ABSTRACT
Soil contamination with perfluorooctanoic acid (PFOA) is a global concern. PFOA in soil can enter plants, affect plant growth and threaten food safety. Therefore, understanding the plant utilization and phytotoxicity of PFOA is conducive to assessing the environmental risk of soil contaminated with PFOA. This review analyzed recent studies about the uptake and effects of PFOA on terrestrial plants from the aspects of absorption, transport, distribution, phytotoxicity, and the underlying mechanism. Based on current research, PFOA was predominantly taken by plant roots and showed adverse effects on plants by affecting the antioxidant system and metabolic process. Research in field or higher terrestrial plants is still very scarce. Given the importance of exploring the potential remediation methods for PFOA, more research with comprehensive consideration of soil and environmental factors should be carried out to tell its specific mechanisms of plant utilization and toxicity action.
1. Introduction
PFOA is concerned because of its widespread use and frequently detected in most regions of the world. Due to its low vapor volatility and extreme difficulty in degradation, PFOA can stay in soil for long period and cause lasting disadvantages to agricultural production [Citation1,Citation2]. PFOA in soil can be absorbed and enriched to plants. Among the perfluorinated compounds (PFASs) detected in crops, PFOA was detected with high frequency in plant samples around the world [Citation3–6]. Meanwhile, the bioaccumulation factors of PFOA in plants were also higher than other long-chain perfluoroalkyl carboxylic acids (PFCAs) [Citation5,Citation7]. Studies stated that PFOA was toxic to various plant species, mainly at physiological and biochemical levels. However, there is too little summary and discussion about the interaction of PFOA with terrestrial plants to clarify the internal mechanism and the deficiencies of existing research. Besides, screening plant varieties that suitable for planting in PFOA contaminated soil or plant species with high accumulation is also important for the safe utilization and remediation of contaminated soil. Here, we review the current most relevant studies to summarize the uptake and transport of PFOA in diverse plant species and provide implications for future research.
2. Uptake and translocation of PFOA in plants
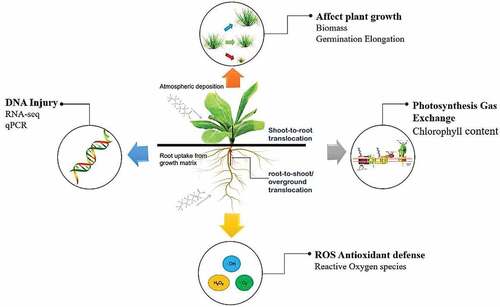
PFOA can enter the plants through root absorption in the soil or leaf deposition from the atmosphere, and then effectively transfer to other organs [Citation8,Citation9]. Due to its strong C-F bond energy, it is difficult to decompose in plants and stays as a complete PFOA molecule when uptake and transfer [Citation9]. Uptake of PFOA varied with plant species and exposure conditions, as summarized in . Among the existing studies, there are still few studies in field and woody plant experiments [Citation10,Citation11]. Besides, there are also limited phytotoxicity tests exposed to PFOA alone. Although mixed exposure is closer to the actual situation, individual exposure is essential to understanding the toxicological mechanism.
Table 1. The summary of existing PFOA uptake in terrestrial plants.
Studies have been carried out on the accumulation and distribution mechanism of PFOA in plant roots, including the distribution characteristics of PFOA from the root surface to the vascular column at tissue and cell level, the accumulation characteristics in subcellular, and the accumulation pathways of apoplast and symplast [Citation12–14]. However, currently, there is no conclusion on the way for plant utilization of PFOA. To summarize, there are two ways: PFOA was found mostly distributed in water-soluble fraction and cell wall, and transported from cortex to vascular bundle via symbiotic pathway [Citation13]; PFOA was also found in the radial movement from epidermal cells to cortex through extracellular and symbiotic pathways [Citation14]. In addition to being directly absorbed by roots, there is another way, metabolic transformation from other perfluorinated compounds [Citation15]. Studies found that precursors such as fluorotelomer-based acrylate polymers (FTACPs) and fluorotelomer alcohols (FTOHs) can be biotransformed to PFOA in soil [Citation15–18] and absorbed by plants. Furthermore, the presence of plants in soil can improve the degradation efficiency of precursors, because root exudates can promote the soil microbial abundance and facilitate the transformation of these precursors to PFOA [Citation15,Citation16,Citation19]. Similar transformation from precursors in plants was also proved in soybean [Citation20] and ryegrass [Citation16].
Research has noticed the relationship between PFOA accumulation and the organic components in roots, but still very limited. Channel inhibitor experiments proved that the absorption of PFOA by maize was a passive absorption process mediated by water channel and anion channel protein carrier [Citation21]. This indicated that proteins played an important role in root accumulation of PFOA, but the key proteins for PFOA transport have not been elucidated yet [Citation21]. Lipid contents of plant roots also played a role. PFOA accumulation in plant roots was shown negatively correlated with root lipid contents (contrary to wetland plants) and positively with that of root protein [Citation14,Citation22]. More studies focusing relationship between plant biological components and PFOA accumulation are needed.
Various factors affect the BCF values, including plant species, exposure doses, exposure time, and culture conditions, as summarized in . BCF values of PFOA varied among 0.012–84, especially higher in solution medium [Citation23–25], indicating that the hydroponic terrestrial plant tests cannot fully reflect the fate of PFOA in real soil. It is essential to have more soil experiments to evaluate theutilization of PFOA in terrestrial plants. Especially, plants with different genotypes showed different absorption and utilization capacity of PFOA [Citation12], reminding people to carry out necessary variety screening when facing PFOA contaminated soil.
PFOA may be filtered out by the Casparian strip at the early entry point, resulting in a shorter translocation distance and faster equilibrium [Citation26]. summarized the root concentration factors (RCFs) and transfer factors (TFs) of PFOA in plants. The majority of PFOA was found in the plant roots, and different plants showed unlike PFOA root accumulation and translocation abilities. RCF values changed in a range of 6.92–108 for those exposed in nutrition solution, 0.03–11.8 for those in soil, and 0.005–0.33 in field, indicating that hydroponic experiments overestimated PFOA accumulation in plant roots. TF values changed in a range of 0–9.68, mostly <0.5, indicating high root accumulation by low transfer to shoot [Citation52]. Although PFOA was more readily taken up in the translocation stream and its accumulation in plant roots was high, its transfer rate from root to stem was low [Citation12,Citation27]. Still, there is no relevant datum on woody plants, unfavorable to assessing their potential in phytoremediation.
Table 2. Translocation of PFOA in terrestrial plant.
Of public concern, corp grains, such as wheat and rice, were found to contain minimal or no PFOA [Citation28,Citation29], indicating a low risk of PFOA entering the human body through the grain crops. The risk of intergenerational transmission of PFOA in plants, influencing the growth and development of the next generation through seeds, might be low, but at present, there is little research in this area, and further research is needed. However, there are vegetables with higher TF values over 1, including cucumber [Citation30], radish [Citation22,Citation31,Citation32,Citation53], tomato [Citation31] and some lettuce varieties [Citation25]. It seems that such vegetables could contribute most to human exposure, indicating a high risk for human exposure to PFOA through dietary intake by vegetables [Citation3].
To develop remediation technologies for PFOA contaminated farmland, it is important to research on the interaction between PFOA and soil composition, including soil physical and chemical properties, as well as coexisting pollutants. However, there is limited research on how such factors affect the PFOA fate in soil-plant system. Liu, et al. [Citation33] found types of dissolved organic matter (DOM) in soil that inhibited the bioaccumulation and transport of PFOA in wheat due to their strong combination with PFOA. Plant root exudates and their low molecular weight organic acid (LMWOA) components also showed a great influence on the linear adsorption-desorption isotherms of PFOA in soil. Wherein, oxalic acid was considered to play a key role in activating PFOA uptake [Citation34]. Exchangeable ions were also important factors. Knight, et al. [Citation35] found that the exchangeable K+ content and cation exchange capacity (CEC) in soil were closely related to the plant accumulation of PFOA. In addition, studies showed that co-existing copper or CuO nanoparticles significantly affected PFOA translocation in plants [Citation32,Citation36]. These studies highlighted the potential to regulate PFOA risk in agricultural field and enhance phytoremediation for PFOA contaminated soil, but more studies are needed to explore feasible technology and the underlying mechanism [Citation32].
3. Phytotoxicity of perfluorooctanoic acid
PFOA phototoxicity showing as agronomical parameters, antioxidant activities, photosynthetic indices, and DNA injuries was studied (). Phytotoxicity of PFOA is related to many factors, including PFOA concentration range, soil properties, and plant species, as summarized in . Its disturbance to antioxidant defense system explained for some studies. Yang, et al. [Citation9] compared the toxicity of sodium fluoride (NaF) and perfluorooctanoic acid to Arabidopsis thaliana and found that PFOA caused oxidative stress, affected cell metabolism and led to reduced biomass, showing higher toxicity than inorganic F. Zhang, et al. [Citation37] found that PFOA inhibited the activities of superoxide dismutase (SOD) and catalase (CAT) and destroyed the antioxidant defense system in J. effusus roots. Studies have been carried out on the metabolic disorder caused by PFOA, and showed that the metabolic disorder of carbohydrates, phenols, amino acids and fatty acids played a central role in plant response to PFOA stress. PFOA inspired the antioxidant defense pathway, interfered the tricarboxylic acid (TCA) cycle, and led to the decline in photosynthesis and final biomass [Citation38–42]. Transcriptome analysis further showed that PFOA could regulate the gene expression in a tissue-specific manner, and provide candidate genes for transporters that involved in PFOA uptake and translocation [Citation43].
Table 3. Phytotoxic effects of plants under exposure of PFOA.
Similarly to plant uptake, the toxicity of PFOA is also related to the growth medium. Compared with the exposure levels in real field conditions, the pollution levels of plants cultured in hydroponics were amplified [Citation24,Citation25]. Soil properties, including organic matter (OM), CEC, pH value, and clay contents were proved to impact the phytotoxicity of PFOA [Citation32,Citation44]. Zhao, et al. [Citation45] found that the toxicity threshold of PFOA to Brassica chinensis grown in soil with low OM or CEC was lower than that in soil with high OM or CEC. Zhou, et al. [Citation46] also found that wheat root growth was more vulnerable to PFOA in soils with lower OM or CEC.
4. Conclusion
Based on previous research, this article describes the PFOA uptake, transport, phytotoxicity in terrestrial plants. Nowadays, there are limited studies carried out with plants exposed to PFOA alone, so it is hard to understand the underlying mechanism for plant utilization and toxicity action. Especially, the key proteins for PFOA transmembrane transport and influencing factors need to be further studied. Current hydroponic experiments overestimated the plant utilization and phytotoxicity of PFOA, so more experiments should be carried out to explore the PFOA risk in real soil conditions, especially field studies that comprehensively consider soil and climate factors. Besides, more attention should be paid to the potential of phytoremediation for PFOA contaminated soil, especially the possibility of the higher woody plants or nanotechnology in enhancing phytoremediation.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Miao Y, Guo X, Dan P, et al. Rates and equilibria of perfluorooctanoate (PFOA) sorption on soils from different regions of China. Ecotoxicol Environ Saf. 2017;139:102–108.
- Choi G-H, Lee D-Y, Song AR, et al. The dietary risk assessment of perfluorooctanoic acid (PFOA) and perfluorosulfonic acid (PFOS) in the root crops from the survey of the residue in agricultural soil and the crops. Appl Biol Chem. 2022;65(60).
- Herzke D, Huber S, Bervoets L, et al. Perfluorinated alkylated substances in vegetables collected in four European countries; occurrence and human exposure estimations. Environ Sci Pollut Res Int. 2013;20(11):7930–7939.
- Sznajder-Katarzynska K, Surma M, Cieslik E, et al. The perfluoroalkyl substances (PFASs) contamination of fruits and vegetables. Food Addit Contam Part A Chem Anal Control Expo Risk Assess. 2018;35(9):1776–1786.
- Zhou Y, Lian Y, Sun X, et al. Determination of 20 perfluoroalkyl substances in greenhouse vegetables with a modified one-step pretreatment approach coupled with ultra performance liquid chromatography tandem mass spectrometry(UPLC-MS-MS). Chemosphere. 2019;227:470–479.
- Li P, Oyang X, Zhao Y, et al. Occurrence of perfluorinated compounds in agricultural environment, vegetables, and fruits in regions influenced by a fluorine-chemical industrial park in China. Chemosphere. 2019;225:659–667.
- Liu Z, Lu Y, Song X, et al. Multiple crop bioaccumulation and human exposure of perfluoroalkyl substances around a mega fluorochemical industrial park, China: implication for planting optimization and food safety. Environ Int. 2019;127:671–684.
- Zhu H, Kannan K. Distribution and partitioning of perfluoroalkyl carboxylic acids in surface soil, plants, and earthworms at a contaminated site. Sci Total Environ. 2019;647:954–961.
- Yang X, Ye C, Liu Y, et al. Accumulation and phytotoxicity of perfluorooctanoic acid in the model plant species Arabidopsis thaliana. Environ Pollut. 2015;206:560–566.
- Huff DK, Morris LA, Sutter L, et al. Accumulation of six PFAS compounds by woody and herbaceous plants: potential for phytoextraction. Int J Phytoremediation. 2020;22(14):1538–1550.
- Mudumbi JB, Ntwampe SK, Muganza M, et al. Susceptibility of riparian wetland plants to perfluorooctanoic acid (PFOA) accumulation. Int J Phytoremediation. 2014;16(7–12):926–936.
- Xiang L, Chen L, Yu LY, et al. Genotypic variation and mechanism in uptake and translocation of perfluorooctanoic acid (PFOA) in lettuce (Lactuca sativa L.) cultivars grown in PFOA-polluted soils. Sci Total Environ. 2018;636:999–1008.
- Wang TT, Ying GG, He LY, et al. Uptake mechanism, subcellular distribution, and uptake process of perfluorooctanoic acid and perfluorooctane sulfonic acid by wetland plant Alisma orientale. Sci Total Environ. 2020;733:139383.
- Wang TT, Ying GG, Shi WJ, et al. Uptake and Translocation of Perfluorooctanoic Acid (PFOA) and Perfluorooctanesulfonic Acid (PFOS) by Wetland Plants: tissue- and Cell-Level Distribution Visualization with Desorption Electrospray Ionization Mass Spectrometry (DESI-MS) and Transmission Electron Microscopy Equipped with Energy-Dispersive Spectroscopy (TEM-EDS). Environ Sci Technol. 2020;54(10):6009–6020.
- Bizkarguenaga E, Zabaleta I, Prieto A, et al. Uptake of 8:2 perfluoroalkyl phosphate diester and its degradation products by carrot and lettuce from compost-amended soil. Chemosphere. 2016;152:309–317.
- Yao Y, Lan Z, Zhu H, et al. Foliar uptake overweighs root uptake for 8:2 fluorotelomer alcohol in ryegrass (Lolium perenne L.): a closed exposure chamber study. Sci Total Environ. 2022;829:154660.
- Rankin K, Lee H, Tseng PJ, et al. Investigating the biodegradability of a fluorotelomer-based acrylate polymer in a soil-plant microcosm by indirect and direct analysis. Environ Sci Technol. 2014;48(21):12783–12790.
- Zhao S, Zhu L. Uptake and metabolism of 10:2 fluorotelomer alcohol in soil-earthworm (Eisenia fetida) and soil-wheat (Triticum aestivum L.) systems. Environ Pollut. 2017;220(Pt A):124–131.
- Martin BC, George SJ, Price CA, et al. The role of root exuded low molecular weight organic anions in facilitating petroleum hydrocarbon degradation: current knowledge and future directions. Sci Total Environ. 2014;472:642–653.
- Zhang H, Wen B, Hu X, et al. Uptake, Translocation, and Metabolism of 8:2 Fluorotelomer Alcohol in Soybean (Glycine max L.Merrill). Environ Sci Technol. 2016;50(24):13309–13317.
- Wen B, Li L, Liu Y, et al. Mechanistic studies of perfluorooctane sulfonate, perfluorooctanoic acid uptake by maize (Zea mays L. cv. TY2). Plant Soil. 2013;370(1–2):345–354.
- Wen B, Wu Y, Zhang H, et al. The roles of protein and lipid in the accumulation and distribution of perfluorooctane sulfonate (PFOS) and perfluorooctanoate (PFOA) in plants grown in biosolids-amended soils. Environ Pollut. 2016;216:682–688.
- Chen CH, Yang SH, Liu Y, et al. Accumulation and phytotoxicity of perfluorooctanoic acid and 2,3,3,3-tetrafluoro-2-(heptafluoropropoxy)propanoate in Arabidopsis thaliana and Nicotiana benthamiana. Environ Pollut. 2020;259:113817.
- Garcia-Valcarcel AI, Molero E, Escorial MC, et al. Uptake of perfluorinated compounds by plants grown in nutrient solution. Sci Total Environ. 2014;472:20–26.
- Felizeter S, Jurling H, Kotthoff M, et al. Influence of soil on the uptake of perfluoroalkyl acids by lettuce: a comparison between a hydroponic study and a field study. Chemosphere. 2020;260:127608.
- Zhang L, Sun H, Wang Q, et al. Uptake mechanisms of perfluoroalkyl acids with different carbon chain lengths (C2-C8) by wheat (Triticum acstivnm L.). Sci Total Environ. 2019;654:19–27.
- Zhang DQ, Wang M, He Q, et al. Distribution of perfluoroalkyl substances (PFASs) in aquatic plant-based systems: from soil adsorption and plant uptake to effects on microbial community. Environ Pollut. 2020;257:113575.
- Yamazaki E, Taniyasu S, Noborio K, et al. Accumulation of perfluoroalkyl substances in lysimeter-grown rice in Japan using tap water and simulated contaminated water. Chemosphere. 2019;231:502–509.
- Sungur Ş, Çevik B, Köroğlu M. Determination of perfluorooctanoic acid (PFOA) and perfluorooctane sulfonic acid (PFOS) contents of compost amended soils and plants grown in these soils. Int J Environ Anal Chem. 2020;102(8):1926–1934.
- Lechner M, Knapp H. Carryover of perfluorooctanoic acid (PFOA) and perfluorooctane sulfonate (PFOS) from soil to plant and distribution to the different plant compartments studied in cultures of carrots (Daucus carota ssp. Sativus), potatoes (Solanum tuberosum), and cucumbers (Cucumis Sativus). J Agric Food Chem. 2011;59(20):11011–11018.
- Blaine AC, Rich CD, Sedlacko EM, et al. Perfluoroalkyl acid distribution in various plant compartments of edible crops grown in biosolids-amended soils. Environ Sci Technol. 2014;48(14):7858–7865.
- Xu Y, Du W, Yin Y, et al. CuO nanoparticles modify bioaccumulation of perfluorooctanoic acid in radish (Raphanus sativus L.). Environ Pollutants Bioavailability. 2022;34(1):34–41.
- Liu S, Zhou J, Guo J, et al. Insights into the impacts of dissolved organic matter of different origins on bioaccumulation and translocation of per- and polyfluoroalkyl substances (PFASs) in wheat. Environ Pollut. 2022;293:118604.
- Xiang L, Chen XT, Yu PF, et al. Oxalic acid in root exudates enhances accumulation of perfluorooctanoic acid in lettuce. Environ Sci Technol. 2020;54(20):13046–13055.
- Knight ER, Braunig J, Janik LJ, et al. An investigation into the long-term binding and uptake of PFOS, PFOA and PFHxS in soil - plant systems. J Hazard Mater. 2021;404(Pt B):124065.
- Zhang L, Wang Q, Chen H, et al. Uptake and translocation of perfluoroalkyl acids with different carbon chain lengths (C2-C8) in wheat (Triticum acstivnm L.) under the effect of copper exposure. Environ Pollut. 2021;274:116550.
- Zhang W, Zhang D, Zagorevski DV, et al. Exposure of Juncus effusus to seven perfluoroalkyl acids: uptake, accumulation and phytotoxicity. Chemosphere. 2019;233:300–308.
- Du W, Liu X, Zhao L, et al. Response of cucumber (Cucumis sativus) to perfluorooctanoic acid in photosynthesis and metabolomics. Sci Total Environ. 2020;724:138257.
- Li P, Li J. Perfluorooctanoic acid (PFOA) caused oxidative stress and metabolic disorders in lettuce (Lactuca sativa) root. Sci Total Environ. 2021;770:144726.
- Li P, Oyang X, Xie X, et al. Phytotoxicity induced by perfluorooctanoic acid and perfluorooctane sulfonate via metabolomics. J Hazard Mater. 2020;389:121852.
- Pietrini F, Passatore L, Fischetti E, et al. Evaluation of morpho-physiological traits and contaminant accumulation ability in Lemna minor L. treated with increasing perfluorooctanoic acid (PFOA) concentrations under laboratory conditions. Sci Total Environ. 2019;695:133828.
- Li P, Xiao Z, Sun J, et al. Metabolic regulations in lettuce root under combined exposure to perfluorooctanoic acid and perfluorooctane sulfonate in hydroponic media. Sci Total Environ. 2020;726:138382.
- Fan L, Tang J, Zhang D, et al. Investigations on the phytotoxicity of perfluorooctanoic acid in Arabidopsis thaliana. Environ Sci Pollut Res Int. 2020;27(1):1131–1143.
- Zhao S, Fan Z, Sun L, et al. Interaction effects on uptake and toxicity of perfluoroalkyl substances and cadmium in wheat (Triticum aestivum L.) and rapeseed (Brassica campestris L.) from co-contaminated soil. Ecotoxicol Environ Saf. 2017;137:194–201.
- Zhao H, Chen C, Zhang X, et al. Phytotoxicity of PFOS and PFOA to Brassica chinensis in different Chinese soils. Ecotoxicol Environ Saf. 2011;74(5):1343–1347.
- Zhou L, Xia M, Wang L, et al. Toxic effect of perfluorooctanoic acid (PFOA) on germination and seedling growth of wheat (Triticum aestivum L.). Chemosphere. 2016;159:420–425.
- Zhao S, Fang S, Zhu L, et al. Mutual impacts of wheat (Triticum aestivum L.) and earthworms (Eisenia fetida) on the bioavailability of perfluoroalkyl substances (PFASs) in soil. Environ Pollut. 2014;184:495–501.
- Blaine AC, Rich CD, Sedlacko EM, et al. Perfluoroalkyl acid uptake in lettuce (Lactuca sativa) and strawberry (Fragaria ananassa) irrigated with reclaimed water. Environ Sci Technol. 2014;48(24):14361–14368.
- Bizkarguenaga E, Zabaleta I, Mijangos L, et al. Uptake of perfluorooctanoic acid, perfluorooctane sulfonate and perfluorooctane sulfonamide by carrot and lettuce from compost amended soil. Sci Total Environ. 2016;571:444–451.
- Lee D-Y, Choi G-H, Rho J-H, et al. Comparison of the plant uptake factor of perfluorooctanoic acid (PFOA) and perfluorooctane sulfonic acid (PFOS) from the three different concentrations of PFOA and PFOS in soil to spinach and Welsh onion. J Appl Biol Chem. 2020;63(3):243–248.
- Mudumbi JBN, Daso AP, Okonkwo OJ, et al. Propensity of Tagetes erecta L., a medicinal plant commonly used in diabetes management, to accumulate pPerfluoroalkyl substances. Toxics. 2019;7(1):1.
- Zhu J, Wallis I, Guan H, et al. Juncus sarophorus, a native Australian species, tolerates and accumulates PFOS, PFOA and PFHxS in a glasshouse experiment. Sci Total Environ. 2022;826:154184.
- Abril C, Santos JL, Martin J, et al. Uptake and translocation of multiresidue industrial and household contaminants in radish grown under controlled conditions. Chemosphere. 2021;268:128823.
- Stahl T, Heyn J, Thiele H, et al. Carryover of perfluorooctanoic acid (PFOA) and perfluorooctane sulfonate (PFOS) from soil to plants. Arch Environ Contam Toxicol. 2009;57(2):289–298.
- Wen B, Li L, Zhang H, et al. Field study on the uptake and translocation of perfluoroalkyl acids (PFAAs) by wheat (Triticum aestivum L.) grown in biosolids-amended soils. Environ Pollut. 2014;184:547–554.
- Dalahmeh S, Tirgani S, Komakech AJ, et al. Per- and polyfluoroalkyl substances (PFASs) in water, soil and plants in wetlands and agricultural areas in Kampala, Uganda. Sci Total Environ. 2018;631-632:660–667.
- Gredelj A, Nicoletto C, Valsecchi S, et al. Uptake and translocation of perfluoroalkyl acids (PFAA) in red chicory (Cichorium intybus L.) under various treatments with pre-contaminated soil and irrigation water. Sci Total Environ. 2020;708:134766.
- Gredelj A, Nicoletto C, Polesello S, et al. Uptake and translocation of perfluoroalkyl acids (PFAAs) in hydroponically grown red chicory (Cichorium intybus L.): growth and developmental toxicity, comparison with growth in soil and bioavailability implications. Sci Total Environ. 2020;720:137333.
- Li MH. Toxicity of perfluorooctane sulfonate and perfluorooctanoic acid to plants and aquatic invertebrates. Environ Toxicol. 2009;24(1):95–101.
- Li P, Xiao Z, Xie X, et al. Perfluorooctanoic acid (PFOA) changes nutritional compositions in lettuce (Lactuca sativa) leaves by activating oxidative stress. Environ Pollut. 2021;285:117246.