Abstract
Dental resin composites (DRCs) have become the first choice among different restorative materials for direct anterior and posterior restorations in the clinic. Though the properties of DRCs have been improved greatly in recent years, they still have several shortcomings, such as volumetric shrinkage and shrinkage stress, biofilm development, lack of radio-opacity for some specific DRCs, and estrogenicity, which need to be overcome. The resin matrix, composed of different monomers, constitutes the continuous phase and determine the performance of DRCs. Thus, the chemical structure of the monomers plays an important role in modifying the properties of DRCs. Numerous researchers have taken to design and develop novel monomers with specific functions for the purpose of fulfilling the needs in dentistry. In this review, the development of monomers in DRCs were highlighted, especially focusing on strategies aimed at reducing volumetric shrinkage and shrinkage stress, endowing bacteriocidal and antibacterial adhesion activities as well as protein-repelling activity, increasing radio-opacity, and replacing Bis-GMA. The influences of these novel monomers on the properties of DRCs were also discussed.
Introduction
Dental resin composites (DRCs) are esthetic restorative materials composed of the resin matrix, inorganic fillers, and coupling agents. Due to their versatility, DRCs have several applications in dentistry, such as restorative materials, cavity liners, pit and fissure sealants, luting materials, endodontic sealers, and root canal posts [Citation1]. With the problems that exist in the clinic and the requirements from clinicians, DRCs still face several challenges regarding the incidence of failed composite restorations, secondary caries, toxicity, etc., even though the properties of DRCs have been improved markedly in recent years [Citation2]. The modification of DRC composition can be approached through tailoring the resin matrix [Citation3–6], the fillers [Citation7,Citation8], and/or the coupling agents [Citation9–11]. Modification of resin matrix has often seemed to be most effective e.g. when attempting to reduce polymerization shrinkage stress [Citation12].
The resin matrix is a mixture of different monomers containing a small amount of an initiation system. After light or heat activation, monomers can polymerize in a short time to form a cross-linked polymeric network constituting a continuous phase which binds the fillers together [Citation13]. Macroscopically, the DRCs transform from a liquid or paste state into a solid state, which can withstand a certain load. The composition and chemical structure of the monomers significantly influence the properties of the polymer, thereby determining the performance of the cured DRCs. Therefore, the design and synthesis of novel monomers for the purpose of fulfilling the needs of dentistry is one of the research hotspots in dental materials science.
Usually, monomers used in thermoset DRCs are di(methyl)acrylate compounds, but in the last two decades, several monomers with other kinds of polymerizable functional groups were introduced into DRCs. This review will focus on the development in the design of monomers for overcoming the drawbacks of DRCs, and the influences of these novel monomers on the properties of DRCs will also be discussed.
Monomers for reducing volumetric shrinkage and shrinkage stress
During the polymerization of cross-linkable thermoset DRCs, weak van der Waals interaction between monomers is replaced by strong covalent bonds, leading to the reduced distance between monomers and thus to volume shrinkage [Citation14–17]. As a critical drawback of DRCs, volumetric shrinkage and related stress on the adjacent tooth structure is a major concern in restorative dentistry [Citation18]. After being applied and light-cured in the prepared cavity, the volumetric shrinkage and elastic modulus of DRC develop and increase gradually during polymerization, but shrinkage stress can be relieved by the rearrangement of the polymer in the early stage of polymerization. At some stage, the gradual development and increase in elastic modulus hinder any further adsorption of stress caused by the volumetric shrinkage and lead to stress being generated within the material, at the tooth/restoration interface and in the tooth structure [Citation19]. If the shrinkage stress is sufficiently concentrated, it may induce interfacial bond failure, microleakage, post-operative sensitivity, deformation of the tooth cusps, marginal staining, and the formation of secondary caries [Citation1,Citation12,Citation17,Citation20–22].
Low volumetric shrinkage methacrylates
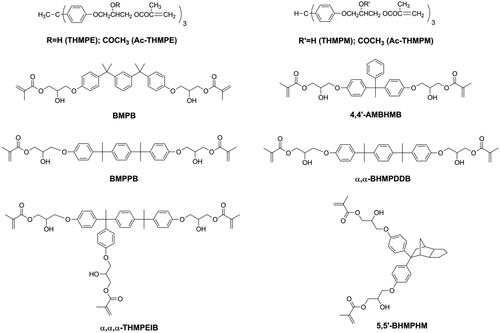
According to the composition and light curing conditions, DRCs exhibit volumetric shrinkage ranging from 1% to 6% [Citation17]. The volumetric shrinkage of DRCs cannot be eliminated, but it can be reduced. As for the resin matrix, it is well known that volumetric shrinkage can be reduced by using monomers with a low reactive group (double bond) concentration, or high molecular weight monomers with the same amount of functional (double bond) groups [Citation23–27]. Using mono-methacrylate is an effective way to reduce double bond concentration, but mono-methacrylate has no contribution to the cross-link density of the polymeric network which might influence the mechanical properties of DRC. Therefore, several methacrylate monomers with high molecular weight (as shown in ) have been synthesized and used to replace Bisphenol A glycidyl methacrylate (Bis-GMA) [Citation28–35], which is the most used monomer in commercial DRCs. All these, newer monomers have been Bis-GMA analogues with more benzene or aliphatic rings in the structure. Due to these rigid groups, the use of these monomers led to reduced volumetric shrinkage of the resin matrix while maintaining mechanical properties, but also to a reduction in double bond conversion. However, filler loading, which also plays an important role for the properties of DRCs [Citation36–38], may have to be lowered due to the high viscosity of these monomers.
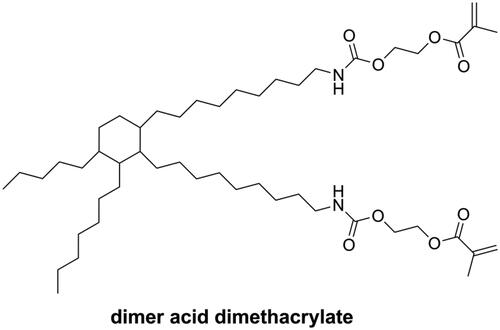
Dimer acid dimethacrylate (as shown in ) is one kind of monomer with high molecular weight but low viscosity. In photopolymerization, dimer acid dimethacrylate showed a high degree of conversion coupled with low volumetric shrinkage and nearly negligible water sorption and is considered a good alternative to Bis-GMA [Citation39]. NĭDurance is one kind of commercial DRCs containing dimer acid dimethacrylate, UDMA (bis-[(2-methacryloyloxy-ethoxycarbonyl)amino]-2,2,4-trimethylhexane), and Bis-EMA (Ethoxylated bisphenol A dimethacrylate). However, NĭDurance has been reported to have higher volumetric shrinkage than Bis-GMA-based commercial DRCs [Citation40].
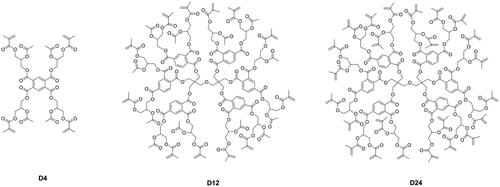
Different from linear monomers, dendritic macromers have a more compact structure [Citation41] and less free volume [Citation42] and can thus reduce the polymerization shrinkage of the resin matrix [Citation43–45]. Viljanen et al. [Citation46–50] prepared dendrimer macromers with 4 and up to 12 or even 24 methacrylate groups (as shown in ), but the high viscosity of these macromers became a problem for curing, and higher concentration of photoinitiation system was needed.
Compared with Bis-GMA (shrinkage 5.2%), the reactive diluent triethylene glycol dimethacrylate (TEGDMA, shrinkage 12.5%) has higher volumetric shrinkage [Citation51]. Hence, developing a low-shrinkage diluent is also an effective way to achieve low-shrinkage DRCs.
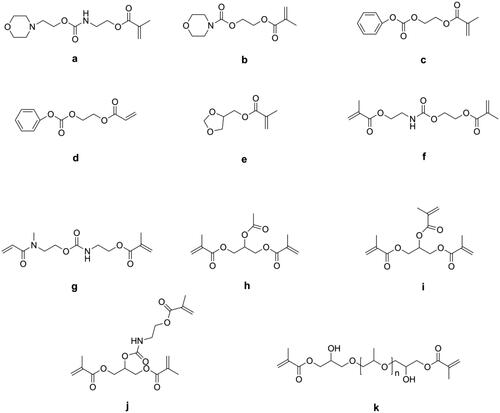
Lu et al. [Citation52] synthesized several mono-(meth)acrylates (as shown in ) as alternatives to TEGDMA and mixed these with Bis-GMA to form dental resin systems. Due to their low double bond concentration, all these new resin systems had lower volumetric shrinkage than Bis-GMA/TEGDMA resin systems, but most of them had lower flexural strength and modulus because of the reduced cross-linking density.
Moszner et al. [Citation53] synthesized five colorless and low-viscosity monomers (as shown in ) and found they had lower volumetric shrinkage than TEGDMA and showed an acceptable performance as a diluent in Bis-GMA/UDMA-containing DRCs. Luo et al. [Citation54] synthesized two methacrylate terminated oligomers named polyropylenglycol bis(2-hydroxy-3-methoxypropyl) dimethacrylates (PPMAs, as shown in ) with different average molecular weights and used these to replace partially TEGDMA in Bis-GMA/TEGDMA resin systems. The results showed that when 20 wt.% of TEGDMA was replaced by PPMAs, the obtained DRCs exhibited significantly lower volumetric shrinkage. However, 10 wt.% of replacement had no effect, because the PPMA-containing DRCs were more homogeneous than the control DRC, which was not beneficial for reducing shrinkage [Citation55].
Ring-opening and cyclopolymerizable monomers
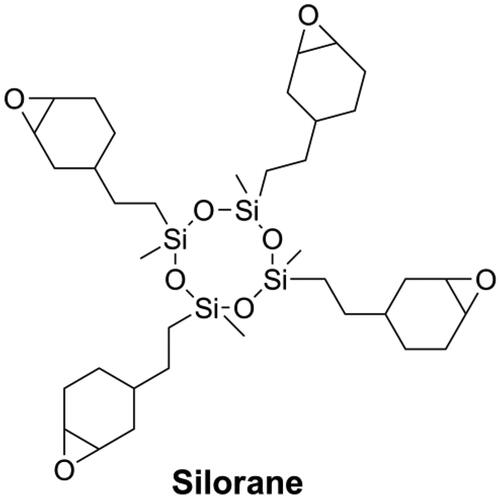
Besides reducing the double bond concentration of the resin matrix, several novel monomers that undergo ring-opening polymerization and cyclopolymerization were developed and introduced in dentistry to reduce polymerization shrinkage of DRCs. During ring-opening polymerization, when a bond that goes from a van der Waals distance to a covalent distance, another bond goes from a covalent distance to a near van der Waals distance, resulting in a certain extent of volumetric expansion, thus offsetting the shrinkage accompanying the chain propagation [Citation56]. Cyclic monomers, such as epoxy [Citation56–58], spiro orthocarbonates [Citation59–62], oxetanes [Citation63,Citation64], and vinylcyclopropanes [Citation21,Citation65–67] can undergo ring-opening polymerization. Silorane (as shown in ) which undergo cationic ring-opening polymerization showed low shrinkage and good mechanical performance [Citation68]. The commercial Silorane-based DRC named Filtek Silorane (3 M-ESPE, Seefeld, Germany) is the only methacrylate-free DRC that has a volumetric shrinkage less than 1% [Citation69]. Nonetheless, Filtek Silorane had lower bond strength to Class-I cavity dentin than conventional methacrylate-based DRCs [Citation70] and did not appear to offer any clinical advantages over methacrylate-based DRCs when used in the restoration of Class-II cavities [Citation71].
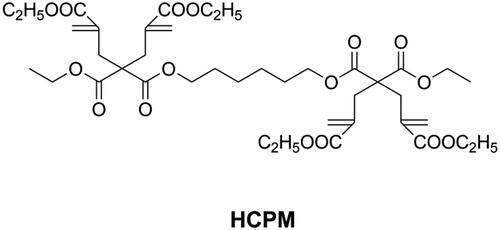
Compared with other cyclic monomers, vinylcyclopropanes are considered to have better resistance to humidity and acidic/basic impurities, which is essential for dental applications [Citation21,Citation66,Citation67]. Catel et al. [Citation66,Citation67] and Tauscher et al. [Citation21], synthesized a series of vinylcyclopropanes (as shown in ) to be used as a base resin and reactive diluents. After composition optimization, vinylcyclopropane-based DRCs showed promising mechanical properties and lower volumetric shrinkage than methacrylate-based DRCs. However, and unfortunately, it is difficult to access vinylcyclopropanes due to their inherent instability and the susceptibility of their double bond to engage in side reactions. Different from ring-opening monomers, cyclopolymerizable monomers can form aliphatic rings during polymerization, resulting in larger free volume and low shrinkage [Citation72]. Peer et al. [Citation73] synthesized a cyclopolymerizable dimalonate (HCPM, as shown in ) and concluded that it could be used as low shrinkage reactive diluent.
Low shrinkage stress monomers
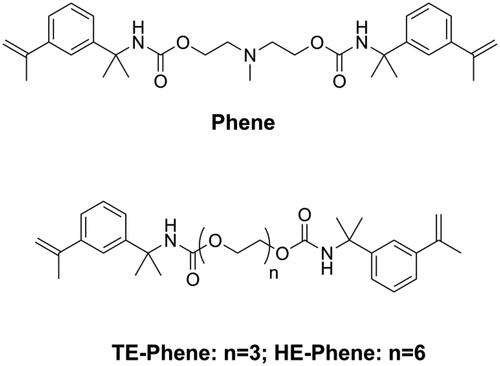
Though volumetric shrinkage is important for DRCs, dental restoration failures are mainly related to the development of shrinkage stress, which is a much more complex phenomenon than the volumetric change of the organic matrix. Shrinkage stress can be reduced through reducing volumetric shrinkage [Citation22,Citation54,Citation74] and polymerization rate [Citation75,Citation76]. He et al. [Citation77,Citation78] synthesized three kinds of novel monomers (Phenes, as shown in ) containing an α-methylstyryl structure as a polymerizable group. Due to the resonance structure that delocalizes the double bonds, the α-methylstyryl group has a lower polymerization rate than the methacrylate group. Moreover, it was reported that polymerization of the α-methylstyryl group had an appreciable depropagation rate even at room temperature [Citation79]. Both these effects could delay the vitrification state of the material, resulting in lower shrinkage stress.
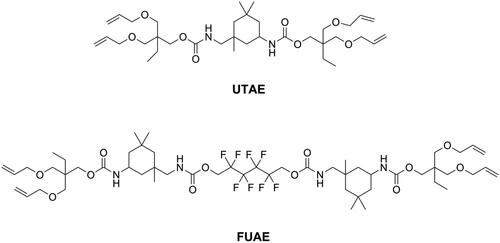
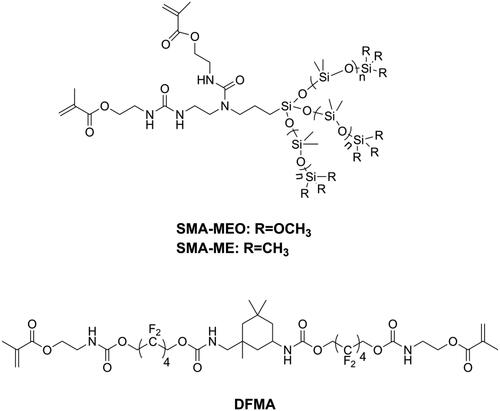
Utilizing monomers which possess unique polymerization mechanisms is also a strategy for achieving low shrinkage stress and might be more effective than reducing volumetric shrinkage. The overall idea is delaying the gel point so that the stress can be released during polymerization. Different from the chain-growth mechanism of methacrylates, thiol-ene resin systems contained thiol and vinyl monomers, which polymerize via a step growth mechanism involving free-radical addition followed by a chain transfer reaction and were found to result in delayed gelation and low shrinkage stress [Citation80–82]. However, since the thiol-ene resin systems showed lower flexural strength and modulus than commonly used Bis-GMA/TEGDMA resin systems [Citation83], they were incorporated into a methacrylate resin system to form a thiol-ene-methacrylate ternary system [Citation84]. To maintain flexural strength, there is a mass fraction limitation of thiol-ene resin systems in thiol-ene-methacrylate ternary systems. Beigi et al. [Citation85] synthesized a urethane terta allyl ether monomer UTAE (as shown in ) and mixed it with commercial thiol pentaerythritol tetra (3-mercaptopropionate) (PETMA) to prepare a thiol-ene system. Subsequently, they added this thiol-ene system into a Bis-GMA/TEGDMA system and found that flexural strength and modulus of the ternary system was reduced significantly when the concentration of thiol-ene was higher than 20 wt.% in the resin. Using a synthesized fluorine-containing urethane-based allyl ether monomer FUAE (as shown in ), Fu et al. [Citation86] were able to raise the amount of a thiol-ene system up to 30 wt.% in a ternary system without impairing flexural strength and modulus. The application of thiol-ene systems in the clinic is still rare, because of the distinct and unpleasant odor of thiol monomers, obvious even after increasing their molecular weight [Citation87].
In recent years, covalent adaptable networks (CANs) have been used to reduce shrinkage stress. When subjected to specific stimuli, the covalent bonds in the network dynamically break, relax, and rearrange to form new bonds, thus allowing stress to be released during network formation [Citation88]. Using addition-fragmentation chain transfer monomers to reduce the shrinkage stress of DRCs is a. type of CANs mechanism. Park et al. [Citation89] incorporated allyl sulfide monomers (as shown in ) into norbornene-methacrylate systems to obtain low shrinkage stress DRCs. The new DRCs showed more than 96% stress reduction compared with Bis-GMA/TEGDMA-based DRCs but also exhibited lower flexural strength. Lamparth et al. [Citation90] and Crob et al. [Citation91] evaluated allyl sulfone urethanes (as shown in ) and allyl sulfide methacrylates (as shown in ) as addition-fragmentation chain transfer agents for low shrinkage stress DRCs. The results revealed that all these agents could reduce shrinkage stress while maintaining flexural strength, but the maximum stress reduction was only around 36% at an agent concentration of 20 mol% in the resin matrix.
Considering the accessibility of raw material and overall properties of the final DRCs, using Phenes and addition-fragmentation chain transfer monomers seem to be the most promising approaches so far.
Monomers as bactericides and anti-bacterial adhesion agents
In contrast to certain other restorative materials, DRCs have no intrinsic antibacterial properties, thus more biofilm accumulation occurs on the surface of DRCs, leading to the high incidence of secondary caries [Citation92,Citation93], which is one of the main reasons for clinical restoration failure [Citation94]. Therefore, it is essential and necessary to endow DRCs with antibacterial properties. Generally, antibacterial properties can be classified as the ability to kill bacteria that surround or are attached to the surface of the DRCs and to inhibit bacteria adherence on the surface. Both these effects can be achieved by adding monomers with specific functional designs. Because the formation of biofilm is initiated by the adsorption of salivary proteins, it is expected that biofilm formation could be minimized by precluding protein adsorption [Citation95].
Bactericidal monomers
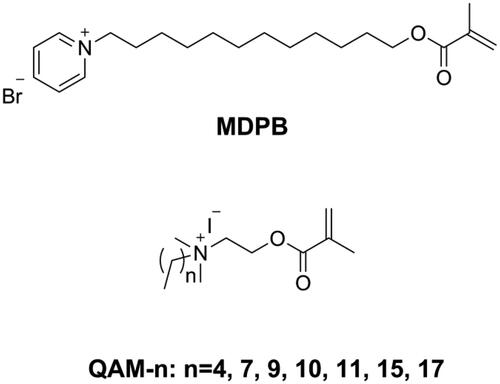
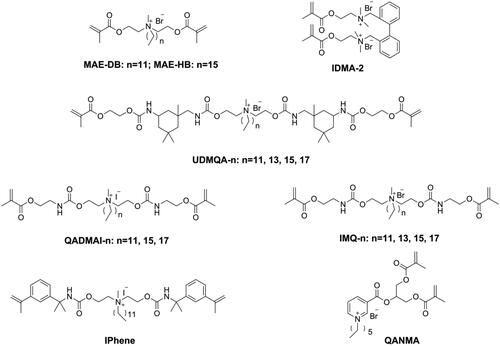
Releasable bactericides like chlorhexidine [Citation96,Citation97], benzalkonium chloride [Citation98], and cetylpyridinium [Citation99] can kill pathogens efficiently, but also have inevitable disadvantages, such as short-term effectiveness, impaired mechanical properties, and induced antibiotic resistance [Citation100]. To achieve long-term antibacterial effectiveness, Imazato introduced a concept of ‘immobilized bactericide’ into dentistry [Citation101]. An immobilizable bactericide is a monomer that contains a bacteriocidal group as well as a polymerizable group in its structure, which can then participate in the polymerization process and immobilize the bacteriocidal group in the polymeric network. The first immobilizable bactericide used in dentistry was methacryloyloxydodecyl pyrimidinium bromide (MDPB, as shown in ) invented by Imazato and co-workers [Citation101–105]. Subsequently, several kinds of immobilized bactericides containing quaternary ammonium as the bacteriocidal group have been synthesized and used in dentistry.
He et al. [Citation106] synthesized a series of quaternary ammonium mono-methacrylates (QAM-n, as shown in ) with an alkyl chain length ranging from 5 to 18 and found that antibacterial activity of the monomer against Streptococcus mutans increased when the alkyl chain length increased from 5 to 16 and decreased when the alkyl chain length increased further to 18, thus showing a typical cut-off effect. After incorporating 5 wt.% of QAM-n (n from 9 to 17) into Bis-GMA/TEGDMA resins, nearly all the obtained resins showed an inhibition effect on 6h young biofilm; only resins with QAM-15 and QAM-17 showed an inhibition effect on 24h mature biofilm, and all QAM-n had no negative effect on mechanical properties [Citation107,Citation108]. Because of a miscible problem with Bis-GMA/TEGDMA resin, 5 wt.% was the maximum mass fraction of QAM-n possible, which meant that the antibacterial activity of the prepared resins was not sufficiently strong. He et al. [Citation109] used Bis-GMA, methyl methacrylate (MMA), and QAM-11 (DDMAI) to prepare resin systems with a high mass fraction (from 15 wt.% to 25 wt.%) and found the resin systems to show strong biofilm inhibitory effect, but also to have significantly lower flexural properties as well as higher water sorption and solubility.
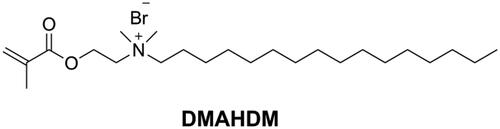
Dimethylaminohexadecyl methacrylate (DMAHDM, as shown in ) synthesized by the reaction between 2-(dimethylamino)ethyl methacrylate (DMAEMA) with 1-bromohexadecane is the most studied quaternary ammonium mono-methacrylate in recent years [Citation110]. Wang [Citation111] added 10 wt.% of DMAHDM into Bis-EMA/PMGDM (pyromellitic dianhydride glycerol dimethacrylate) resin matrix to prepare a DRC. The obtained DRC showed an excellent antibacterial rate (more than 90%) without compromising flexural properties. In another study [Citation112] it was found that even 5 wt.% of DMAHDM in Bis-GMA/TEGDMA endowed the DRC with strong antibacterial activity while maintaining flexural properties and that the effect lasted for 6 months in distilled water. However, Cherchali et al. [Citation113] reported that a DRC with 10 wt.% of DMAHDM in the resin matrix (Bis-GMA/TEGDMA) had good antibacterial activity but lower flexural strength than the control DRC. Vidal et al. [Citation114] also reported that 5 wt.% and 10 wt.% of DMAHDM in DRCs did not influence mechanical properties in dry condition. However, after 30 d of water immersion, the mechanical properties of the DMAHDM containing DRCs were lower than those of the control DRC. The different results in the above-mentioned studies might be due to the differences in matrix composition, filler characteristics and filler content.
Addressing the issues of poor miscibility and lack of contribution to cross-linking density of quaternary ammonium mono-methacrylate, quaternary ammonium monomers with multi-polymerizable groups (as shown in ) were designed and synthesized [Citation115,Citation116]. However, due to the reduced quaternary ammonium concentration in the structure, a higher mass fraction (from 10 wt.% to 50 wt.%) was needed to guarantee sufficient antibacterial activity of these monomers, lowering the mechanical properties [Citation117–122]. Therefore, further optimization work is needed for these monomers.
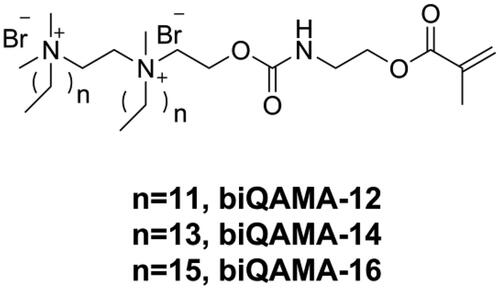
Because the antibacterial activity of DRCs is dependent on the concentration of the incorporated quaternary ammonium structure, using a monomer with high quaternary ammonium concentration might endow DRCs with strong antibacterial activity at a low-dose drug loading. Bis-quaternary ammonium monomers with a higher concentration of quaternary ammonium structure might have the potential to provide sufficient antibacterial activity even at a low molar concentration [Citation123]. He et al. [Citation124] designed three kinds of bi-quaternary ammonium mono-methacrylates (biQAMAs, as shown in ) and added 5 wt.% of these into Bis-GMA/TEGDMA resins to prepare DRCs. The results showed that all biQAMA-containing DRCs exhibited strong antibacterial activity (more than 90% antibacterial rate against S. mutans) and comparable physicochemical properties as the control DRC, except that the water sorption of biQAMA-containing DRCs was higher than that of the control. However, only biQAMA with a side alkyl chain length of 12 had no influence on the cytotoxicity of the DRC, whereas the other two biQAMAs increased the cytotoxicity of the DRC.
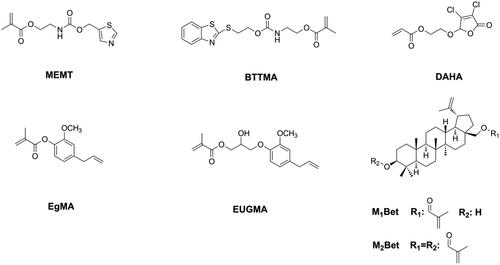
Although the quaternary ammonium group has strong and broad-spectrum antibacterial activity, its hydrophilic structure can increase the water sorption of DRCs and influence the water resistance of DRCs. Moreover, the antibacterial activity of quaternary ammonium methacrylate-containing DRCs can be reduced due to the electrostatic interactions between quaternary ammonium salts and proteins in saliva [Citation125,Citation126]. Therefore, other bactericidal structures, such as heterocyclic rings [Citation127–129] and natural bactericidal structures [Citation130–135], were chosen to be connected with the (meth)acrylate group and then applied in dentistry as immobilizable bactericides (as shown in ). All these monomers endowed DRCs with antibacterial activity without sacrificing physicochemical properties [Citation127–135], thus showing great potential for clinical applications.
All the above studies focused on how to kill bacteria effectively without considering the influence of the bactericides on the oral microbiome. However, as an unbalanced oral microbiome may be detrimental to general health [Citation136], developing antibacterial agents that are friendly to the commensal microbiome should be the aim of future research.
Anti-bacterial adhesion monomers
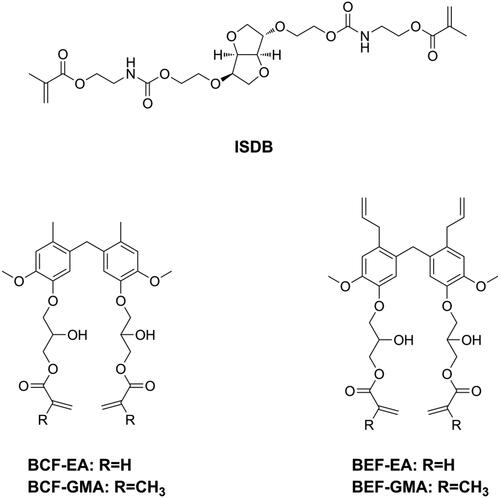
Bacterial adhesion is reversable and considered to be the most important step in biofilm formation [Citation137]. Thus, modification of DRCs to be anti-adhesive against cariogenic bacteria should be an effective way to reduce biofilm formation. According to surface thermodynamics, bacterial adhesion will not be favored if it causes the free energy function to increase [Citation138]. Under salivary conditions, it is considered that bacteria with high surface free energy have difficulty in accumulating on surfaces with low surface free energy. Because bacteria in early plaque such as S. mutans, Streptococcus sanguis, and V. parvula are all high surface energy bacteria [Citation139,Citation140], modifying DRCs to have low surface energy might be beneficial in preventing secondary caries. Silicon-based materials is one kind of materials with low surface free energy and have been widely used in antifouling coating [Citation141,Citation142]. Tong et al. [Citation143] prepared two kinds of silicone methacrylates (SMA-ME and SMA-MEO, as shown in ) and incorporated these into Bis-GMA/TEGDMA. The obtained resins did not only display an anti-bacterial adhesion effect but also resistance to protein adsorption, due to decreased surface free energy and increased hydrophobicity. However, silicone methacrylates displayed significantly lower flexural strength and modulus. Experimental silicone methacrylate-containing DRCs may have promising mechanical properties, reduced biofilm formation, acceptable cytotoxicity, and good bond strength to adhesive-treated dentin [Citation144,Citation145]. Fluorinated polymers are typical materials with low surface free energy and are attractive for dental application due to their advantages such as good biocompatibility, resistance to a wide range of chemicals, and potential resistance to bacterial adhesion [Citation3]. Zhang et al. [Citation146] synthesized a fluorine-containing methacrylate (DFMA, as shown in ) and used it to replace Bis-GMA as a base resin in DRCs. It was found that DFMA-based DRCs exhibited anti-bacterial adhesion effects, resistance to protein adsorption as well as better physicochemical properties than a Bis-GMA-based DRC.
Figure 16. Structures of methacrylates for endowing dental resin composites with antibacterial adhesion activity.
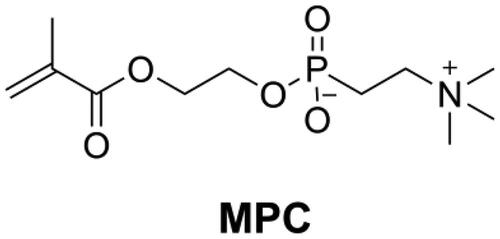
2-Methacryloyloxyethyl phosphorylcholine (MPC, as shown in ) is a monomer that has an excellent ability to repel protein adsorption and prevent bacterial adhesion. Because of the high hydrophilicity of MPC, there would be an abundance of free water on the surface of MPC-containing polymers that can detach proteins effectively [Citation147,Citation148]. Incorporation of MPC into DRCs reduced protein adsorption and bacteria attachment on the surface of DRCs [Citation148]. The combined use of antibacterial monomer quaternary ammonium dimethylaminohexadecyl methacrylate (DMAHDM) and MPC, led to a DRC with a strong biofilm inhibitory effect [Citation149]. However, the concentration of MPC in DRCs should be optimized, because too high an MPC concentration will reduce mechanical properties and increase water sorption.
Monomers as radio-opaque agents
Radio-opacity is an essential property for DRCs, allowing restoration to be easily distinguished from tooth tissue through X-ray examination. This distinction is beneficial for precisely localizing the interface between restoration and tooth and for assessing the clinical effectiveness of the restoration [Citation150]. It is also worth noting that too high a radio-opacity may cause problems in novel medical imaging systems such as cone-beam x-ray system and magnetic resonance imaging [Citation151,Citation152].
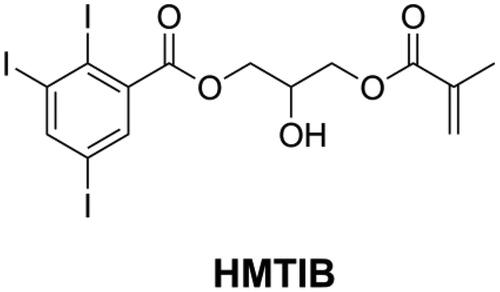
Usually, the radio-opacity of DRCs is dependent on the type and amount of inorganic fillers. However, some specific DRCs like flowable DRCs and E-glass fiber reinforced DRCs may have insufficient radio-opacity [Citation153,Citation154] because of their limited filler loading. For this reason, it is necessary to increase the radio-opacity of the resin matrix. The attenuation of X-rays in materials is associated with the density of the materials and with the atomic number and electron density of the elements that constitute the materials. Atoms with high atomic number can cause significant attenuation of X-rays [Citation155]. Iodine is one kind of high atomic number atom with high electronic density and can thus block X-rays effectively. He et al. [Citation107,Citation109,Citation118,Citation120] synthesized several kinds of quaternary ammonium methacrylate with iodine anion and found that all these monomers endow dental resins with both antibacterial activity and radio-opacity. However, these monomers had a low concentration of iodine anion and therefore limited capacity of improving radio-opacity. He et al. [Citation156] synthesized a monomer named 2-hydroxy-3-methacryloyloxypropyl- (2,3,5-triiodobenzoate) (HMTIB, as shown in ) with a high concentration of iodine element and mixed with Bis-GMA and MMA to prepare E-glass fiber reinforced composites (EFRCs). The results showed that HMTIB increased the radio-opacity of EFRCs significantly, and the obtained EFRCs had higher flexural strength, higher modulus, and lower water sorption.
Monomers as Bis-GMA substituents
Since being invented, Bis-GMA, which is a derivative of Bisphenol A (BPA), have dominated as the main monomer in DRCs. BPA is an endocrine-disrupting compound that can cause several diseases [Citation157–161]. Although BPA is not a component of dental materials, and although alternative synthetic routes of Bis-GMA no longer require BPA, several studies still found the presence of BPA in patients’ urine and saliva after dental procedures [Citation162]. Moreover, the latest studies showed that BPA may be released during the grinding and degradation of dental materials [Citation163,Citation164]. Consequently, numerous BPA-free monomers have been developed and used as alternatives to Bis-GMA, and most of them have shown promising properties and great potential in dentistry [Citation74,Citation146,Citation165–177]. However, the estrogenicity of these new developed BPA-free monomers has not been investigated. Jun et al. [Citation178] used isosorbide to synthesize light polymerizable isosorbide-derived biomonomers (ISDBs, as shown in ) and prepared Bis-GMA free sealants with ISDBs. They found the ISD-based sealants to have properties comparable to a Bis-GMA-based sealant and no estrogenicity. Sun et al. [Citation179,Citation180] tested the estrogenicity of two bio-based phenols and used these two phenols to prepare a series of BPA-free monomers (as shown in ), after confirming the absence of estrogenicity. Subsequently, they used these monomers to prepare Bis-GMA-free DRCs. Though these Bis-GMA-free DRCs exhibited acceptable physicochemical properties, certain properties still need to be improved to maintain the same level of performance as Bis-GMA-based DRCs.
Conclusion
Tailoring monomers is an effective way of endowing dental resin composites with specific functions that prolong the service life of the restorations and reduce their potential harm to patients. However, so far, improving some properties has inevitably led to the impairment of other properties. Therefore, greater efforts should be made to develop novel monomers or to optimize the composition using existing monomers for the purpose of achieving low shrinkage and shrinkage stress, inhibited biofilm formation, resistance to enzymatic and hydrolytic degradation, or remineralization.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Ferracane JL. Resin composite – state of the art. Dent Mater. 2011;27(1):29–38.
- Vallittu PK. An overview of development and status of fiber-reinforced composites as dental and medical biomaterials. Acta Biomater Odontol Scand. 2018;4(1):44–55.
- Moszner N, Salz U. New developments of polymeric dental composites. Prog Polym Sci. 2001;26(4):535–576.
- Moszner N, Hirt T. New polymer-chemical developments in clinical dental polymer materials: enamel-dentin adhesives and restorative materials. J Polym Sci A Polym Chem. 2012;50(21):4369–4402.
- Zhu XX, Lavigueur C. Recent advances in development of dental composite resins. RSC Adv. 2012;2:59–63.
- Pfeifer CS, Fugolin APP. New resins for dental composites. J Dent Res. 2017;96:1085–1092.
- Habib E, Wang R, Wang Y, et al. Inorganic fillers for dental resin composites: present and future. ACS Biomater Sci Eng. 2016;2(1):1–11.
- Wang Y, Zhu M, Zhu XX. Functional fillers for dental resin composites. Acta Biomater. 2021;122:50–65.
- Karabela MM, Sideridou ID. Effect of the structure of silane coupling agent on sorption characteristics of solvents by dental resin-nanocomposites. Dent Mater. 2008;24(12):1631–1639.
- Matinlinna JP, Vallittu PK, Lassila LVA. Effects of different silane coupling agent monomers on flexural strength of an experimental filled resin composite. J Adhes Sci Technol. 2011;25(1–3):179–192.
- Nihei T. Dental applications for silane coupling agents. J Oral Sci. 2016;58(2):151–155.
- Meereis CTW, Münchow EA, Rosa WLO, et al. Polymerization shrinkage stress of resin-based dental materials: a systematic review and meta-analyses of composition strategies. J Mech Behav Biomed Mater. 2018;82:268–281.
- Ferracane JL, Pfeifer CS, Hilton TJ. Microstructural features of current resin composite materials. Curr Oral Health Rep. 2014;1(4):205–212.
- Tobolsky AV, Leonard F, Roeser GP. Use of polymerizable ring compounds in constant volume polymerizations. J Polym Sci. 1948;3(4):604–606.
- Ge J, Trujillo M, Stansbury J. Synthesis and photopolymerization of low shrinkage methacrylate monomers containing bulky substituent groups. Dent Mater. 2005;21(12):1163–1169.
- Ellakwa A, Cho N, Lee IB. The effect of resin matrix composition on the polymerization shrinkage and rheological properties of experimental dental composites. Dent Mater. 2007;23(10):1229–1235.
- Soares CJ, Faria-E-Silva AL, Rodrigues MdP, et al. Polymerization shrinkage stress of composite resins and resin cements – what do we need to know? Braz Oral Res. 2017;31(Suppl 1):49–63.
- Rees JS, Jacobsen PH. The polymerization shrinkage of composite resin. Dent Mater. 1989;5(1):41–44.
- Peutzfeldt A, Asmussen E. Determinants of in vitro gap formation of resin composites. J Dent. 2004;32(2):109–115.
- Ferracane JL, Hilton TJ. Polymerization stress-is it clinically meaningful? Dent Mater. 2016;32(1):1–10.
- Tauscher S, Catel Y, Fässler P, et al. Development of low shrinkage composites based on novel crosslinking vinylcyclopropanes. J Appl Polym Sci. 2017;134(48):45577.
- He J, Garoushi S, Vallittu PK, et al. Effect of low-shrinkage monomers on the physicochemical properties of experimental composite resin. Acta Biomater Odontol Scand. 2018;4(1):30–37.
- Patel MP, Braden M, Davy KWM. Polymerization shrinkage of methacrylate esters. Biomaterials. 1987;8(1):53–56.
- Venhoven BAM, de Gee AJ, Davidson CL. Polymerization contraction and conversion of light-curing BisGMA-based methacrylate resins. Biomaterials. 1993;14(11):871–875.
- Yap AU, Soh MS. Post-gel polymerization contraction of “low shrinkage” composite restoratives. Oper Dent. 2004;29:182–187.
- Dewaele M, Truffier-Boutry D, Devaux J, et al. Volume contraction in photocured dental resins: the shrinkage-conversion relationship revisited. Dent Mater. 2006;22(4):359–365.
- de Oliveira DC, Rovaris K, Hass V, et al. Effect of low shrinkage monomers on physicochemical properties of dental resin composites. Braz Dent J. 2015;26(3):272–276.
- Chung CM, Kim MS, Kim JG, et al. Synthesis and photopolymerization of trifunctional methacrylates and their application as dental monomers. J Biomed Mater Res. 2002;62(4):622–627.
- Kim JG, Chung CM. Trifunctional methacrylate monomers and their photocured composites with reduced curing shrinkage, water sorption, and water solubility. Biomaterials. 2003;24(21):3845–3851.
- He J, Luo Y, Liu F, et al. Synthesis, characterization and photopolymerization of a new dimethacrylate monomer based on (α-mehtyl-benzylidene)bisphenol used as root canal sealer. J Biomater Sci Polym Ed. 2010;21(8–9):1191–1205.
- He J, Luo Y, Liu F, et al. Synthesis and characterization of a new trimethacrylate monomer with low polymerization shrinkage and its application in dental restoration materials. J Biomater Appl. 2010;25(3):235–249.
- He J, Liao L, Liu F, et al. Synthesis and characterization of a new dimethacrylate monomer based on 5,5’-bis(4-hydroxylphenyl)-hexahydro-4,7-methanoindan for root canal sealer application. J Mater Sci Mater Med. 2010;21:1135–1142.
- He J, Liu F, Luo Y, et al. Synthesis and characterization of a dimethacrylates monomer with low shrinkage and water sorption for dental application. J Appl Polym Sci. 2012;125(1):114–120.
- He J, Liu F, Vallittu PK, et al. Synthesis of dimethacrylates monomers with low polymerization shrinkage and its application in dental composite materials. J Polym Res. 2012;19(8):9932.
- He J, Liu F, Vallittu PK, et al. Synthesis and characterization of new dimethacrylate monomer and its application in dental resin. J Biomater Sci Polym Ed. 2013;24(4):417–430.
- Kim KH, Ong JL, Okuno O. The effect of filler loading and morphology on the mechanical properties of contemporary composites. J Prosthet Dent. 2002;87(6):642–649.
- Bociong K, Szczesio A, Krasowski M, et al. The influence of filler amount on selected properties of new experimental resin dental composite. Open Chem. 2018;16(1):905–911.
- Liu J, Zhang H, Sun H, et al. The development of filler morphology in dental resin composites: a review. Materials. 2021;14(19):5612.
- Trujillo-Lemon M, Ge J, Lu H, et al. Dimethacrylate derivatives of dimer acid. J Polym Sci A Polym Chem. 2006;44(12):3921–3929.
- Boaro LCC, Gonçalves F, Guimarães TC, et al. Polymerization stress, shrinkage and elastic modulus of current low-shrinkage restorative composites. Dent Mater. 2010;26(12):1144–1150.
- Potschke D, Ballauff M, Lindner P, et al. Analysis of structure of dendrimers in solution by small-angle neutron scattering including contrast variation. Macromolecules. 1999;32(12):4079–4087.
- Sendijarevic I, McHugh AJ. Effects of molecular variables and architecture on the rheological behavior of dendritic polymers. Macromolecules. 2000;33(2):590–596.
- Wan Q, Schricker SR, Culbertson BM. Methacrylate derivatized hyperbranched polyester. 2. Photo-polymerization and properties for dental resin systems. J Macromol Sci Part A.2000;37(11):1317–1331.
- Dewaele M, Leprince JG, Fallais I, et al. Benefits and limitation of adding hyperbranched polymers to dental resins. J Dent Res. 2012;91(12):1178–1183.
- Yu B, Liu F, He J. Preparation of low shrinkage methacrylate-based resin system without bisphenol a structure by using a synthesized dendritic macromer (G-IEMA). J Mech Behav Biomed Mater. 2014;35:1–8.
- Viljanen EK, Skrifvars M, Vallittu PK. Degree of conversion of an experimental monomer and methyl methacrylate copolymer for dental applications. J Appl Polym Sci. 2004;93(4):1908–1912.
- Viljanen EK, Lassila LVJ, Skrifvars M, et al. Degree of conversion and flexural properties of a dendrimer/methyl methacrylate copolymer: a statistical modeling. Dent Mater. 2005;21(2):172–177.
- Viljanen EK, Skrifvars M, Vallittu PK. Dendrimer/methyl methacrylate copolymers: residual methyl methacrylate and degree of conversion. J Biomater Sci Polym Ed. 2005;16(10):1219–1231.
- Viljanen EK, Langer S, Skrifvars M, et al. Analysis of residual monomers by HPLC and HS-GC/MS. Dent Mater. 2006;22(9):845–851.
- Viljanen EK, Skrifvars M, Vallittu PK. Dendritic copolymers and particulate filler composites for dental applications: degree of conversion and thermal properties. Dent Mater. 2007;23(11):1420–1427.
- Braga RR, Ballester RY, Ferracane JL. Factors involved in the development of polymerization shrinkage stress in resin-composites: a systematic review. Dent Mater. 2005;21(10):962–970.
- Lu H, Stansbury JW, Nie J, et al. Development of highly reactive mono-(meth)acrylates as reactive diluents for dimethacrylate-based dental resin systems. Biomaterials. 2005;26(12):1329–1336.
- Moszner N, Fischer UK, Angermann J. New diluents for dental composites. Macromol Mater Eng. 2016;301(6):750–759.
- Luo S, Liu F, He J. Preparation of low shrinkage stress dental composite with synthesized dimethacrylate oligomers. J Mech Behav Biomed Mater. 2019;94:222–228.
- Yamasaki L, De Vito Moraes AG, Barros M, et al. Polymerization development of “low-shrink” resin composites: reaction kinetics, polymerization stress and quality of network. Dent Mater. 2013;29(9):e169–e179.
- Vitale A, Sangermano M, Bongiovanni R, et al. Visible light curable restorative composites for dental application based on epoxy monomer. Materials. 2014;7(1):554–562.
- Li Z, Zhang H, Xiong G, et al. A low-shrinkage dental composite with epoxy-polyheral oligomeric silsesquioxane. J Mech Behav Biomed Mater. 2020;103:103515.
- Xing A, Sun Q, Meng Y, et al. A hydroxyl-containing hyperbranched polymer as multi-purpose modifier for a dental epoxy. React Funct Polym. 2020;149:104505.
- Sun X, Li Y, Xiong J, et al. Shrinkage properties of a modified dental resin composites containing a novel sprio-orthocarbonate expanding monomer. Mater Lett. 2011;65(23–24):3586–3589.
- Fu J, Liu W, Hao Z, et al. Characterization of a low shrinkage dental composite containing bismethylene spiroorthocarbonate expanding monomer. IJMS. 2014;15(2):2400–2412.
- Duarte MLB, Medina LAR, Reyes PT, et al. Dental restorative composites containing methacrylic spiroorthocarbonate monomers as antishrinking matrixes. J Appl Polym Sci. 2018;135:47114.
- Wang Z, Zhang X, Yao S, et al. Development of low-shrinkage dental adhesive via blending with spiroorthocarbonate expanding monomer and unsaturated epoxy resin monomer. J Mech Behav Biomed Mater. 2022;133:105308.
- Marx P, Wiesbrock F. Expanding monomers as anti-shrinkage additives. Polymers. 2021;13(5):806.
- Lai H, Peng X, Li L, et al. Novel monomers for photopolymer networks. Prog Polym Sci. 2022;128:101529.
- Catel Y, Fischer U, Fässler P, et al. Bis(4-methoxybenzoyl)diethylgermane: a highly efficient photoinitiator for the polymerization of vinylcyclopropanes. Macromol Chem Phys. 2016;217(24):2686–2691.
- Catel Y, Fässler P, Fischer U, et al. Evaluation of difunctional vinylcyclopropanes as reactive diluents for the development of low-shrinkage composites. Macromol Mater Eng. 2017;302(7):1700021.
- Catel Y, Fässler P, Fischer U, et al. Synthesis and polymerization of vinylcyclopropanes bearing urethane groups for the development of low-shrinkage composites. Eur Polym J. 2018;98:439–447.
- Boaro LC, Goncalves F, Guimaraes TC, et al. Sorption, solubility, shrinkage and mechanical properties of “low shrinkage” commercial resin composites. Dent Mater. 2013;29(4):398–404.
- Weinmann W, Thalacker C, Guggenberger R. Siloranes in dental composites. Dent Mater. 2005;21(1):68–74.
- Van Ende A, De Munck J, Mine A, et al. Does a low-shrinkaing composite induce less stress at the adhesive interface? Dent Mater. 2010;26(3):215–222.
- Gönülol N, Kalyoncuoğlu E, Ertaş E, et al. Clinical evaluation of a low-shrinkage resin composite in endodontically treated premolar: 3-year follow-up. Clin Oral Investig. 2019;23(5):2323–2330.
- Stansbury JW. Cyclopolymerizable monomers for use in dental resin composites. J Dent Res. 1990;69(3):844–848.
- Peer G, Kury M, Gorsche C, et al. Revival of cyclpolymerizable monomers as low-shrinkage cross-linkers. Macromolecules. 2020;53(19):8374–8381.
- Luo S, Liu F, Yu B, et al. Preparation of low shrinkage stress Bis-GMA free dental resin composites with a synthesized urethane dimethacrylate monomer. J Biomater Sci Polym Ed. 2019;30(2):137–149.
- Wang X, Huyang G, Palagummi SV, et al. High performance dental resin composites with hydrolytically stable monomers. Dent Mater. 2018;34(2):228–237.
- González-López JA, Fonseca-García A, Acosta-Ortiz R, et al. Photopolymerization dental composite resins with lower shrinkage stress and improved hydrolytic and hydroscopic behavior with a urethane monomer used as an additive. J Mech Behav Biomed Mater. 2022;130:105189.
- He J, Garoushi S, Säilynoja E, et al. The effect of adding a new monomer “phene” on the polymerization shrinkage reduction of a dental resin composite. Dent Mater. 2019;35(4):627–635.
- He J, Garoushi S, Säilynoja E, et al. Physicochemical properties of dimethacrylate resin composites with comonomer of hexa/tri-ethylene glycol bis(carbamate-isoproply-α-methylstyrene). J Mech Behav Biomed Mater. 2020;108:103832.
- Lowry GG. Radical-initiated homopolymerization of α-Methylstyrene. J Polym Sci. 1958;31(122):187–188.
- Lu H, Carioscia JA, Stansbury JW, et al. Investigation of step-growth thiol-ene polymerization for novel dental restoratives. Dent Mater. 2005;21(12):1129–1136.
- Lee TY, Smith Z, Reddy SK, et al. Thiol-allyl ether-methacrylate ternary systems. Polymerization mechanism. Macromolecules. 2007;40(5):1466–1472.
- Reinelt S, Tabatabai M, Moszner N, et al. Synthesis and photopolymerization of thiol-modified triazine-based monomers and oligomers for use in thiol-ene-based dental composites. Macromol Chem Phys. 2014;215(14):1415–1425.
- Cramer NB, Couch CL, Schreck KM, et al. Investigation of thiol-ene and thiol-ene-methacrylate based resins as dental restorative materials. Dent Mater. 2010;26(1):21–28.
- Cramer NB, Couch C, Schreck KM, et al. Properties of methacrylate-thiol-ene formulations as dental restorative materials. Dent Mater. 2010;26:799–806.
- Beigi S, Yeganeh H, Atai M. Evaluation of fracture toughness and mechanical properties of ternary thiol-ene-methacrylate systems as resin matrix for dental restorative composites. Dent Mater. 2013;29(7):777–787.
- Fu W, Wang L, He J. Evaluation of mechanical properties and shrinkage stress of thiol-ene-methacrylate dental composites with synthesized fluorinated allyl ether. J Mech Behav Biomed Mater. 2019;95:53–59.
- Yu B, He J, Garoushi S, et al. Enhancing toughness and reducing volumetric shrinkage for Bis-GMA/TEGDMA resin systems by using hyperbranched thiol oligomer HMDI-6SH. Materials. 2021;14(11):2817.
- Shah PK, Stansbury JW, Bowman CN. Application of an addition-fragmentation-chain transfer monomer in di(meth)acrylate network formation to reduce polymerization shrinkage stress. Polym Chem. 2017;8(30):4339–4351.
- Park HY, Kloxin CJ, Abuelyaman AS, et al. Novel dental restorative materials having low polymerization shrinkage stress via stress relaxation by addition-fragmentation chain transfer. Dent Mater. 2012;28(11):1113–1119.
- Lamparth I, Wottawa D, Angermann J, et al. Synthesis of allyl sufones bearing urethane groups as efficient addition-fragmentation chain transfer agents for the development of low-shrinkage composites. Eur Polym J. 2021;158:110672.
- Grob B, Frieser B, Liska R, et al. Evaluation of allyl sulfides bearing methacrylate group as addition-fragmentation chain transfer agents for low shrinkage dental composites. Eur Polym J. 2022;181:111699.
- Auschill TM, Arweiler NB, Brecx M, et al. The effect of dental restorative materials on dental biofilm. Eur J Oral Sci. 2002;110(1):48–53.
- Beyth N, Domb AJ, Weiss E. An in vitro quantitative antibacterial analysis of amalgam and composite resins. J Dent. 2007;35(3):201–206.
- Zhang K, Wang S, Zhou X, et al. Effect of antibacterial dental adhesive on multispecies biofilms formation. J Dent Res. 2015;94(4):622–629.
- Al-Qarni FD, Tay F, Weir MD, et al. Protein-repelling adhesive resin containing calcium phosphate nanoparticles with repeated ion-recharge and re-releases. J Dent. 2018;78:91–99.
- Boaro LCC, Campos LM, Varca GHC, et al. Antibacterial resin-based composite containing chlorhexidine for dental applications. Dent Mater. 2019;35(6):909–918.
- Lahdenperä MS, Puska MA, Alander PM, et al. Release of chlorhexidine digluconate and flexural properties of glass fibre reinforced provisional fixed partial denture polymer. J Mater Sci Mater Med. 2004;15:1349–1353.
- Al-Musallam TA, Evans CA, Drummond JL, et al. Antimicrobial properties of an orthodontic adhesive combined with cetylpyridinium chloride. Am J Orthod Dentofacial Orthop. 2006;129(2):245–251.
- Othman HF, Wu CD, Evans CA, et al. Evaluation of antimicrobial properties of orthodontic composite resins combined with benzalkonium chloride. Am J Orthod Dentofacial Orthop. 2002;122(3):288–294.
- Chen L, Sun BI, Yang J. Antibacterial dental restorative materials: a review. Am J Dent. 2018;31:6B–12B.
- Imazato S. Immobilization of an antibacterial component in composite resin. Dent Jpn. 1993;30:63–68.
- Imazato S, Torii M, Tsuchitani Y, et al. Incorporation of bacterial inhibitor into resin composite. J Dent Res. 1994;73(8):1437–1443.
- Imazato S, Kinomoto Y, Tarumi H, et al. Incorporation of antibacterial monomer MDPB in dentin primer. J Dent Res. 1997;76(3):768–772.
- Imazato S, Ehara A, Torii M, et al. Antibacterial activity of dentine primer containing MDPB after curing. J Dent. 1998;26(3):267–271.
- Imazato S, Torii M, Takatsuka T, et al. Bactericidal effect of dentin primer containing antibacterial monomer methacryloloxydodecylpyridinium bromide (MDPB) against bacteria in human carious dentin. J Oral Rehabil. 2001;28(4):314–319.
- He J, Söderling E, Österblad M, et al. Synthesis of methacrylate monomers with antibacterial effects against S. mutans. Molecules. 2011;16(11):9755–9763.
- He J, Söderling E, Lassila LVJ, et al. Incorporation of antibacterial and radiopaque monomer into dental resin system. Dent Mater. 2012;28(8):e110–e117.
- He J, Söderling E, Vallittu PK, et al. Investigation of double bond conversion, mechanical properties, and antibacterial activity of dental resins with different alkyl chain length quaternary ammonium methacrylate monomers (QAM). J Biomater Sci Polym Ed. 2013;24(5):565–573.
- He J, Söderling E, Vallittu PK, et al. Preparation and evaluation of dental resin with antibacterial and radio-opaque functions. IJMS. 2013;14(3):5445–5460.
- Duarte de Oliveira FJ, Ferreira da Silva Filho PS, Costa MJF, et al. A comprehensive review of the antibacterial activity of dimethylaminohexadecyl methactylate (DMAHDM) and its influence on mechanical properties of resin-based dental materials. Jpn Dent Sci Rev. 2021;57:60–70.
- Wang L, Xie X, Imazato S, et al. A protein-repellent and antibacterial nanocomposite for Class-V restorations to inhibit periodontits-related pathogens. Mater Sci Eng C. 2016;67:702–710.
- Zhang N, Zhang K, Melo MAS, et al. Effects of long-term water-aging on novel anti-biofilm and protein-repellent dental composite. IJMS. 2017;18(1):186.
- Cherchali FZ, Mouzali M, Tommasino JB, et al. Effectiveness of the DHMAI monomer in the development of antibacterial dental composite. Dent Mater. 2017;33(12):1381–1391.
- Vidal ML, Rego GF, Viana GM, et al. Physical and chemical properties of model composites containing quaternary ammonium methacrylates. Dent Mater. 2018;34(1):143–151.
- Huang L, Xiao Y, Xing X, et al. Antibacterial activity and cytotoxicity of two novel cross-linking antibacterial monomers on oral pathogens. Arch Oral Biol. 2011;56(4):367–373.
- Antonucci JM, Zeiger DN, Tang K, et al. Synthesis and characterization of dimethacrylates containing quaternary ammonium functionalities for dental application. Dent Mater. 2012;28(2):219–228.
- Liang X, Huang Q, Liu F, et al. Synthesis of novel antibacterial monomer (UDMQA) and their potential application in dental resin. J Appl Polym Sci. 2013;129(6):3373–3381.
- He J, Söderling E, Lassila LVJ, et al. Synthesis of antibacterial and radio-opaque dimethacrylate monomers and their potential application in dental resin. Dent Mater. 2014;30(9):968–976.
- Liang X, Soderling E, Liu F, et al. Optimizing the concentration of quaternary ammonium dimethacrylate monomer in bis-GMA/TEGDMA dental resin system for antibacterial activity and mechanical properties. J Mater Sci mater Med. 2014;25(5):1387–1393.
- He J, Soderling E, Lassila LVJ, et al. Preparation of antibacterial and radio-opaque dental resin with new polymerizable quaternary ammonium monomer. Dent Mater. 2015;31(5):575–582.
- Li S, Yu X, Liu F, et al. Synthesis of antibacterial dimethacrylate derived from niacin and its application in preparing antibacterial dental resin system. J Mech Behav Biomed Mater. 2020;102:103521.
- Huang Q, He J, Lin Z, et al. Physical and chemical properties of an antimicrobial Bis-GMA free dental resin with quaternary ammonium dimethacrylate monomers. J Mech Behav Biomed Mater. 2016;56:68–76.
- Fanfoni L, Marsich E, Turco G, et al. Development of dimethacrylate quaternary ammonium monomers with antibacterial activity. Acta Biomater. 2021;129:138–147.
- He X, Ye L, He R, et al. Antibacterial dental resin composites (DRCs) with synthesized bis-quaternary ammonium monomethacrylates as antibacterial agents. J Mech Behav Biomed Mater. 2022;135:105487.
- Ebi N, Imazato S, Noiri Y, et al. Inhibitory effects of resin composite containing bactericide-immobilized filler on plaque accumulation. Dent Mater. 2001;17(6):485–491.
- Imazato S, Ebi N, Takahashi Y, et al. Antibacterial activity of bactericide-immobilized filler for resin-based restoratives. Biomaterials. 2003;24(20):3605–3609.
- Luo W, Huang Q, Liu F, et al. Synthesis of antibacterial methacrylate monomer derived from thiazole and its application in dental resin. J Mech Behav Biomed Mater. 2015;49:61–68.
- Zhu W, Lao C, Luo S, et al. Mechanical and antibacterial properties of benzothiazole-based dental resin materials. J Biomater Sci Polym Ed. 2018;29(6):635–645.
- Caneli G, Chen Y, Sungsoo N, et al. A dental filling composite resin restorative with improved antibacterial function and hardness. J Compos Mater. 2021;55(2):159–168.
- Almaroof A, Rojo L, Mannocci F, et al. A resin composite material containing an eugenol derivative for intracanal post cementation and core build-up restoration. Dent Mater. 2016;32(2):149–160.
- Almaroof A, Niazi SA, Rojo L, et al. Influence of a polymerizable eugenol derivative on the antibacterial activity and wettability of a resin composite for intracanal post cementation and core build-up restoration. Dent Mater. 2016;32(7):929–939.
- Al-Odayni AB, Saeed WS, Ahmed AYBH, et al. New monomer based on eugenol methacrylate, synthesis, polymerization and copolymerization with methyl methacrylate-characterization and thermal properties. Polymers. 2020;12(1):160.
- Al-Odayni AB, Alotaibi DH, Saeed WS, et al. Eugenyl-2-hydroxypropyl methacrylate-incorporated experimental dental composite: degree of polymerization and in vitro cytotoxicity evaluation. Polymers. 2022;14(2):277.
- Alrahlah A, Al-Odayni AB, Saeed WS, et al. Water sorption, water solubility, and rheological properties of resin-based dental composites incorporating immobilizable eugenol-derivative monomer. Polymers. 2022;14(3):366.
- Zhang L, Ma Z, Wang R, et al. Synthesis and characterization of methacrylate-funcitonalized botulin derivatives as antibacterial comonomer for dental restorative resins. ACS Biomater Sci Eng. 2021;7(7):3132–3140.
- Kilian M, Chapple ILC, Hannig M, et al. The oral microbiome-an update for oral healthcare professionals. Br Dent J. 2016;221(10):657–666.
- Gyo M, Nikaido T, Okada K, et al. Surface response of fluorine polymer-incorporated resin composites to cariogenic biofilm adherence. Appl Environ Microbiol. 2008;74(5):1428–1435.
- Absolom DR, Lamberti FV, Policova Z, et al. Surface thermodynamics of bacterial adhesion. Appl Environ Microbiol. 1983;46(1):90–97.
- Uyen M, Busscher HJ, Weerkamp AH, et al. Surface free energies of oral streptococci and their adhesion to solids. FEMS Microbiol Lett. 1985;30:103–106.
- Quirynen M, Marechal M, Busscher HJ, et al. The influence of surface free energy and surface roughness on early plaque formation. J Clin Periodontol. 1990;17(3):138–144.
- Santiago A, Martin L, Iruin JJ, et al. Microphase separation and hydrophobicity of urethane/siloxane copolymers with low siloxane content. Prog Org Coat. 2014;77:798–802.
- Santiago A, Irusta L, Schafer T, et al. Resistance to protein sorption as a model of antifouling performance of poly(siloxane-urethane) coatings exhibiting phase separated morphologies. Prog Org Coat. 2016;99:110–116.
- Tong H, Liao M, Huang X, et al. Physicochemical properties, anti-adhesion effect against S. mutans, and resistance to mucin adsorption of dental resins contained synthesized silicone methacrylates. Silicon. 2022;14:5835–5845.
- Tong H, Yu X, Shi Z, et al. Physicochemical properties, bond strength and dual-species biofilm inhibition effect of dental resin composites with branched silicone methacrylate. J Mech Behavr Biomed Mater. 2021;116:104368.
- Liao M, Tong H, Huang X, et al. Mechanical properties, biocompatibility and anti-bacterial adhesion property evaluation of silicone-containing resin composite with different formulae. J Renew Mater. 2022;10:3201–3215.
- Zhang S, Liao M, Liu F, et al. Preparation of Bis-GMA free dental resin composites with anti-adhesion effect against Streptococcus mutans using synthesized fluorine-containing methacrylate (DFMA). J Mech Behav Biomed Mater. 2022;131:105263.
- Zhang N, Melo MAS, Bai Y, et al. Novel protein-repellent dental adhesive containing 2-methacryloyloxyethyl phosphorylcholine. J Dent. 2014;42:1284–1291.
- Zhang N, Chen C, Melo MAS, et al. A novel protein-repellent dental composite containing 2-methacryloyloxyethyl phosphorylcholine. Int J Oral Sci. 2015;7:103–109.
- Zhang N, Ma J, Melo MAS, et al. Protein-repellent and antibacterial dental composite to inhibit biofilm and caries. J Dent. 2015;43:225–234.
- Pekkan G, Pekkan K, Hatipoglu MG, et al. Comparative radiopacity of ceramics and metals with human and bovine dental tissues. J Prosthet Dent. 2011;106:109–117.
- Rendenbach C, Schöllen M, Bueschel J, et al. Evaluation and reduction of magnetic resonance imaging artifacts induced by distinct plates for osseous fixation. an in vitro study @3T. Dentomaxillofac Radiol. 2018;47:20170361.
- Kuusisto N, Huumonen S, Kotiaho A, et al. Intensity of artefacts in cone beam CT examinatiuons caused by titanium and glass fiber-reinforced composite implants. Dentomaxillofac Radiol. 2019;48:20170471.
- Yildirim D, Ermis RB, Gormez O, et al. Comparison of radiopacities of different flowable resin composites. J Oral Maxillofac Radiol. 2014;2:21–25.
- Garoushi S, Vallittu P, Lassila L. Mechanical properties and radiopacity of flowable fiber-reinforced composite. Dent Mater J. 2019;38:196–202.
- Issa SAM. Effective atomic number and mass attenuation coefficient of PbO-BaO-B2O3 glass system. Radiat Phys Chem. 2016;120:33–37.
- He J, Vallittu PK, Lassila LV. Preparation and characterization of high radio-opaque E-glass fiber-reinforced composite with iodine containing methacrylate monomer. Dent Mater. 2017;33:218–225.
- Diamanti-Kandarakis E, Bourguignon JP, Giudice LC, et al. Endocrine-disrupting chemicals: an endocrine society scientific statement. Endocr Rev. 2009;30:293–342.
- Li YJ, Song TB, Cai YY, et al. Bisphenol a exposure induces apoptosis and upregulation of fas/FasL and caspase-3 expression in the testes of mice. Toxicol Sci. 2009;108:427–436.
- Meeker JD, Ehrlich S, Toth TL, et al. Semen quality and sperm DNA damage in relation to urinary bisphenol a among men from an infertility clinic. Reprod Toxicol. 2010;30:532–539.
- Li DK, Zhou Z, Miao M, et al. Urine bisphenol-A (BPA) level in relation to semen quality. Fertil Steril. 2011;195:625–630.
- Izumi Y, Yamaguchi K, Ishikawa T, et al. Molecular changes induced by bisphenol-A in rat sertoli cell culture. Syst Biol Reprod Med. 2011;57:228–232.
- Fugolin AP, de Paula AB, Dobson A, et al. Alternative monomer for BisGMA-free resin composites formulations. Dent Mater. 2020;36:884–892.
- Polydorou O, Schmidt OC, Spraul M, et al. Detection of bisphenol a in dental wastewater after grinding of dental resin composites. Dent Mater. 2020;36:1009–1018.
- De Nys S, Duca RC, Verliet P, et al. Bisphenol a as degradation product of monomers used in resin-based dental materials. Dent Mater. 2021;37:1020–1029.
- Liang X, Liu F, He J. Synthesis of none bisphenol a structure dimethacrylate monomer and characterization for dental composite applications. Dent Mater. 2014;30:917–925.
- Yu B, Liu D, Liu F, et al. Preparation and characterization of light-cured dental resin without methacrylate monomers derived from bisphenol A. Adv Polym Technol. 2014;33:21417.
- Yin M, Guo S, Liu F, et al. Synthesis of fluorinated dimethacrylate monomer and its application in preparing Bis-GMA free dental resin. J Mech Behav Biomed Mater. 2015;51:337–344.
- Yuan S, Liu F, He J. Preparation and characterization of low polymerization shrinkage and Bis-GMA-free dental resin system. Adv Polym Technol. 2015;34:21503.
- Yu B, Liu F, He J, et al. Preparation of Bis-GMA-free dental restorative composites with dendritic macromer (G-IEMA). Adv Polym Technol. 2015;34:21519.
- Vaidyanathan TK, Vaidyanathan J. Visible light cure characteristics of cycloaliphatic polyester dimethacrylate alternative oligomer to bisGMA. Acta Biomater Odontol Scand. 2015;1:59–65.
- Ding Y, Li B, Wang M, et al. Bis-GMA free dental material based on UDMA/SR833s dental resin system. Adv Polym Technol. 2016;35:396–401.
- Yin M, Liu F, He J. Preparation and characterization of Bis-GMA free dental resin system with synthesized dimethacrylate monomer TDDMMA derived from tricyclo[5.2.1.0(2,6)]-decanedimethanol. J Mech Behav Biomed Mater. 2016;57:157–163.
- He J, Kopperud HM. Preparation and characterization of Bis-GMA-free dental composites with dimethacrylate monomer derived from 9,9-Bis[4-(2-hydroxyethoxy)- phenyl]fluorene. Dent Mater. 2018;34:1003–1013.
- Liu X, Wang Z, Zhao C, et al. Synthesis, characterization and evaluation of a fluorinated resin monomer with low water sorption. J Mech Behav Biomed Mater. 2018;77:446–454.
- Wang T, Matinlinna JP, He J, et al. Biomechanical and biological evaluations of novel BPA-free fiber-reinforced composites for biomedical applications. Mater Sci Eng C. 2020;117:111309.
- Yoshinaga K, Yoshihara K, Yoshida Y. Development of new diacrylate monomers as substitutes for Bis-GMA and UDMA. Dent Mater. 2021;37:e391–e398.
- Zhang X, Ma X, Liao M, et al. Properties of Bis-GMA free bulk-filled resin composite based on high refractive index monomer Bis-EFMA. J Mech Behav Biomed Mater. 2022;134:105372.
- Jun S, Cha J-R, Knowles JC, et al. Development of Bis-GMA-free biopolymer to avoid estrogenicity. Dent Mater. 2020;36:157–166.
- Sun Y, Zhou Z, Jiang H, et al. Preparation and evaluation of novel bio-based Bis-GMA-free dental composites with low estrogenic activity. Dent Mater. 2022;38(2):281–293.
- Sun Y, Sun L, Hong L, et al. Bio-based non-estrogenic dimethacrylate dental composites from cloves. J Dent Res. 2022;101:1613–1619.