a very complex interaction between different systems including soil and pesticide melding, physical factors, biological factors and types of pesticides. The extent of interlinking and sorption rate between the soil particle and pesticide decides their persistent nature depending on the characteristic feature of pesticide. The mode of action varies from different groups of pesticides and even within same group with similar structures. Degradation is highly influenced by soil type, pH, organic carbon content in soil and bioactivity of microbial community [Citation78]. There are several techniques developed to degrade and efface the pesticide contaminant from environment, which are discussed in the following sections.
4.1. Physical degradation
The two important factors that contribute mostly in physical degradation of pesticide process is light and temperature. The basic physical process includes photolysis. Photolytic degradation is critical for organic compounds. Low freezing temperatures are used sometimes to aid in pesticide degradation [Citation79]. Thermal desorption at low temperatures of 300 F to 1000 F is a efficient technique in physical degradation. This works by volatilizing the pesticides with further treatment of contaminated gas stream extruded in a separate burner to immobilize the targeted pollutants. However, the system is quite costly and requires advanced facilities. Another common process of physical degradation is incineration. Incineration completely destroys the organic contaminants by two phase heating instead of deactivating the contaminants [Citation80].
4.2. Chemical degradation
The reactive components present in pesticides are targeted for chemical degradation. The process may involve simple amendment of pH, which may ease out in detoxification of pH-sensitive components. Some reactive oxygen forms like ozone, peroxide and superoxide also aids in chemical degradation [Citation79]. Chemical degradation process of pesticide exclusion can be quite expensive depending upon the course of treatment. Generally, the contaminated samples are pre-treated and processed chemically to obtain the less toxic intermediate compounds, which are potentially less harmful to environment. In many cases, chemical degradation is collaborated with physical degradation treatments to get the desired results. The following table explains some techniques involved in chemical degradation process of pesticides.
Advanced oxidation process (AOP) is a very common technique, which works by oxidation of almost all type of organic substrate [Citation81]. Hydroxyl radical plays the crucial role in the process. Highly reactive free hydroxyl radical reacts either with molecular oxygen forming peroxyl radical and generates a stream of oxidation reaction or pounce the halogen containing aromatic rings of the pesticides to finally mineralize to produce H2O and CO2. UV-mediated degradation involves enhancement of AOP reactions in presence of strong and controlled UV light exposure on the pollutants [Citation82]. This is mostly suitable for treating pollutants in aqueous system. High energy UV radiations help to loosen the chemical bonds, thus facilitating the oxidation reaction stream. This process can be UV-peroxide (H2O2) or UV-ozone system. Although a very effective technique, still turbidity and UV wavelength might interfere the efficiency. Sometimes reducing agents like zero-valent iron (Fe0) are used to reduce the pesticide pollutants. This technique can be employed in soil as well as for aquatic systems. However, efficiency of Fe0 decreases with time as the active sites gets blocked due to formation of oxide layer. Successful mineralization was obtained by combination of Fe0 with magnetite (Fe3O4), aluminium sulfate (Al2(SO4)3) or acetic acid (CH3COOH) [Citation83,Citation84]. Additionally, some catalysts (semiconductor oxides) like titanium dioxide and zinc oxide are used along with UV, to overcome the drawback of time consumption by the processes stated earlier in this section [Citation85]. Moreover, these metal oxides being inert in nature, cumbers less to the environment safety.
4.3. Nanoparticle-mediated degradation
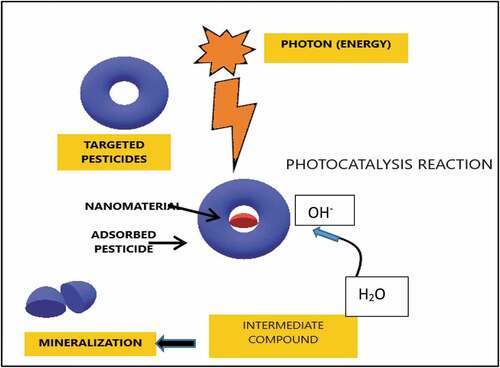
Nanoparticle-mediated pesticide degradation is a promising technology which will have a great potential in nullifying the toxic effects of recalcitrant compounds from environment [Citation4]. Photocatalysis is the basic backbone of this method. It basically involves high-tech oxidative process in presence of photo energy leading to mineralization of hydrocarbon [Citation86]. This technology involves different photocatalytic reactor systems where the photocatalysts are applied either in suspended or immobilised form [Citation87]. Kanan et al. have discussed about TiO2 as a photocatalyst, which can be utilized in pure form or doped with some other components and was reported to be an excellent candidate for degradation of a wide spectrum of pesticides [Citation88]. Recent explorations are done on these doping materials, which consist of different nanoparticles. Nanoparticles have raised a great response as it bears special features with large specific surface area, little resistance for diffusion, high capacity of adsorption and rapid adsorption equilibrium [Citation89,Citation90].
Nanoparticles like gold (Au) and silver (Ag) have colossal response for photocatalytic activity [Citation91]. Their optical and electronic properties get modulated to a wide extent by alteration of the size, shape and surface charge [Citation92]. A simplified pictorial representation of the basic nanoparticle-mediated remediation system is shown in . According to recent reports, gold nanoparticles (AuNPs) supported onto TiO2, leads to visible illumination due to photocatalysis as the organic compounds in aqueous environment gets oxidized [Citation93]. shows a list of the nanoparticles usage against various pesticides degradation.
Table 8. Nanoparticle-mediated pesticide degradation.
However, this method of pesticide degradation is relatively new and complex compared to other techniques. Polymeric, inorganic or carbonaceous nanoparticles are widely being researched. Suitability of the same has not been tallied in actual field condition [Citation94]. There are only scarce work existing due to arduous work of delineating and preparation of nanoparticles. Additionally, exhilarating the photocatalytic reaction, often UV light is used, which may lead to detrimental condition to researcher without proper care.
4.4. Microbe-assisted degradation
Microbial community of a particular area plays major role in maintaining the environment quality [Citation104]. Researchers have explored a wide array of microbes isolated from different sources that were potent enough to degrade toxic pollutants from the soil and water. However, there are both pros and cons for this degradation process. Although it is cost-effective and bears almost negligible side effect on environment apparently [Citation79], it may also produce some more toxic and persistent metabolite in course of degradation. Additionally, the microbes incorporated into the soils often shows less viability in actual environment condition due to several physical parameters [Citation105]. There are several factors affecting the microbe-assisted pesticide degradation.
The type of micro-organism employed for the purpose, their metabolic pattern and their extent of adaptation to the tainted condition [Citation106].
The chemical skeleton of the targeted pesticide, which includes molecular weight, presence of functional groups, nature of bio-available residual component (Complex polymer composites or simple compounds) determines the degradation of pesticides by microbes [Citation107].
Micro-organisms generally degrade the complex compounds by their enzymatic machinery, the proper functioning of which depends on temperature, pH, nutrient and substrate availability, balanced C: N: P in the locale [Citation108].
shows a list of microbe succored pesticide degradation and the extent of their efficiency.
Table 9. Degradation efficiency of some potential microbes.
4.5. Enzymatic degradation
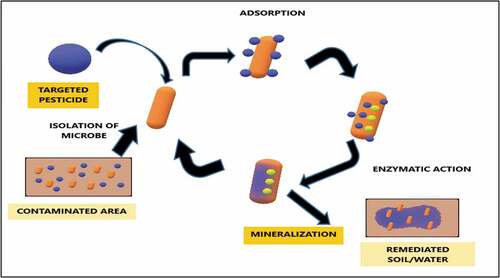
Another current approach to break down toxic pesticides in most natural and congenial way is the implication of microbial enzymes in the process. Unlike the technological methods, which often involve a huge cost and mostly inefficient in real-time utility, this microbial enzyme detoxification is way more efficient. This enzymatic degradation was found suitable in both soil and aquatic environment. The schematic representation of the enzymatic action is represented in . Additionally, soil fertility enhancement, crop growth and nutrient richness were improved as reported. Some of the recent reports are listed in .
Table 10. Enzymatic strategies of pesticide biodegradation.
5. Conclusion
This review covers the recent spectra from the research fields of detection and degradation of pesticides. The various detection techniques with their simple working principles, comparative analyses are summarized. Researchers are focusing on the improvisation and fabrication of sensors for upgradation and accuracy. The detection sensors should be upgraded in terms of sensitivity and specificity annexing femto level detection. Advancements can be designed by combining multiple technologies, hence minimizing the limitations behind each technique. Currently, integrative technologies are coming up like combination of smartphone and electrochemical sensors for real-time monitoring. Sufficient literature exists for degradation strategies too. However, detailed metabolic pathways need to be explored for advanced monitoring and detoxification of pesticides from environment. The intermediate degradation products and compound kinetics should be explored more to determine the pathway of elimination of pesticide from the environment. Accurate detection techniques, followed by precise degradation, may help in solving the problems created by unavoidable pesticide usage. Beside all these scientific commodities, a proper legislative control and awareness can lead to the eradication of this pesticide pollution[Citation122Citation123.
Acknowledgments
The authors thank Vellore Institute of Technology, Vellore for providing the necessary support and encouragement.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
References
- Ali, N., Khan S, Khan, M.A., Waqas, M., and Yao, H.,2019. Endocrine disrupting pesticides in soil and their health risk through ingestion of vegetables grown in Pakistan. Environmental Science and Pollution Research. 26(9), pp.8808-8820.
- Singh, N.S., Sharma, R, Parween, T. and Patanjali, P.K., 2018. Pesticide contamination and human health risk factor. In: Modern age environmental problems and their remediation.pp. 49-68. Springer, Cham.
- Simonelli A, Basilicata P, Miraglia N, et al. Analytical method validation for the evaluation of cutaneous occupational exposure to different chemical classes of pesticides. J Chromatogr B. 2007;860(1):26–33.
- Rani L, Thapa K, Kanojia N, et al. An extensive review on the consequences of chemical pesticides on human health and environment. J Clean Prod. 2021;283:124657.
- Kim KH, Kabir E, Jahan SA. Exposure to pesticides and the associated human health effects. SciTotal Environ. 2017;575:525–535.
- Sabarwal A, Kumar K, Singh RP. Hazardous effects of chemical pesticides on human health–Cancer and other associated disorders. Environ Toxicol Pharmacol. 2018;63:103–114.
- Lu Y, Song S, Wang R, et al. Impacts of soil and water pollution on food safety and health risks in China. Environ Int. 2015;77:5–15.
- Erinle KO, Jiang Z, Ma B, et al. Exogenous calcium induces tolerance to atrazine stress in Pennisetum seedlings and promotes photosynthetic activity, antioxidant enzymes and psbA gene transcripts. Ecotoxicol Environ Saf. 2016;132(Supplement C):403–412.
- Chowdhury A, Pradhan S, Saha M, et al. Impact of pesticides on soil microbiological parameters and possible bioremediation strategies. Ind J Microbiol. 2008;48(1):114–127
- Rosenbom S, Costa V, Alaraimi N, et al. Genetic diversity of donkey populations from the putative centers of domestication.[J]. Anim Genet. 2015;46(1):30–36.
- Andleeb S, Jiang Z, Rehman K, et al. Influence of soil pH and temperature on atrazine bioremediation. Journal of Northeast Agricultural University (English Edition). 2016;23(2):12–19.
- Kaur R, Mavi GK, Raghav S, et al. Pesticides classification and its impact on environment. International Journal of Current Microbiology and Applied Sciences. 2019;8(3):1889–1897.
- Wang DZ, Kong LF, Li YY, et al. Environmental microbial community proteomics: status, challenges and perspectives. Int J Mol Sci. 2016;17(8):1275.
- Yadav IC, Devi NL. Pesticides classification and its impact on human and environment. Environmental science and engineering. 2017;6:140–158.
- Bilal M, Iqbal HM, Barceló D. Persistence of pesticides-based contaminants in the environment and their effective degradation using laccase-assisted biocatalytic systems. SciTotal Environ. 2019;695:133896.
- Kerle E.A., Jenkins, J.J, and Vogue, P.A. (2007). Understanding Pesticide Persistence and Mobility for Groundwater and Surface Water Protection. Oregon State University Extension Services, EM8561-E.
- Deer HM. Pesticide adsorption and half-life. AG/Pesticides. 1999;15:1.
- Zacco E, Pividori MI, Alegret S, et al. Electrochemical magnetoimmunosensing strategy for the detection of pesticides residues. Anal Chem. 2006;78(6):1780.
- Alcantara-Duran J, Moreno-Gonzalez D, Gilbert-Lopez B, et al. Matrix-effect free multi-residue analysis of veterinary drugs in food samples of animal origin by nanoflow liquid chromatography high resolution mass spectrometry. Food Chem. 2018;245:29–38.
- Jia M, Zhai F, Bing X. Rapid multi-residue detection methods for pesticides and veterinary drugs. Molecules. 2020;25(16):3590.
- Van Dyk JS, Pletschke B. Review on the use of enzymes for the detection of organochlorine, organophosphate and carbamate pesticides in the environment. Chemosphere. 2011;82(3):291–307.
- Bapat G, Labade C, Chaudhari A, et al. Silica nanoparticle based techniques for extraction, detection, and degradation of pesticides. Adv Colloid Interface Sci. 2016;237:1–14.
- Bhadekar R, Pote S, Tale V, et al. Developments in analytical methods for detection of pesticides in environmental samples. American Journal of Analytical Chemistry. 2011;2(8):1.
- Qiu L, Lv P, Zhao C, et al. Electrochemical detection of organophosphorus pesticides based on amino acids conjugated nanoenzyme modified electrodes. Sens Actuators B Chem. 2019;286:386–393.
- Pérez-Fernández B, Costa-García A, Muñiz ADLE. Electrochemical (bio) sensors for pesticides detection using screen-printed electrodes. Biosensors (Basel). 2020;10(4):32.
- Anu Prathap MU, Chaurasia AK, Sawant SN, et al. Polyaniline-based highly sensitive microbial biosensor for selective detection of lindane. Anal Chem. 2012;84(15):6672–6678.
- Anu Prathap MU, Sun S, Wei C, et al. A novel non-enzymatic lindane sensor based on CuO–MnO2 hierarchical nano-microstructures for enhanced sensitivity. Chemical Communications. 2015;51(21):4376–4379.
- Rahemi V, Garrido JMPJ, Borges F, et al. Electrochemical determination of the herbicide bentazone using a carbon nanotube β-cyclodextrin modified electrode. Electroanalysis. 2013;25:2360–2366.
- Kalyani, N., Goel, S., and Jaiswal, S., 2021. On-site sensing of pesticides using point-of-care biosensors: a review. Environmental Chemistry Letters, 19(1), pp.345-354.
- Drechsel L, Schulz M, von Stetten F, et al. Electrochemical pesticide detection with AutoDip–a portable platform for automation of crude sample analyses. Lab Chip. 2015;15(3):704–710.
- Kumaravel A, Vincent S, Chandrasekaran M. Development of an electroanalytical sensor for γ-hexachlorocyclohexane based on a cellulose acetate modified glassy carbon electrode. Anal Methods. 2013;5(4):931–938.
- Fayemi OE, Adekunle AS, Ebenso EE. A sensor for the determination of lindane using PANI/Zn, Fe(III) Oxides and Nylon 6,6/MWCNT/Zn, Fe(III) oxides nanofibers modified glassy carbon electrode. J Nanomater. 2016;2016:1–10.
- Beland, F. A., Farwell, S. O., Robocker, A. E., and Geer, R. D., 1976. Electrochemical reduction and anaerobic degradation of lindane. Journal of Agricultural and Food Chemistry, 24(4), pp.753-756.
- Songa EA, Somerset VS, Waryo T, et al. Amperometric nanobiosensor for quantitative determination of glyphosate and glufosinate residues in corn samples. Pure Appl Chem. 2009;81(1):123–139.
- Sánchez-Bayo F, Hyne RV, Desseille KL. An amperometric method for the detection of amitrole, glyphosate and its aminomethyl-phosphonic acid metabolite in environmental waters using passive samplers. Anal Chim Acta. 2010;675(2):125–131.
- Noori JS, Mortensen J, Geto A. Recent development on the electrochemical detection of selected pesticides: a focused review. Sensors. 2020;20(8):2221.
- Coutinho CFB, Coutinho LFM, Mazo LH, et al. Copper microelectrode as liquid chromatography detector for herbicide glyphosate. Electroanalysis. 2007;19(11):1223–1226.
- Méndez MA, Súarez MF, Cortés MT, et al. Electrochemical properties and electro-aggregation of silver carbonate sol on polycrystalline platinum electrode and its electrocatalytic activity towards glyphosate oxidation. Electrochem Commun. 2007;9(10):2585–2590.
- Do MH, Florea A, Farre C, et al. Molecularly imprinted polymer-based electrochemical sensor for the sensitive detection of glyphosate herbicide. Int J Environ Anal Chem. 2015;95(15):1489–1501.
- Cahuantzi-Muñoz SL, González-Fuentes MA, Ortiz-Frade LA, et al. Electrochemical biosensor for sensitive quantification of glyphosate in maize kernels. Electroanalysis 2019;31(5):927–935.
- Noori JS, Dimaki M, Mortensen J, et al. Detection of glyphosate in drinking water: a fast and direct detection method without sample pretreatment. Sensors. 2018;18(9):2961.
- Arduini F, Cinti S, Scognamiglio V, et al. Nanomaterials in electrochemical biosensors for pesticide detection: advances and challenges in food analysis. Mikrochim Acta. 2016;183(7):2063–2083.
- Lee JH, Park JY, Min K, et al. A novel organophosphorus hydrolase-based biosensor using mesoporous carbons and carbon black for the detection of organophosphate nerve agents. Biosens Bioelectron. 2010;25(7):1566–1570.
- Aydogan A. Boronic acid-fumed silica nanoparticles incorporated large surface area monoliths for protein separation by nano-liquid chromatography, Anal. Bioanal Chem. 2016;408(29):8457–8466
- Ahmed M, Yajadda MMA, Han Z, et al. Single-walled carbon nanotube-based polymer monoliths for the enantioselective nano-liquid chromatographic separation of racemic pharmaceuticals. J Chromatogr A. 2014;1360:100–109.
- Zhao K, Fu W, Qiu Q, et al. Spatial patterns of potentially hazardous metals in paddy soils in a typical electrical waste dismantling area and their pollution characteristics. Geoderma. 2019;337:453–462.
- Yin Z, Zhao W, Tian M, et al. A capillary electrophoresis-based immobilized enzyme reactor using graphene oxide as a support via layer by layer electrostatic assembly. Analys.t. 2013;139(8):1973–1979.
- Weiser D, Bencze LC, Bánóczi G, et al. Phenylalanine ammonia-lyase-catalyzed deamination of an acyclic amino acid: enzyme mechanistic studies aided by a novel microreactor filled with magnetic nanoparticles. ChemBioChem. 2015;16(16):2283–2288.
- Gong A, Zhu CT, Xu Y, et al. Moving and unsinkable graphene sheets immobilized enzyme for microfluidic biocatalysis. Sci Rep. 2017;7(1):1–15.
- Evans D, Gabriel EFM, Benavidez TE, et al. Modification of microfluidic paper-based devices with silica nanoparticles. Analyst. 2014;139(21):5560–5567.
- Anitha K, VenkataMohan S, JayaramaReddy S. Development of acetylcholinesterase silica sol-gel immobilized biosensor-an application towards Oxydemeton methyl detection. Biosens Bioelectron. 2004;20(4):848–856.
- Dhull V, Gahlaut A, Dilbaghi N, et al. Acetylcholinesterase biosensors for electrochemical detection of organophosphorus compounds: a review. Biochem Res Int. 2013;2013:1–18.
- Yang L, Wang GC, Liu YJ, et al. Development of a stable biosensor based on a SiO2 nanosheet–Nafion–modified glassy carbon electrode for sensitive detection of pesticides. Anal Bioanal Chem. 2013;405(8):2545–2552.
- Du D, Chen S, Cai J, et al. Immobilization of acetylcholinesterase on gold nanoparticles embedded in sol-gel film for amperometric detection of organophosphorous insecticide. Biosens Bioelectron. 2007;23(1):130–134.
- Luckham RE, Brennan JD. Bioactive paper dipstick sensors for acetylcholinesterase inhibitors based on sol–gel/enzyme/gold nanoparticle composites. Analyst. 2010;135(8):2028–2035.
- Carullo P, Cetrangolo GP, Mandrich L, et al. Fluorescence spectroscopy approaches for the development of a real-time organophosphate detection system using an enzymatic sensor. Sensors. 2015;15(2):3932–3951.
- Sun X, Xia K, Liu B. Design of fluorescent self-assembled multilayers and interfacial sensing for organophosphorus pesticides. Talanta. 2008;76(4):747–751.
- Dong J, Yang H, Li Y, et al. Fluorescence sensor for organophosphorus pesticide detection based on the alkaline phosphatase-triggered reaction. Anal Chim Acta. 2020;1131:102–108.
- Pang STR, Yang TX, He LL. Review of surface enhanced Raman spectroscopic (SERS) detection of synthetic chemical pesticides. TrAC-Trend. Anal Chem. 2016;85:73–82.
- Cialla D, März A, Böhme R, et al. Surface-enhanced 598 Raman spectroscopy (SERS): progress and trends. Anal Bioanal Chem. 2012;403(1):27–599 54.
- Vessman J, Stefan RI, Van Staden JF, et al. Selectivity in analytical chemistry (IUPAC Recommendations 2001). Pure Appl Chem. 2001;73(8):1381–1386.
- Bernat A, Samiwala M, Albo J, et al. Challenges in SERS-based pesticide detection and plausible solutions. J Agric Food Chem. 2019;67(45):12341–12347.
- Li DW, Zhai WL, Li YT, et al. Recent progress in surface enhanced Raman spectroscopy for the detection of environmental pollutants. Mikrochim Acta. 2014;181(1–2):23–43.
- Zhang YY, Wang XP, Pemer S, et al. Effect of antigen retrieval methods on nonspecific binding of antibody-metal nanoparticle conjugates on formalin-fixed paraffin-embedded tissue. Anal Chem. 2018;90(1):760–768.
- Moldovan R, Iacob BC, Farcău C, et al. Strategies for SERS detection of organochlorine pesticides. Nanomaterials. 2021;11(2):304.
- Li X, Yang T, Song Y, et al. Surface-enhanced Raman spectroscopy (SERS)-based immunochromatographic assay (ICA) for the simultaneous detection of two pyrethroid pesticides. Sens Actuators B Chem. 2019;283:230–238.
- Vikrant K, Tsang DCW, Raza N, et al. Potential utility of metal–organic framework-based platform for sensing pesticides. ACS Appl Mater. Interfaces 2018. 2018;10(10):8797–8817. ().
- Zhu C, Meng G, Zheng P, et al. A hierarchically ordered array of silver-nanorod bundles for surface-enhanced raman scattering detection of phenolic pollutants. Adv Mater. 2016;28(24):4871–4876.
- Mariño-Lopez A, Sousa-Castillo A, Blanco-Formoso M, et al. Microporous plasmonic capsules as stable molecular sieves for direct SERS quantification of small pollutants in natural waters. Chem Nanomater Energy Biol More. 2019;5:46–50.
- Zhou X, Zhao Q, Liu G, et al. Kinetically-controlled growth of chestnut-like au nanocrystals with high-density tips and their high SERS performances on organochlorine pesticides. Nanomaterials. 2018;8(7):560.
- Guerrini L, Izquierdo-Lorenzo I, Garcia-Ramos JV, et al. Self-assembly of α,ω-aliphatic diamines on Ag nanoparticles as an effective localized surface plasmon nanosensor based in interparticle hot spots. Phys Chem Chem Phys. 2009;11(34):7363–7371.
- Kubackova J, Fabriciova G, Miskovsky P, et al. Sensitive surface-enhanced Raman Spectroscopy (SERS) detection of organochlorine pesticides by alkyl dithiol-functionalized metal nanoparticles-induced plasmonic hot spots. Anal Chem. 2015;87(1):663–669.
- Qu Y, He L. Development of a facile rolling method to amplify an analyte’s weak SERS activity and its application for chlordane detection. Anal Methods. 2020;12(4):433–439.
- Kim A, Barcelo SJ, Li Z. SERS-based pesticide detection by using nanofinger sensors. Nanotechnology. 2014;26(1):15502.
- Xu Y, Kutsanedzie FY, Hassan M, et al. Mesoporous silica supported orderly-spaced gold nanoparticles SERS-based sensor for pesticides detection in food. Food Chem. 2020;315:126300.
- Umapathi R, Ghoreishian SM, Sonwal S, et al. Portable electrochemical sensing methodologies for on-site detection of pesticide residues in fruits and vegetables. Coord Chem Rev. 2022;453:214305.
- Hong T, Liu W, Li M, et al. Recent advances in the fabrication and application of nanomaterial-based enzymatic microsystems in chemical and biological sciences. Anal Chim Acta. 2019;1067:31–47.
- Kah M, Beulke S, Brown CD. Factors influencing degradation of pesticides in soil. J Agric Food Chem. 2007;55(11):4487–4492.
- Verma JP, Jaiswal DK, Sagar R. Pesticide relevance and their microbial degradation: a-state-of-art. Rev Environ Sci Bio/Technol. 2014;13(4):429–466.
- Parte SG, Mohekar AD, Kharat AS. Microbial degradation of pesticide: a review. Afr J Microbiol Res. 2017;11(24):992–1012.
- Chiron S, Fernandez-Alba A, Rodriguez A, et al. Pesticide chemical oxidation: state-of-the-art. Water Res. 2000;34(2):366–377.
- Marican A, Durán-Lara EF. A review on pesticide removal through different processes. Environ Sci Pollut Res. 2018;25(3):2051–2064.
- Shea PJ, Machacek T, Comfort S. Accelerated remediation of pesticide-contaminated soil with zerovalent iron. Environ Pollut. 2004;132(2):183–188.
- Shoiful A, Ueda Y, Nugroho R, et al. Degradation of organochlorine pesticides (OCPs) in water by iron (Fe)-based materials. J Water Process Eng. 2016;11:110–117. https://doi.org/10.1016/j.jwpe.2016.02.011.
- Cheng M, Zeng G, Huang D, et al. Hydroxyl radicals based advanced oxidation processes (AOPs) for remediation of soils contaminated with organic compounds: a review. Chem Eng J. 2016;284:582–598.
- Bai H, Zhou J, Zhang H, et al. Enhanced adsorbability and photocatalytic activity of TiO2-graphene composite for polycyclic aromatic hydrocarbons removal in aqueous phase. Colloids Surf B Biointerfaces. 2017;150:68–77.
- Ray S, Lalman JA. Fabrication and characterization of an immobilized titanium dioxide (TiO2) nanofiber photocatalyst. Mater Today Proc. 2016;3(6):1582–1591.
- Kanan S, Moyet MA, Arthur RB, et al. Recent advances on TiO2-based photocatalysts toward the degradation of pesticides and major organic pollutants from water bodies. Catalysis Reviews. 2020;62(1):1–65.
- Buzea C, Pacheco I, Robbie K. Nanomaterials and nanoparticles: sources and toxicity. Biointerphases. 2007;2(4):MR17–MR71.
- Ma Y, Zheng Y, Chen JP. A zirconium based nanoparticle for significantly enhanced adsorption of arsenate: synthesis, characterization and performance. J Colloid Interface Sci. 2011;354(2):785–792.
- Carabineiro SA. Supported gold nanoparticles as catalysts for the oxidation of alcohols and alkanes. Front Chem. 2019;7:702.
- Perera M, Wijenayaka LA, Siriwardana K, et al. Gold nanoparticle decorated titania for sustainable environmental remediation: green synthesis, enhanced surface adsorption and synergistic photocatalysis. RSC Adv. 2020;10(49):29594–29602.
- Ide Y, Matsuoka M, Ogawa M. Efficient visible-light-induced photocatalytic activity on gold-nanoparticle-supported layered Titanate. J Am Chem Soc. 2010;132(47):16762–16764.
- Guerra FD, Attia MF, Whitehead DC, et al. Nanotechnology for environmental remediation: materials and applications. Molecules. 2018;23(7):1760.
- Tian H, Li J, Mu Z, et al. Effect of pH on DDT degradation in aqueous solution using bimetallic Ni/Fe nanoparticles. Sep Purif Technol. 2009;66(1):84–89.
- El-Temsah YS, Sevcu A, Bobcikova K, et al. DDT degradation efficiency and ecotoxicological effects of two types of nano-sized zerovalent iron (nZVI) in water and soil. Chemosphere. 2016;144:2221–2228.
- Joo SH, Zhao D. Destruction of lindane and atrazine using stabilized iron nanoparticles under aerobic and anaerobic conditions: effects of catalyst and stabilizer. Chemosphere. 2008;70(3):418–425.
- Thomas J, Kumar KP, Chitra KR. Synthesis of Ag doped Nano TiO2 as efficient solar photocatalyst for the degradation of endosulfan. Adv Sci Lett. 2011;4(1):108–114.
- Budarz JF, Cooper EM, Gardner C, et al. Chlorpyrifos degradation via photoreactive TiO2 nanoparticles: assessing the impact of a multi-component degradation scenario. J Hazard Mater. 2019;372:61–68.
- Khan SH, Pathak B, Fulekar M. Synthesis, characterization and photocatalytic degradation of chlorpyrifos by novel Fe: znO nanocomposite material. Nanotechnology for Environmental Engineering. 2018;3(1):13.
- Das A, Singh J, Yogalakshmi K. Laccase immobilized magnetic iron nanoparticles: fabrication and its performance evaluation in chlorpyrifos degradation. Int Biodeterior Biodegrad. 2017;117:183–189.
- Srivastava M, Abhilash PC, Singh N. Remediation of lindane using engineered nanoparticles. J Biomed Nanotechnol. 2011;7(1):172–174.
- Rosales GG, Ávila-Pérez P, Reza-García JO, et al. Nanoparticle beads of Chitosan-Ethylene Glycol Diglycidyl Ether/Fe for the removal of aldrin. J Chem. 2021;2021:1–13.
- Wang S, Wang J, Shang F, et al. A GA-BP method of detecting carbamate pesticide mixture based on three-dimensional fluorescence spectroscopy. Spectrochim Acta A Mol Biomol Spectrosc. 2020;224:117396.
- Boudh, S. and Singh, J. S., 2019. Pesticide contamination: environmental problems and remediation strategies. In: Emerging and eco-friendly approaches for waste management. Springer, Singapore.
- Sartoros C, Yerushalmi L, Béron P, et al. Effects of Surfactant and Temperature on Biotransformation Kinetics of Anthracene and Pyrene. Chemosphere. 2015;61(7):1042–1050.
- Bhattacharya J, Islam M, Cheong YW. Microbial growth and action: implications for passive bioremediation of acid mine drainage. J Mine Water Environ. 2006;25(4):233–240
- Nakajima T, Shigeno Y. Polyester plastic-degrading microorganism, polyester plastic-degrading enzyme and polynucleotide encoding the enzyme. EP 1849859B1. 2014 Jan 21
- Huang Y, Xiao L, Li F, et al. Microbial degradation of pesticide residues and an emphasis on the degradation of cypermethrin and 3-phenoxy benzoic acid: a review. Molecules. 2018;23(9):2313.
- Bose S, Kumar PS, Vo DVN. A review on the microbial degradation of chlorpyrifos and its metabolite TCP. Chemosphere. 2021;283:131447.
- Yang J, Feng Y, Zhan H, et al. Characterization of a pyrethroid-degrading Pseudomonas fulva strain P31 and biochemical degradation pathway of D-phenothrin. Front Microbiol. 2018;9:1003.
- Bhatt P, Huang Y, Rene ER, et al. Mechanism of allethrin biodegradation by a newly isolated Sphingomonas trueperi strain CW3 from wastewater sludge. Bioresour Technol. 2020;305:123074.
- Bhatt P, Huang Y, Zhang W, et al. Enhanced cypermethrin degradation kinetics and metabolic pathway in Bacillus thuringiensis strain SG4. Microorganisms. 2020;8(2):223.
- Bhatt P, Zhang W, Lin Z, et al. Biodegradation of allethrin by a novel fungus Fusarium proliferatum strain CF2, isolated from contaminated soils. Microorganisms. 2020;8(4):593.
- Narayanan M, Kumarasamy S, Ranganathan M, et al. Enzyme and metabolites attained in degradation of chemical pesticides ß Cypermethrin by Bacillus cereus. Mater Today Proc. 2020;33:3640–3645.
- Logeshwaran P, Krishnan K, Naidu R, et al. Purification and characterization of a novel fenamiphos hydrolysing enzyme from Microbacterium esteraromaticum MM1. Chemosphere. 2020;252:126549.
- Sirajuddin S, Khan MA, Qader SAU, et al. A comparative study on degradation of complex malathion organophosphate using of Escherichia coli IES-02 and a novel carboxylesterase. Int J Biol Macromol. 2020;145:445–455.
- Dash DM, Osborne JW. Biodegradation of monocrotophos by a plant growth promoting Bacillus aryabhattai (VITNNDJ5) strain in artificially contaminated soil. Int J Environ Sci Technol. 2020;17(3):1475–1490.
- Cardozo M, de Almeida JS, Cavalcante SFDA, et al. Biodegradation of organophosphorus compounds predicted by enzymatic process using molecular modelling and observed in soil samples through analytical techniques and microbiological analysis: a comparison. Molecules. 2020;25(1):58.
- Aswathi A, Pandey A, Sukumaran RK. Rapid degradation of the organophosphate pesticide–Chlorpyrifos by a novel strain of Pseudomonas nitroreducens AR-3. Bioresour Technol. 2019;292:122025.
- Senko O, Maslova O, Efremenko E. Optimization of the use of His6-OPH-based enzymatic biocatalysts for the destruction of chlorpyrifos in soil. Int J Environ Res Public Health. 2017;14(12):1438.
- Rani M, Shanker U, Jassal V. Recent strategies for removal and degradation of persistent & toxic organochlorine pesticides using nanoparticles: a review. J Environ Manage. 2017;190:208–222.
- Li, D., and Wang, Y., 2017, 'Plasmonic Nanostructures as Surface-Enhanced Raman Scattering (SERS) Substrate for Protein Biomarker Sensing', in G. Barbillon (ed.), Nanoplasmonics - Fundamentals and Applications, IntechOpen, London. 10.5772/intechopen.68164.