Abstract
Pharmacies in low- and middle-income countries play an important role in increasing the availability of medical abortion to individuals for self-use. We aimed to document the costs to users of medical abortion products at outlets across geographies and understand the diversity of available products, primarily in low- and middle-income countries or in places where access to abortion is restricted. A descriptive analysis of price data was completed for identified medical abortion products at retail outlets visited in 44 countries from November 2017 to February 2018. Median prices and ranges are reported in $US for mifepristone 200 mg tablets, misoprostol 200 mcg tablets, and combipacks. Misoprostol, mifepristone, and combipacks were found in 44, 19, and 16 countries, respectively. Nearly two-thirds of products (321/508) required a prescription. The median price of misoprostol was $0.63 per tablet (range $0.09–$27.63) based on 304 price points. Mifepristone and combipacks had fewer price points available (n = 59 and n = 44, respectively). Median prices were $11.78 per mifepristone tablet (range $1.77–$37.83) and $11.18 per combipack (range $3.50–$35.86). Overall, prices were highest in Latin America and lowest in South/Southeast Asia. Only 11.5% (7/61) of the total unique misoprostol brands were quality-assured (i.e. approved by a stringent regulatory authority or pre-qualified by the World Health Organization), compared to 25.0% (4/16) of unique combipack products. There was wide variation in product pricing and availability across settings. The infrequent availability of mifepristone and combipacks, in addition to the limited availability of quality-assured medicines and high cost of abortion medications, are important factors affecting access to high-quality abortion care.
Résumé
Dans les pays à revenu faible et intermédiaire, les pharmacies jouent un rôle important pour élargir la disponibilité de l’avortement médicamenteux pour les individus en auto-administration. Nous souhaitions documenter les coûts pour les utilisateurs de produits d’avortement médicamenteux dans des points de vente dans différents lieux et comprendre la diversité des produits disponibles, principalement dans les pays à revenu faible ou intermédiaire ou là où l’accès à l’avortement est restreint. Une analyse descriptive des données sur les prix a été complétée pour des produits identifiés de l’avortement médicamenteux dans des points de vente au détail visités dans 44 pays de novembre 2017 à février 2018. Les prix médians et les fourchettes sont exprimés en dollars US pour les comprimés de 200 milligrammes de mifépristone, les comprimés de 200 microgrammes de misoprostol et les combipacks des deux produits. Le misoprostol, la mifépristone et les combipacks étaient disponibles dans 44, 19 et 16 pays, respectivement. Près des deux tiers des produits (321/508) exigeaient une ordonnance. Le prix médian du misoprostol était de $0,63 par comprimé (fourchette $0,09-$27,63) sur la base de 304 prix référencés. La mifépristone et les combipacks avaient moins de prix référencés disponibles (n = 59 et n = 44, respectivement). Les prix médians étaient de $11,78 par comprimé de mifépristone (fourchette $1,77-$37,83) et $11,18 par combipack (fourchette $3,50-$35,86). Dans l’ensemble, les prix étaient les plus élevés en Amérique latine et les moins chers en Asie du Sud/du Sud-Est. À peine 11,5% (7/61) des marques de misoprostol seul bénéficiaient d’une assurance qualité (c’est-à-dire qu’elles étaient approuvées par une autorité régulatrice rigoureuse ou présélectionnées par l’Organisation mondiale de la santé), contre 25,0% (4/16) des combipacks. On a observé de larges variations dans le prix des produits et leur disponibilité selon les endroits. La faible disponibilité de la mifépristone et des combipacks, en plus de la rareté des médicaments à qualité garantie et du coût élevé des médicaments pour l’avortement, sont des facteurs importants qui contrarient l’accès à des soins de qualité en cas d’avortement.
Resumen
Las farmacias en los países de bajos y medianos ingresos desempeñan un papel importante en aumentar la disponibilidad de pastillas de aborto con medicamentos para que las personas puedan autogestionar su aborto. Procuramos documentar los gastos de las usuarias en productos de aborto con medicamentos en puntos de venta en todas las regiones geográficas y entender la diversidad de los productos disponibles, principalmente en países de bajos y medianos ingresos o en lugares con acceso restringido a los servicios de aborto. Se realizó un análisis descriptivo de los datos de precios de los productos de aborto con medicamentos identificados en establecimientos minoristas visitados en 44 países entre noviembre de 2017 y febrero de 2018. La mediana de precios y gama de precios son reportadas en $US para tabletas de 200 mg de mifepristona, tabletas de 200 mcg de misoprostol y paquetes combinados. Misoprostol, mifepristona y paquetes combinados fueron encontrados en 44, 19 y 16 países, respectivamente. Para casi dos terceras partes de los productos (321/508) se necesitaba una receta médica. La mediana de precios de misoprostol fue de $0.63 por cada tableta (gama de $0.09 a $27.63) basada en 304 precios. Había menos precios disponibles para la mifepristona y los paquetes combinados (n = 59 y n = 44, respectivamente). La mediana de precios fue de $11.78 por cada tableta de mifepristona (gama de $1.77 a $37.83) y $11.18 por cada paquete combinado (gama de $3.50 a $35.86). En general, los precios más altos se encontraron en Latinoamérica y los más bajos en el sur y sudeste de Asia. Solo el 11.5% (7/61) del total de marcas únicas de misoprostol tenían calidad garantizada (es decir, estaban aprobadas por una autoridad normativa estricta o precalificadas por la Organización Mundial de la Salud), comparadas con el 25.0% (4/16) de los productos únicos de paquetes combinados. Hubo gran variación en los precios y en la disponibilidad de los productos en los diferentes entornos. La disponibilidad poco frecuente de mifepristona y paquetes combinados, así como la disponibilidad limitada de medicamentos de calidad garantizada y el alto costo de los medicamentos para inducir el aborto, son factores importantes que afectan el acceso a los servicios de aborto de alta calidad.
Introduction
Increasing availability of mifepristone and misoprostol and established evidence on the safety and effectiveness of medical abortion regimens have enabled abortion access outside of health facilities and expanded opportunities for self-use in recent years.Citation1 A number of online resources and educational tools support informed self-use of mifepristone and misoprostol or misoprostol only by individuals at 12 weeks gestation or less.Citation2,Citation3 Drug registrations with national regulatory authorities have opened the door to availability in many countries over the past decade.Citation4,Citation5 However, a number of factors still affect access to and use of these drugs by individuals. Depending on the setting, these factors include legal restrictions for access to abortion care, poor access to quality medicines, lack of information among providers and women, provider reluctance to offer services, stigma, requirements for prescription, and cost to the consumer.Citation2,Citation6–8
In order to realise the full potential of self-use of medical abortion, access to affordable, quality-assured mifepristone and misoprostol is critical. Information on price to the consumer is necessary to understand market-related factors and to identify actions to ensure affordable pricing and thus increase equitable access to these commodities. Availability of data on price is also needed to address a gap in evidence on the comparative cost and cost-effectiveness of these essential medicines.Citation1,Citation9 While the International Planned Parenthood Federation’s Medical Abortion Commodities DatabaseCitation10 provides information on the availability of quality medical abortion drugs in over 100 countries, existing literature lacks robust and detailed information on the price of medical abortion commodities at point of purchase or use, particularly for mifepristone and combined packages of misoprostol and mifepristone in many of these settings.
This paper presents a descriptive analysis of price data on medical abortion drugs for sale in pharmacies and retail outlets in 44 countries, including mostly low- and middle-income countries or in places where access to abortion is restricted. Pharmacies in many of these settings play an important role in increasing the availability of medical abortion to individuals.Citation11 The primary aims of this analysis were to understand the diversity of medical abortion products available at outlets and pricing during the period from November 2017 to February 2018. This information can support a broader understanding of the medical abortion landscape to inform abortion programmes, policy initiatives, and investment decisions to increase access to good quality and affordable medical abortion commodities.
Methods
This secondary analysis draws from data collected on the availability of mifepristone 200 mg, misoprostol 200 mcg, and combined mifepristone–misoprostol (combipack) products for the development of a global Medical Abortion Commodities Database.Citation10 From November 2017 to February 2018, the International Planned Parenthood Federation, in partnership with Gynuity Health Projects and Concept Foundation, undertook data collection to populate an inventory of medical abortion products available around the world.
Data collection was done using a convenience sample. Regions and countries were selected primarily based on their low- or middle-income status and data collection feasibility. A few high-income countries, located in the Latin America and the Caribbean (LAC) region, were also visited (i.e. Chile, Uruguay, and Barbados) to capture data from different contexts. In each country, data collection was conducted in the capital city and one other town or city, in up to five outlets in each location that were known or likely to sell medical abortion commodities. To the extent possible, data collectors attempted to include a mix of the various types of sites while giving priority to those that were registered or licensed. Standardised data collection forms and accompanying standard operating procedures were developed and translated from English into Spanish and French.
The majority of data collectors were health professionals and project managers, and all were familiar with the local medical abortion landscape. Upon arrival at an outlet, data collectors presented themselves and explained that they were part of a global research team that was collecting information on medical abortion products. Data collectors recorded details of the products identified during site visits, including the brand name, manufacturer name, dosage, packaging type, pack size, and/or number of pills. The type of outlet visited was also recorded (pharmacy attached to a public health facility, pharmacy attached to a private health facility, pharmacy attached to an NGO health facility, private pharmacy, drug shop, drug seller, or other). The data collector asked outlet staff to confirm all available brands of medical abortion commodities that were available for sale, and would request to purchase the product, where possible, and only if the product had not been purchased elsewhere during visits to other outlets in that country. Product prices were recorded in the local currency. In cases where the purchase of products was not possible (e.g. the pharmacist’s refusal to sell the product or stock-out of the item), data collectors asked to take photos of the product packaging, if available, and recorded any details that the outlet staff were willing to share. Data collectors noted whether a prescription was requested by the outlet staff to obtain the pills and any other comments relevant to their experience with the purchase of the commodities. Data did not include any identifiers or information about the pharmacy staff who assisted with the purchase. As the primary purpose of this exercise was to build an inventory of products for potential inclusion in the Medical Abortion Commodities Database, information on price and prescription was defined as “secondary”.
Analysis
The main outcomes analysed were the number and type of medical abortion commodities identified, their price per unit, and whether a prescription was required (i.e. requested by outlet staff). For each type of medical abortion commodity (misoprostol 200 mcg, mifepristone 200 mg, and combipack), the number of unique brands was tallied based on the research team’s review of brand names and manufacturer details captured in the data forms. A brand was considered “unique” if the brand name was different from other products in the database. Products with the same brand names but manufactured by different companies were also considered unique. The brand and manufacturer names are not specified in this analysis given that permission from each manufacturer was not requested.
This analysis presents information on the individual price per tablet for misoprostol 200 mcg and for mifepristone 200 mg. The price analysis excludes products that were obtained for free and products for which we had inadequate information to calculate the price per tablet. Price points per tablet were calculated by dividing the product’s overall price by the number of pills sold. For instance, outlets would often sell strips, boxes, or containers of misoprostol and mifepristone pills (of varying pill quantities); thus, the total price paid by the data collector was divided by the number of pills to estimate the price per tablet for misoprostol 200 mcg and mifepristone 200 mg. International price guides for medicines, such as the one published by Management Sciences for Health, calculates the unit (per tablet) cost for misoprostol in the same way, which enables comparison of our findings with price guides that include information on buyer costs.Citation12 The price per combipack reflects the price per pack, which consists of one mifepristone tablet and four misoprostol pills. The unit price of each product was converted from its local currency into US dollars using the relevant historical exchange rate for each country from the midpoint of data collection (10 January 2018), using the online currency converter: https://www.xe.com/currencytables/.
Data were entered into an Excel database and transferred to SPSS to run descriptive analyses, primarily percentages and number counts. Prices are reported in mean, median, and range. Ultimately, median prices were used to characterise the data patterns due to outliers in prices. To undertake the price analysis, the research team agreed, a priori, to explore the average price of each commodity by different subgroups. These include the availability of multiple brands and products, legal status of abortion, country’s income status (based on the World Bank’s 2017 classificationsCitation13), quality assurance status of misoprostol, outlet type, requirement for prescription (based on prescription requests by outlet staff), and region. The final countries included in the analysis were categorised by region (Africa, LAC, Eastern Europe/Central Asia, and South/Southeast Asia). Classification of countries’ legal status of abortion was based on the 2014 Center for Reproductive Rights’ (CRR) Map on Abortion Laws, which categorises the legality of abortion in each country on a scale of I–IV (I = most restricted; IV = least restrictedCitation14). Categories were updated in our analysis for two African countries to reflect changes to the legal status of abortion that occurred since publication of CRR’s 2014 Map on Abortion Laws and prior to the time period of data collection for this project.
Variation in price was also explored in relation to the number of brands available and their quality assurance status. For each country, the number of unique misoprostol brands was tallied and then this number was used to designate the country as one with few brands available (i.e. 1–2 brands available) or one with multiple brands available (3 or more). This type of analysis was done for misoprostol only, due to the greater variation in the number of misoprostol brands per country, compared to mifepristone and combipack, of which there was often only one brand available. We also explored how the price of misoprostol varied according to the availability of mifepristone and/or combipack.
Price analyses were also conducted in relation to misoprostol and combipack products that were defined as “quality-assured” and “non-quality-assured”. Misoprostol products were considered “quality-assured” if they had been approved by a stringent regulatory authority or had met standards by the World Health Organization’s (WHO) Prequalification of Medicines Programme, which deems products as “pre-qualified” for purchase by the United Nations and its agencies based on WHO’s assessment of the product and the good manufacturing practices.Citation15 The quality designation for combipacks refers only to the misoprostol component. “Non-quality-assured” misoprostol and combipack products are those not meeting these criteria. There is no evidence to suggest degradation or stability problems with mifepristoneCitation16; therefore, no assessment was undertaken to determine the quality of mifepristone.
Results
Information on medical abortion products was collected during visits to outlets in 46 countries (Africa (n = 17), LAC (n = 11), Eastern Europe/Central Asia (n = 11), and South/Southeast Asia (n = 7)). A total of 403 outlets were visited, and, on average, data collection was carried out at 9 outlets per country. Private, retail pharmacies were the most frequent type of outlet visited (61.8%; n = 249), followed by pharmacies attached to public hospitals (12.7%; n = 51), pharmacies attached to private hospitals (8.2%; n = 33), pharmacies attached to NGO clinics (5.5%; n = 22), drug shops (6.7%; n = 27), and those categorised as other (5.2%; n = 21). The final analysis includes 528 identified medical abortion products in 44 countries, including misoprostol (n = 380), mifepristone (n = 87), and combipack (n = 61). The analysis excludes two countries where no products were identified during visits to outlets. Overall, half of the countries in this analysis (n = 22) had less restrictive laws on abortion; however, the number of countries varied per region (e.g. in Africa 6/17 (35.3%), LAC 4/10 (40%), Eastern Europe/Central Asia 10/10 (100%), and South/Southeast Asia 2/7 (28.6%)).
Product availability
In this sample for analysis, misoprostol was identified in all 44 countries ( and ). In contrast, mifepristone was found in fewer than half of the countries (43.2%; 19/44) and combipacks were identified in approximately one-third of the countries (36.4%; 16/44) (). In seven countries (15.9%), mifepristone and combipacks were both available. Among the subset of countries with more liberal laws on abortion, mifepristone and combipacks were identified in 16/22 (72.7%) and 11/22 (50.0%) countries, respectively.
Table 1. Overview of medical abortion commodities and the total number of unique brands identified by region and overall
Table 2. Median price of each medical abortion commodity by region and by countryTable Footnotea
Overall, 61 unique misoprostol brands, 18 mifepristone brands, and 16 combipacks were identified. Among the 61 brands of misoprostol, 11.5% (7/61) were quality-assured. One-quarter of the combipacks were also quality-assured (25.0%; 4/16).
The total number of unique misoprostol brands varied by region (). The Eastern Europe/Central Asia region had the lowest number of unique misoprostol brands (5), in comparison to Africa, LAC, and South/Southeast Asia which had 17, 20, and 23, respectively. There was only one misoprostol brand, produced by the same company, found in all four regions; most unique misoprostol brands were only found in one region or in one country. Nearly one-third of countries had only one misoprostol brand available (29.5%; 13/44).
Regional differences in the availability of mifepristone were also observed. LAC and Africa regions had two and three unique mifepristone brands respectively; whereas Eastern Europe/Central Asia and South/Southeast Asia regions each had seven unique brands of mifepristone. Mifepristone was available in all the Eastern European and Central Asian countries included in this analysis (100%; 10/10). In contrast, a smaller proportion of countries (26.5%; 9/34) across the other three regions (LAC, Africa, and South/Southeast Asia) had mifepristone available. Among the 19 countries with mifepristone available, 63.2% (12/19) had one brand only and for the most part, brands were sold in specific countries or regions and not multi-regionally.
Among the 16 countries with combipacks, three had multiple brands, and the remainder had only one brand. Africa had the highest number of unique brands (7), followed by South/Southeast Asia with 6, and LAC and Eastern Europe/Central Asia each had 3 unique brands (). The most frequently identified combipack brand was found in three out of the four regions, including in Africa, Eastern Europe/Central Asia, and South/Southeast Asia regions. Yet, the total number of countries where this common brand was found was low (15.9%, 7/44).
Price analysis
Misoprostol
Price information was recorded for 304 misoprostol products in 39 countries. The median price per misoprostol tablet was $0.63. The price per tablet ranged from $0.09 to $27.63 and varied widely within and between countries and regions ( and ). The median price of misoprostol was highest in LAC ($1.47) and lowest in South/Southeast Asia ($0.09).
Seventy per cent (214/304) of the price data on misoprostol was collected in private, retail pharmacies; the median price of misoprostol at this outlet type was $0.64 (range $0.09–$20.00). In comparison, misoprostol obtained from pharmacies attached to private and public hospitals or NGO clinics had a slightly lower median price ($0.46, range $0.09–$4.80). The price of misoprostol was considerably higher when accessed at drug shops or other locations where the median prices per tablet were $1.73 and $1.14, respectively, although notably fewer price points were collected from these locations (). The most expensive misoprostol ($27.63) in this sample was purchased informally in a country where abortion was highly restricted.
Table 3. Price of misoprostol 200 mcg tablets stratified by location and other factors (N = 304)Table Footnotea
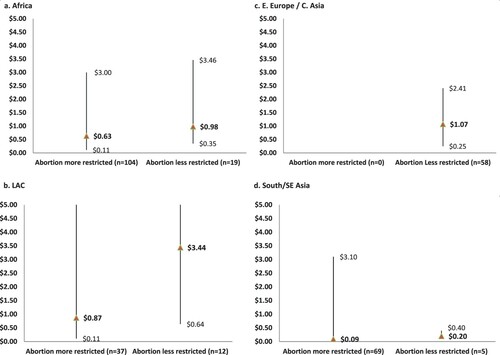
In countries with less restrictive abortion laws, the median price of a misoprostol tablet was $1.07 (range $0.13–$5.19), compared to a lower median price of $0.56 (range $0.09–$27.63) in more restrictive settings. This data trend was also found when comparing median misoprostol prices between countries with more and less restrictive laws on abortion, stratified by region (). The median price of misoprostol was also noted to be higher in places where mifepristone and/or combipacks were sold compared to countries where neither of those products was available ().
Figure 1. Median (min–max) price of misoprostol in settings where laws on abortion are more vs. less restrictive by region
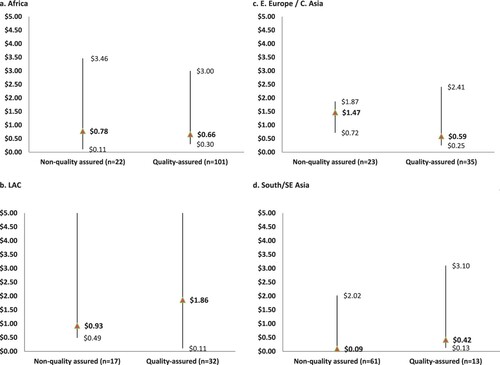
Variations in misoprostol pricing were also examined in relation to product packaging, quality assurance status and the number of brands available per country. Eighty-seven per cent (254/292) of misoprostol products were packed in alu/alu blisters, which are recommended to prevent degradation of individual tablets from exposure to moisture.Citation17 The median price for a misoprostol tablet in an alu/alu blister was $0.57, compared to a median price of $1.44 for misoprostol that was packed in a plastic/alu blister, a bottle, or another packaging type/unspecified (). Overall, quality- and non-quality-assured misoprostol products had similar median prices in this analysis ($0.63 per tablet for quality-assured misoprostol compared to $0.62 for non-quality-assured products). The median price of misoprostol also did not vary greatly if multiple brands were available (i.e. ≥3) or there were ≤2 brands identified ($0.64 and $0.61, respectively, ). However, some differences were noted in the median prices of quality- and non-quality-assured products in each region (). For example, results from LAC and South/Southeast Asia showed the median price of quality-assured misoprostol was notably higher, compared to non-quality assured brands (). On the other hand, quality-assured misoprostol in Africa and Eastern Europe/Central Asia regions had a lower median price than non-quality-assured products. Of note, African countries in this analysis contributed the highest proportion of price points on quality-assured misoprostol products (55.7%; 103/185); the second highest was from the Eastern Europe/Central Asia region (20.0%; 37/185).
Mifepristone
The price per tablet of mifepristone was analysed based on 59 price points recorded in 17 countries. Nearly three-quarters of the price data on mifepristone (43/59) came from the Eastern Europe/Central Asia region where mifepristone was confirmed available in the 10 countries included in this analysis (). Including all regions, the calculated median price per tablet was $11.78 and ranged from $1.77 to $37.83. Similar to the price pattern for misoprostol when analysed by region, the median price of mifepristone was highest in LAC ($24.47) and lowest in South/Southeast Asia ($5.20), though fewer price points from these regions contributed to this analysis. In Africa, mifepristone was identified in only four countries in the sample and prices ranged from $6.00 to $21.86 (). Countries with a higher number of unique misoprostol brands available (≥3 brands) had a lower median price of mifepristone ($9.39), compared to a median price of $12.96 in places with fewer misoprostol brands. The median price of mifepristone was higher when obtained from a pharmacy attached to a public hospital (n = 6) in comparison to other outlet types. However, the limited data points contributing to this analysis make it difficult to draw any conclusions.
Table 4. Price of mifepristone 200 mg tablets and combipacks stratified by location and other factors
Combipacks
Combipacks had a median price per pack of $11.14 (range $3.50–$35.86), based on 44 price points in 13 countries. The median price of a combipack was lowest in the South/Southeast Asia region ($5.40), compared to other regions where combipacks had a median price of $10.25 in Africa, $12.29 in Eastern Europe/Central Asia, and $27.70 in LAC (). Only two price points were confirmed for combipacks in LAC. Overall, the median price of combipacks ranged from $11 to $12 in private, stand-alone pharmacies, and in pharmacies attached to public or private hospitals or NGO clinics; whereas the median price per kit was lower in other locations, including drug shops ().
Nearly 40% of combipacks in this analysis were quality-assured products. The median price of quality-assured combipacks was lower than the median price for non-quality-assured combipacks (quality $9.52 vs. non-quality $11.33); these results are mostly driven by data from the Africa region. The overall median price of combipack was lower in countries where ≥3 misoprostol brands were identified ($7.79), compared to countries where only 1 or 2 misoprostol brands were identified ($12.46) ().
Prescription and price
Prescriptions were reported as required for 63.6% (234/368), 60.5% (49/81), and 64.4% (38/59) of misoprostol, mifepristone, and combipack products, respectively. In settings with more restrictive laws on abortion, 65.6% (112/247) of products were reported requiring a prescription by outlet staff, compared to 60.9% (159/261) in places with fewer restrictions. Prescriptions were reported as required at the point of sale at a similar frequency among private outlets (67.8%, 211/311), pharmacies attached to private hospitals or NGO clinics (61.2%, 52/85), and pharmacies attached to public facilities (59.2%, 42/71), compared with drugs shops and other outlet types that less often reported the need for a prescription (46.2% (12/26) and 26.7% (4/15), respectively). A closer look at quality-assured products in this analysis shows that prescriptions were requested for over three-quarters of quality-assured products (76.8%, 192/250), compared to 45.2% (80/177) of non-quality-assured products.
The overall median price of a misoprostol tablet was $0.10 higher for products requiring a prescription compared to those that did not ($0.66 and $0.56, respectively) (). Similarly, the median price of a combipack was higher with a prescription ($14.54), compared to $9.80 without a prescription (). The opposite trend was noted for mifepristone with the median price being slightly lower when a prescription was requested ($10.44), compared to a median price of $13.20 when not requested ().
Discussion
Medical abortion using the combination of mifepristone and misoprostol or with misoprostol only has the potential to increase access to safe abortion but relies upon expanded availability of high-quality products globally. This secondary analysis revealed wide variation in the pricing and availability of medical abortion products across regions and countries. Misoprostol tablets were found to be the most accessible medical abortion commodity in low- and middle-income countries in this analysis (identified in all 44 countries). In contrast, mifepristone tablets were identified in less than half of the countries; one-third of countries had combipacks for sale, and even fewer countries (16%) had both products. The less frequent availability of mifepristone and combipacks and inconsistent access to both products highlights the need for continued advocacy at the country level to remove legal barriers to abortion, as well as increased efforts among manufacturers and national drug regulators to expand registrations of these products.
Not surprisingly, mifepristone and combipacks were found at a higher frequency in places where the legal environment supported abortion. Nevertheless, certain regions had a higher concentration of one of these products over the other, which is likely due, in part, to donor investment and strategic partnerships between commercial entities and social marketing organisations to support differential investments in product registrations, and to expand distribution efforts in specific countries or regions.Citation18 For example, in African countries in this sample, 10 unique brands of combipacks were found compared to only three brands of mifepristone. Notably, a 2019 landscape report on the availability of combipacks found that there were no distributors of mifepristone alone in ten Sub-Saharan countries.Citation18 In the Eastern European/Central Asia region in this sample, the opposite pattern was observed, where a higher number of mifepristone brands were available compared to combipacks (). This is likely due to less investment from donors and social marketing organisations in combipack registration and distribution in countries where mifepristone is readily available. In LAC, there were notable gaps in the availability of both mifepristone and combipacks, compared to other regions, given the slower progress in advancing rights to legal abortion in LAC compared to other settings.Citation19
In countries where products are already registered and available, concerted efforts are needed to educate and encourage provider outlets to diversify their stock of medical abortion commodities. It was noted in this analysis that 5 out of 29 outlets in African countries, where combipacks were available, did not have misoprostol independently for sale. The lack of misoprostol could pose a problem to providers and women who may need access to additional doses to complete an abortion, particularly for second trimester abortions, or for treatment of incomplete abortion. Additionally, the stocking of all three products (misoprostol, mifepristone, and combipacks) is important in case of supply challenges or requests to purchase products individually for other indications, such as the use of misoprostol for postpartum haemorrhage. Stock-outs of misoprostol and pharmacy staff’s lack of knowledge about indications for its use remain key barriers to improving access to this medicine.Citation20–22 Further work is necessary at the national level to address weaknesses in supply chains and train pharmacy workers on the different purposes of these medicines and the benefits of stocking all three products.
While multiple brands of misoprostol were noted in this dataset (61 in total), global brands (i.e. brands available across multiple regions) were largely uncommon. Most misoprostol brands were sold within a specific region or country. One exception is the quality-assured misoprostol brand that was found in all four regions and in 28 out of the 44 countries (Supplementary Table S1). The retail price for this global brand varied widely, ranging from $0.09 to $27.63 per tablet (median $0.64), which suggests additional market factors leading to pricing inconsistencies across geographies and the need for further research to better understand these factors. In comparison, price ranges per tablet were less variable for the second and third most frequently identified misoprostol brands (median $1.48 (range $0.72–$1.87) and median $0.61 (range $0.30–$1.54)), which were found in specific regions (Eastern Europe/Central Asia and Africa, respectively) (Supplementary Table S1). Of note, the median prices per tablet for the three most common brands of misoprostol found in this sample were substantially higher than the median price per misoprostol as listed in Management Science for Health’s international price guide, which reported a median buyer price of $0.20 per misoprostol tablet.Citation12
The unit prices of mifepristone and combipacks also varied greatly. The most commonly identified mifepristone brand had a median price to the consumer of $10.35 per tablet and a price range from $3.02 to $17.91. The price range for the most frequently identified combipack brand was also wide ($4.02–$20.05); this product had a median price per pack of $15.44 and was found in three out of four regions (Supplementary Table S1). While increased brand diversity may help drive down prices of these products via competition, such a trend could not be explored in this analysis due to the limited number of mifepristone and combipack products in each setting. Africa was the only region in this sample where combipack products appeared to have a price-advantage over mifepristone (). It is likely that external subsidies and product pricing strategies by distributors contribute to this finding.Citation18
Until combipacks or mifepristone become increasingly available, a misoprostol only abortion may be a more accessible option and a reasonable alternative for many medical abortion users.Citation23 The present findings show that it may be a cheaper alternative. For example, in Africa, LAC, and South/Southeast Asia regions, the median price to purchase pills for an abortion up to 12 weeks’ gestation using misoprostol only is lower than the median price of purchasing a combipack or one mifepristone tablet plus four misoprostol tablets (Supplementary Table S2). Importantly though, only 17% of misoprostol brands in these settings were quality-assured, in comparison with 44% of combipacks that met this criterion. Also, depending on the region, the price of quality-assured misoprostol tablets was often higher than their non-quality-assured counterparts (); whereas quality-assured combipacks had a lower median price than non-quality-assured combipacks (). Limited availability of quality-assured medicines and the high consumer cost of medical abortion continue to be important factors affecting access to high-quality abortion care in many settings.
Indeed, international partners, manufacturing companies, and procurement agencies, including national governments, are encouraged to work together and develop marketing strategies that result in greater availability of quality-assured products while maintaining their low cost to users.Citation17,Citation24 Overall, within this dataset, the median prices for quality-assured and non-quality-assured misoprostol were similar (). However, regional differences were noted with the median price of quality-assured misoprostol higher than that of non-quality-assured misoprostol in the LAC and South/Southeast Asia regions, and the opposite trend was found in Africa and Eastern Europe/Central Asia regions (). While external subsidies may play a role in temporarily keeping the prices of quality-assured products lower in some settings,Citation18 it is not possible to draw a clear relationship in the higher median price of quality-assured misoprostol that was found in LAC and South/Southeast Asia regions. These regional differences warrant further investigation to verify these findings and, secondly, to identify if there are other factors that may be influencing consumer prices at the point of sale in these settings.
The requirement for a prescription is another potential barrier to accessing medical abortion. In this dataset, prescriptions were reported as required by outlet staff for approximately two-thirds of misoprostol, mifepristone, and combipack samples. However, regional variation was noted for this requirement depending on the type of product requested. For example, over 80% of misoprostol samples in Africa required a prescription; whereas prescription requests were lower in the other three regions for misoprostol (ranging from 50% to 55%). In three of the four regions, prescriptions were also more frequently reported as required for quality-assured products, including misoprostol and combipacks, compared to non-quality-assured products of these commodities.
Limitations
An important limitation of this analysis is that the data collectors’ experiences, including the pricing and prescription information they obtained, may not fully reflect what an individual seeking abortion care might encounter when trying to purchase the same products at these sites. Data collectors occasionally recorded comments to clarify that the price or prescription requirement was negotiated or depended on other factors, or would document the reasons why the product was not available. However, this contextual information was not uniformly collected in all settings and therefore could not be formally analysed. For instance, data collectors did not document socio-economic information about the neighbourhoods or populations served by the retail outlets that they visited in each country; and hence, it was not possible to understand whether medical abortion products accessed by regular clients at these sites are affordable to them. Data collectors also aimed to visit registered or licensed outlets as part of this exercise, and it is therefore plausible that pricing might have been different if purchased from unregistered outlets. Future studies should take into account socio-economic variables and setting-specific details to carry out additional costing analyses.
Another key limitation is that data on the availability of medicines and their prices were collected at a specific point in time and thus may not reflect the average availability over time or current pricing. These results are also not generalisable to all low- and middle-income countries nor do they include all products that are available in a country or region. Rather this analysis presents a snapshot of identified medical abortion commodities with analysable data on their unit prices. None of the products were tested to confirm if they were genuine. Lastly, the summary findings and totals should be interpreted cautiously as some countries and regions provided more data than others. For instance, Africa contributed a disproportionate number of data points on misoprostol given that 17 countries were visited in this region, whereas only 7 countries were included from the South/Southeast Asia region in this analysis. In fact, pricing information from some countries which are known to have a large number of medical abortion commodities (i.e. India, China) was not collected.
Conclusions
Overall, this dataset contributes to the body of evidence on the price of medical abortion pills at point of purchase for users. The insights gained from this assessment may help in planning future research that dives deeper into the medical abortion commodity landscape in a specific country or region. Given the wide variation in product availability and pricing between settings, country or region-specific landscape assessments will be useful for creating strategies to make these products more affordable and available. Indeed, many of the data points were likely a reflection of regional or national market practices and external funding that offers subsidies to the pricing of the product. Thus, the constantly changing landscape of medical abortion products warrants further research to determine how price and product availability at different outlet types change over time. Undertaking a regular price-to-user survey may provide independent data to assess the suitability of resource investments by funders and programmes and to ensure that resources are aimed to improve the availability and affordability of these essential and safe drugs.
Understanding the intricacies of medical abortion commodity pricing and availability will help to develop a roadmap for increasing product choice and more equitable access to medical abortion. The limited availability of mifepristone and combipacks in this assessment underscores the need for greater donor investment and global advocacy for the inclusion of quality-assured products on national essential medicines lists. Strategic partnerships between commercial entities, social marketing organisations and national drug authorities will be important for achieving the goal of increased registrations of medical abortion products at the country level. Until mifepristone becomes available (either alone or in combipacks) in addition to misoprostol, large segments of the population do not have access to the optimum regimen for medical abortion. Continued efforts by funders, international partners, and governments, are also needed to work with manufacturers to support their development of high-quality products that meet the requirements to be quality-assured. National health systems are also encouraged to procure and supply high-quality products to pharmacies and to educate their staff on the importance of stocking the different commodities for medical abortion as well as for other health indications.
Supplementary Tables
Download MS Word (48 KB)Acknowledgements
We are grateful for the data collectors’ hard work and persistence in each country to identify and purchase medical abortion products. We also thank Ms. Jennifer Blum and Concept Foundation for their input and technical guidance on the Medical Abortion Commodities Database project and contribution to data collection and acknowledge Julia Habbe for her contributions to data cleaning.
Disclosure statement
The authors declare no conflicts of interest.
Data availability statement
The data that support the findings of this study are available upon reasonable request to the authors.
Additional information
Funding
References
- World Health Organization. Medical management of abortion. Geneva: World Health Organization; 2018.
- World Health Organization. WHO recommendations on self-care interventions: self-management of medical abortion (WHO/SRH/20.11). Geneva: World Health Organization; 2020.
- ReproAction. Understanding and advocating for self-managed abortion; [cited 2020 Sept. 24]. Available from: https://reproaction.org/campaign/self-managed-abortion/
- Gynuity Health Projects. Map of mifepristone approvals; [cited 2020 Sept. 24]. Available from: https://gynuity.org/assets/resources/mapmife-en.pdf
- Gynuity Health Projects. Map of misoprostol approvals; [cited 2020 Sept. 24]. Available from: https://gynuity.org/assets/resources/mapmiso__en.pdf
- Doran F, Nancarrow S. Barriers and facilitators of access to first-trimester abortion services for women in the developed world: a systematic review. J Fam Plann Reprod Health Care. 2015;41:170–180.
- Winikoff B, Sheldon W. Use of medicines changing the face of abortion. Int Perspect Sex Reprod Health. 2012;38(3):164–166.
- Diamond-Smith N, Percher J, Saxena M, et al. Knowledge, provision of information and barriers to high quality medication abortion provision by pharmacists in Uttar Pradesh, India. BMC Health Serv Res. 2019;19(1):476.
- World Health Organization (WHO). Essential medicines list application mifepristone–misoprostol for medical abortion; [cited 2020 Sept. 24]. Available from: https://www.who.int/selection_medicines/committees/expert/22/applications/s22.1_mifepristone-misoprostol.pdf?ua=1
- International Planned Parenthood Federation. Medical abortion commodities database; [cited 2020 Sept. 24]. Available from: https://www.medab.org/
- Footman K, Keenan K, Reiss K, et al. Medical abortion provision by pharmacies and drug sellers in low- and middle-income countries: A systematic review. Stud Fam Plann. 2018;49(1):57–70.
- MSH (Management Sciences for Health). International medical products price guide. 2015 ed. Medford, MA: MSH; 2016.
- World Bank. World Bank country income’s classification status; [cited 2020 Sept. 24]. Available from: https://blogs.worldbank.org/opendata/new-country-classifications-income-level-2017-2018
- Center for Reproductive Rights. The world's abortion laws map 2014; [cited 2020 Sept. 24]. Available from: https://www.reproductiverights.org/sites/default/files/documents/AbortionMap2014.PDF
- UNFPA. Quality assurance policy for reproductive health medicines, November, 2012. Available from: https://www.unfpa.org/resources/reproductive-health-medicines.
- Murtagh C, Wells E, Raymond EG, et al. Exploring the feasibility of obtaining mifepristone and misoprostol from the internet. Contraception. 2018;97:287–291.
- Hall P. Quality of medicines: quality of misoprostol products. WHO Drug Inf. 2016;30:1–109.
- Mann Global Health. Landscape assessment: leveraging the role of national distributors to increase access to medical abortion combi-packs in Africa. Report to the Reproductive Health Supplies Coalition, January 2019 Available from: https://www.rhsupplies.org/uploads/tx_rhscpublications/Landscape_Assessment_Combi-Packs_RHSC_01.pdf.
- Singh S, Remez L, Sedgh G, et al. Abortion worldwide 2017: uneven progress and unequal access. New York: Guttmacher Institute, 2018.
- Reiss K, Footman K, Burke E, et al. Knowledge and provision of misoprostol among pharmacy workers in Senegal: a cross sectional study. BMC Pregnancy Childbirth. 2017;17(1):211.
- Ganle JK, Busia NT, Maya E. Availability and prescription of misoprostol for medical abortion in community pharmacies and associated factors in Accra, Ghana. Int J Gynaecol Obstet. 2019;144(2):167–173.
- Weaver G, Schiavon R, Collado ME, et al. Misoprostol knowledge and distribution in Mexico city after the change in abortion law: a survey of pharmacy staff. BMJ Sex Reprod Health. 2020;46:46–50.
- Raymond EG, Harrison MS, Weaver MA. Efficacy of misoprostol alone for first-trimester medical abortion: A systematic review. Obstet Gynecol. 2019;133(1):137–147.
- Schocken C. Business case: investing in production of high-quality misoprostol for low-resource settings, Jhpiego; 2014.