 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Age-related osteoporosis affects people of both genders at any age especially postmenopausal women. The manifestation of reduced bone strength leads to increased risk of bone fracture. Currently, many have sought for plant-based supplements for the management of bone health. Red clover (Trifolium pratense) contains phytoestrogenic isoflavones which are known to limit osteoclastic activity and reduce bone resorption. Our study aimed to optimize the condition for the preparation of red clover ethanolic extract to obtain highest yield while retaining the desired bioactivity. In this study, relative aggregated metric (RAM) was designed to ease the selection process of extraction formulation based on yield and SaOS-2 cell viability. The selected ethanolic red clover extract obtained from the optimum condition (RC-D) exerted high cell viability in SaOS-2 cells which is comparable to that of 17β-estradiol. RC-D also showed promising dose-dependent alkaline phosphatase activity in SaOS-2 cells. Besides, the RANKL, OPG, ALP and Col-A gene expression analysis revealed that RC-D potentially halts the osteoclastic activity via downregulation of RANKL mRNA expression. In short, RC-D prepared and selected via RAM developed in this study has shown promising anti-osteoporotic characteristics in SaOS-2 cells and might be considered as a functional food for bone quality management.
Introduction
The research of age-related osteoporosis, especially postmenopausal osteoporosis, are maturing and are now being spun into increased clinical and commercial interest due to the global aging population (Lems and Raterman Citation2017). Many have sought for plant-based supplements as a safer, more economical and natural alternative to relieve this postmenopausal symptom (Cao et al. Citation2014). The isoflavones found in red clover (RC) extract, including formononetin, biochanin A, daidzein and genistein, have been widely studied by researchers all over the world for its estrogenic and osteoblastic effect in in vitro (Lee and Choi Citation2005; Chen and Wong Citation2006; Gautam et al. Citation2011) and in vivo models (Kawakita et al. Citation2009; Kaczmarczyk-Sedlak et al. Citation2013). A recently published research reported that these isoflavones can form complexes with receptor activator of nuclear factor-kappa B ligand (RANKL) which could eventually attenuate osteoclastic activity (Zakłos-Szyda et al. Citation2020). These isoflavones were more commonly known to be found in soybean (Zheng et al. Citation2016) than in clover (Su et al. Citation2013). While Zakłos-Szyda et al. (Citation2020) also reported that the pairing of formononetin and biochanin A showed similar affinity for RANKL as of osteoprotegrin (OPG), and clover extract contains mainly formononetin and biochanin A. RANKL and OPG are the ligands that interact with RANK receptor. Osteoblasts and bone marrow stromal cells were responsible for synthesizing and secreting these ligands (Kobayashi et al. Citation2009). Osteoclast differentiation was initiated when RANK is activated by the RANKL, which results in bone resorption. OPG, which acts as a decoy receptor for RANKL, prevents RANKL-RANK interaction and inhibits the activation of osteoclasts (Infante et al. Citation2019). The balance between expressions of RANKL and OPG in osteoblasts and bone marrow stromal cells regulates bone resorption (Zhang et al. Citation2014).
Previous literature has reported promising dose-dependent alkaline phosphatase (ALP) activity of RC water extract and chloroform extract on human osteoblastic osteosarcoma (HOS58) cell line (Wende et al. Citation2004). ALP is the most widely recognized biochemical marker for osteoblastic activity. This enzyme is secreted by osteoblasts and it is essential for bone mineralization (Han and Wang Citation2017). In our study, ethanol and water formulation were selected as the extraction solvents. Ethanol is one of the most used biosolvent by the manufacturers as the products can be safely consumed internally by the consumers (Dog Citation2008). As far as we know, no previous research has investigated the effects of ethanolic RC extract in human osteoblastic (SaOS-2) cells. Here we aimed to acquire extraction method which can obtain the maximum yield of RC extract with highest SaOS-2-stimulating ability. Besides, we also aim to evaluate the ALP activity and the regulatory effect of selected RC extract on the gene expression in SaOS-2 cells for the first time. HPLC analysis was also done to determine the amount of the key isoflavones in the ethanolic RC extract.
Materials and methods
Preparation of ethanolic extract of red clover (Trifolium pratense)
To prepare the ethanolic extract of red clover (Trifolium pratense), the macerated RC flower of 10 g was added to 300 mL of extraction solvent: 0, 5, 10, 30, 50, 70 and 100% ethanol, labeled as RC-A, RC-B, RC-C, RC-D, RC-E, RC-F and RC-G, respectively. The sample was extracted twice for 3 h at 80°C on a heating mantle (GLHMD-F200). The extract was cooled to room temperature and ethanol was removed and concentrated using a vacuum rotary evaporator. The concentrated extract was lyophilized using a freeze dryer (Operon, FDB-8603).
SaOS-2 cell viability assay
For the cell viability study, the SaOS-2 cells were seeded into a 96-well plate at a cell density of 1 × 104 cells/well in phenol red-free RPMI1640 medium supplemented with 5% charcoal-stripped FBS and 1% penicillin/streptomycin for 24 h. The cells were then treated with RC, pomegranate extract (P) and 17β-estradiol (E) at different concentrations for 48 h. The 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay was used, as described previously (Denizot and Lang Citation1986). Pomegranate extract (purchased from Gülsan Gıda San. Tic. Inc., Turkey) and 17β-estradiol (Sigma) were used as positive controls.
Determination of alkaline phosphatase (ALP) activity in SaOS-2 cells
To determine the ALP activity, SaOS-2 cells were seeded into 24 wells plate (1.2 × 103 cells/well) for 48 h before treatment. After 48 h of incubation, samples at different concentrations were added into the individual wells and incubated for 48 hrs. After the treatment, the cell culture medium was analyzed directly using the ALP assay kit (ab83369, Abcam, Cambridge, UK) according to the manufacturer’s instructions.
Osteoblastic and osteoclastic gene expression in SaOS-2 cells using real-time reverse transcriptase polymerase chain reaction (qRT-PCR)
Briefly, total RNA was extracted from cells using TRIzol reagent and used for cDNA synthesis with the cDNA EcoDry Premix (Takara). Relative mRNA levels were determined by cycler CFX96 (Bio-Rad). Samples of total cDNA were subjected to PCR amplification with respective gene-specific primers (Figure (C)). Amplifications were performed for 30 cycles at 94°C for 30 sec, annealing at the optimized temperature of the respective genes for 30 sec, and 72°C for 30 sec.
HPLC analysis
The freeze-dried RC-D sample was dissolved at 200 mg/ml in acetonitrile (ACN) and filtered with 0.45 µm syringe filters. This experiment employed a reversed-phase Fortis Tech. C18 (250 × 4.6 mm, 5 µm) column eluted with 40% water as solvent A and 60% ACN as solvent B (both were acidified with 0.5% phosphoric acid) using an isocratic elution with a flow rate of 1 ml/min and analyzed at 254 nm wavelength.
Statistical analysis
The results were reported as mean and their standard deviation, as well as one-way ANOVA (analysis of variation) and Tukey test for differences between means demonstrated statistical significance of the data (p < 0.05).
Results and discussion
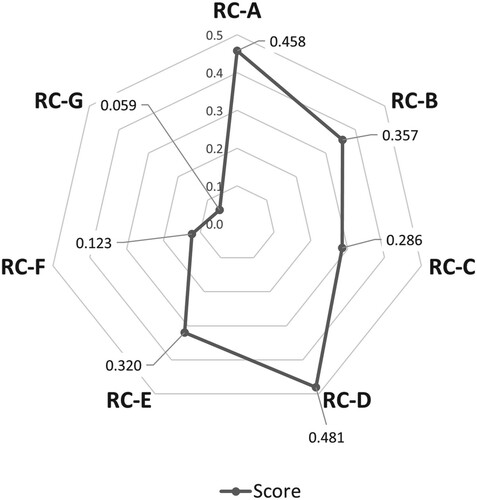
In this study, the preparation method to obtain ethanolic RC extract was evaluated by the yield of the recovered product and their effects on SaOS-2 cell viability. The yield of water extract (RC-A) was the highest among the 7 extracts followed by 30% ethanol extract (RC-D). Water extraction (RC-A) and 30% ethanol extraction (RC-D) yielded 40.5% and 37.5% of the weight of macerated RC plant, respectively. The resultant RC extracts were subjected to cell viability analysis against SaOS-2 cells using MTT assay. In order to determine the most effective extraction method to yield RC extract which possess desirable osteoblastic activity, a standardized sample concentration at 300 µg/ml was used for SaOS-2 cell treatment. In view of the commercialization relevance for the selection of extraction method and effect of RC extracts, commercialized pomegranate extract (P) was used as a positive control in our study apart from 17β-estradiol (E). As shown in Figure (A), RC-D displayed the highest SaOS-2 cell viability (94.2%) compared to other extracts. Although RC-A showed higher product yield among the extracts, RC-D showed more promising bioactivity in osteoblastic SaOS-2 cells. Besides, SaOS-2 cell viability was 2.25-fold higher when treated by RC-D than that of P treatment (41.9%).
The data collected from these two experiments show that there is a trade-off between sample yield and cell viability. RC-A gave the best yield, while RC-D showed the highest cell viability. In other words, RC-A has 8% higher yield than RC-D, but RC-D exhibited 18.5% higher cell viability than RC-A. If both yield and cell viability are equality important, RC-D would be more preferable. However, this study attempts to overcome this dilemma by defining a metric inspired by the F1-score to aggregate the 2 metrics (yield and cell viability) into a single metric for direct comparison. This new metric was defined as below:
where mi is the i-th metric’s score.
When the individual metric, mi (in this case mi could be the yield or cell viability) becomes larger, the RAM’s denominator will become smaller, hence RAM will become larger.
In statistical analysis of classification, F1-score was designed to combine 2 important metrics (precision and recall) into a single matrix in the field of statistical analysis of classification (Sasaki Citation2007). The F1 score is given as below:
where 0 ≤ recall ≤ 1, 0 ≤ precision ≤ 1
Both recall and precision are very important in measuring the performance of a statistical model, where higher recall and higher precision imply better performance (Li et al. Citation2008). Referring to the equation above, higher recall and higher precision will both lead to higher F1-score. Therefore, F1-score is reflective of the model performance. In fact, F1-score is the harmonic mean of recall and precision. A general expression of harmonic mean is given as follows:
where xi is the i-th real number
This research requires a standardized scoring system to determine the optimal extraction method. The score is based on Yield (in grams) and Viability (%). At first glance, the harmonic mean could be used directly to obtain the desired score as shown below:
However, this score would be significantly bias toward either the Yield or the Viability, whichever has the larger magnitude. Moreover, both the Yield and Viability are not under same unit of measurement. In order to overcome these constraints, both Yield and Viability must be normalized and bounded between 0 and 1, similar to that of the F1-score. The normalization method used in this research to normalize Yield and Viability is known as Min–Max Normalization. This is because the output of Min–Max Normalized is bounded in between 0 and 1. The general expression of Min–Max Normalization is given as
where x ∈ X, X are real numbers
Therefore, normalizing both the Yield and Viability by Min–Max Normalization before computing their harmonic solves both the magnitude constraint and the unit of measurement constraint. Since the term ‘n’ in the harmonic mean expression is a scalar, it can be removed to simplify the expression. This scoring system is known as the relative aggregated metric (RAM).
The advantage of RAM is that it introduces non-linearity into the selection process. The limitation of using RAM is that the score is a relative score between methods presented within a study, hence it should not be compared directly to the RAM from other studies. This is contributed mainly by the Min–Max Normalization component. Since the terms ‘min(X)’ and ‘max(X)’ are local, in other words, specific only to the experiment at hand, these terms vary across different research studies, hence the results are not comparable across different research studies. However, this can be overcome simply by setting a consensus value for the terms ‘min(X)’ and ‘max(X)’.
According to the RAM, RC-D has a score of 0.481, higher than that of RC-A (0.458), as shown in Figure . Therefore, the newly defined metric also rated RC-D as optimal and was chosen for the subsequent analyses. A worksheet showing each step in the RAM computation was provided (Supplementary Table 1). The workings are presented as cell functions in the worksheet.
Figure 1. Scores of RC extract calculated by relative aggregated metric (RAM) using the normalized yield scores and SaOS-2 cell viability scores.
ALP activity can be considered as an indicator of osteoblastic differentiation in SaOS-2 cells (Chen and Wong Citation2006). From Figure (B), the ALP activity of RC-D showed a drastic increase in SaOS-2 cells when the sample concentration increases from 100 to 300 µg/ml. On the other hand, P and E treatments only exhibited marginal increment of ALP activity in SaOS-2 cells. This finding was in line with our qRT-PCR analysis on ALP gene expression in SaOS-2 cell line (Figure (D)). SaOS-2 cells treated with 300 µg/ml RC-D showed substantial increase in ALP gene expression, but P did not show dose-dependent induction of ALP gene. Although SaOS-2 cells treated with E showed dose-dependent ALP activity (Figure (B)), the results shown in Figure (D) indicate that the mRNA expression of ALP did not increase in response to the increasing dose of E which agrees with the report from previous study (Chen and Wong Citation2006).
Figure 2. The effects of RC extracts on cell viability (A) and ALP activity (B) in SaOS-2 cells. The cells were treated by 300 µg/ml extracts (P: pomegranate extract; RC-A to RC-G: RC extracted using different percentage of ethanol) and 0.1 nM of E for 48 h. The percentage of cell viability and ALP activity were calculated in comparison to the untreated control cells (NC). The primer sequences and the condition for qRT-PCR analysis (D). The osteoblastic and osteoclastic gene expression in SaOS-2 cells treated with R (RC-D), P (pomegranate extract) at 100, 300 µg/ml and E (17β-estradiol) at 0.1 and 1.0 nM. Values were expressed as mean ± SEM, ┼p < 0.05 compared to P, #p < 0.05, ##p < 0.01 and ###p < 0.0001 compared to E; *p < 0.05.
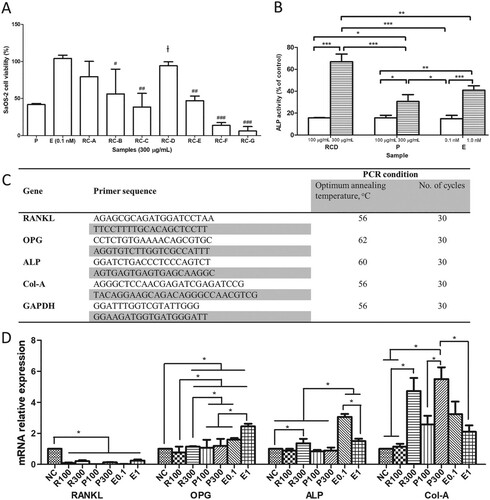
From the qRT-PCR results, RC-D, P and E significantly downregulated mRNA level of RANKL expression in SaOS-2 cells (Figure (D)). OPG gene expression, on the other hand, was only marginally induced by RC-D (300 µg/ml) and P (100 and 300 µg/ml). A desirable upregulation of OPG gene expression was in SaOS-2 when treated with 0.1 and 1 nM of E. It was previously reported that RANKL, OPG and ALP were expressed by osteoblastic cells (Zhang et al. Citation2017), whereby OPG competes with RANKL for interacting with RANK receptor. RANKL:RANK receptor interaction will activate osteoclasts and as a result will induce osteoclastic activity. Our qRT-PCR results suggest that RC-D could slow down osteoclastic activity by suppressing RANKL gene expression yet did not significantly induce OPG expression in human osteoblast-like SaOS-2 cells. To examine whether RC-D induces differentiation in osteoblasts, we determined the type 1 collagen (Col-A) mRNA expression in SaOS-2 cells. Our results showed that Col-A gene was upregulated in SaOS-2 by RC-D and P at 300 µg/ml at 4.73- and 5.50-fold compared to NC, respectively. The upregulation of Col-A mRNA expression observed in SaOS-2 cells treated by RC-D and P treatment suggests early differentiation of SaOS-2 cells. This might be the implication of the onset of differentiation of mature osteoblasts which are transitioning toward an osteocyte (Prideaux et al. Citation2014).
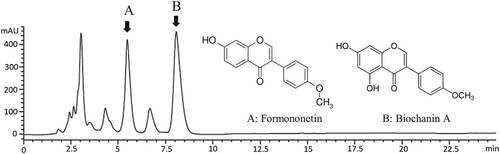
Finally, we analyzed the constituents of RC-D using the HPLC analysis. Isoflavones, namely formononetin and biochanin A, have been commonly known as the indicative compounds in red clover extract (Heinonen et al. Citation2004; Booth et al. Citation2006). Formononetin and biochanin A in RC extract were identified using the HPLC analysis with the amount of 0.67 and 0.40 µg/mg RC extract dry weight, respectively (Figure ). Red clover chloroform extract was reported to stimulate the activity of osteoblastic osteosarcoma (HOS58) cells in the previous finding. Their HPLC analysis identified formononetin and biochanin A as the major free isoflavonoids in the crude chloroform extract (Wende et al. Citation2004). Besides, Ramos et al. (Citation2012) also reported that high levels of formononetin and biochanin A were found in red clover ethanolic extract which displayed in vitro anti-inflammatory activity in rats. A recent research has evaluated the interaction of the RANKL with pairs of formononetin and biochanin A. Their results showed that the curve of titration of RANKL with the solution of these two isoflavones was the most similar to that of titration with OPG solution (Zakłos-Szyda et al. Citation2020). Their results also suggest that these two isoflavones can act in common as an effective RANKL decoy. Therefore, although RC-D did not show induction in OPG gene expression, the ability of formononetin and biochanin A to interact with RANKL in this plant extract could attenuate the RANKL:RANK interaction and as a result, hinder bone resorption.
Figure 3. Representative HPLC chromatogram for RC-D. Eluent: ACN:water (60%:40%) acidified with 0.5% phosphoric acid at 1 ml/min detected at 254 nm. Arrows indicate peaks for formononetin (A) and biochanin A (B). Chromatogram of the standard of formononetin and biochanin A (inset).
Based on the metric system derived from F1-score, our results suggest that RC extract obtained by 30% ethanol extraction (RC-D) was the most optimum extraction method to obtain high sample yield and exhibited substantial cell viability in SaOS-2 cells compared to other extraction formulation. RC-D could potentially halt osteoclastic activity by suppressing the expression of RANKL gene in human osteoblast-like SaOS-2 cells. When used at 300 µg/ml, RC-D was able to slightly induce OPG gene expression which may further impede the activation of osteoclastic cells. Besides, at the same concentration, RC-D showed significant induction in ALP gene.
Supplemental Material
Download MS Excel (11.1 KB)Acknowledgements
This research was financially supported by the Ministry of Small and Medium-sized Enterprises (SMEs) and Startups (MSS), Korea, under the ‘Regional Specialized Industry Development Program (R&D, P0002954)’ supervised by the Korea Institute for Advancement of Technology (KIAT). The manuscript will be based on a part of the first author’s doctoral dissertation from Kyungpook National University.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data that support the findings of this study are available from the corresponding author, Seung-Chun Park, upon reasonable request. Supplementary material containing the worksheet of RAM computation is available at https://doi.org/10.6084/m9.figshare.12250919.
Additional information
Funding
References
- Booth NL, Overk CR, Yao P, Burdette JE, Nikolic D, Chen S-N, Bolton JL, van Breemen RB, Pauli GF, Farnsworth NR. 2006. The chemical and biologic profile of a red clover (Trifolium pratense L.) phase II clinical extract. J Altern Complement Med. 12(2):133–139. doi: https://doi.org/10.1089/acm.2006.12.133
- Cao PC, Xiao WX, Yan YB, Zhao X, Liu S, Feng J, Zhang W, Wang J, Feng YF, Lei W. 2014. Preventive effect of crocin on osteoporosis in an ovariectomized rat model. Evid Based Complement Alternat Med. 2014:825181.
- Chen WF, Wong MS. 2006. Genistein modulates the effects of parathyroid hormone in human osteoblastic SaOS-2 cells. Br J Nutr. 95(6):1039–1047. doi: https://doi.org/10.1079/BJN20061735
- Denizot F, Lang R. 1986. Rapid colorimetric assay for cell growth and survival: Modifications to the tetrazolium dye procedure giving improved sensitivity and reliability. J. Immunol. Methods. 89:271–277. doi: https://doi.org/10.1016/0022-1759(86)90368-6
- Dog TL. 2008. Smart talk on supplements and botanicals. Altern Complement Ther. 14(5):227–230. doi: https://doi.org/10.1089/act.2008.14502
- Gautam AK, Bhargavan B, Tyagi AM, Srivastava K, Yadav DK, Kumar M, Singh A, Mishra JS, Singh AB, Sanyal S, et al. 2011. Differential effects of formononetin and cladrin on osteoblast function, peak bone mass achievement and bioavailability in rats. J Nutr Biochem. 22(4):318–327. doi: https://doi.org/10.1016/j.jnutbio.2010.02.010
- Han J, Wang W. 2017. Effects of tanshinol on markers of bone turnover in ovariectomized rats and osteoblast cultures. Plos One. 12(7):e0181175. doi: https://doi.org/10.1371/journal.pone.0181175
- Heinonen SM, Wahala K, Adlercreutz H. 2004. Identification of urinary metabolites of the red clover isoflavones formononetin and biochanin A in human subjects. J Agric Food Chem. 52(22):6802–6809. doi: https://doi.org/10.1021/jf0492767
- Infante M, Fabi A, Cognetti F, Gorini S, Caprio M, Fabbri A. 2019. RANKL/RANK/OPG system beyond bone remodeling: involvement in breast cancer and clinical perspectives. J Exp Clin Cancer Res. 38(1):12. doi: https://doi.org/10.1186/s13046-018-1001-2
- Kaczmarczyk-Sedlak I, Wojnar W, Zych M, Ozimina-Kaminska E, Taranowicz J, Siwek A. 2013. Effect of formononetin on mechanical properties and chemical composition of bones in rats with ovariectomy-induced osteoporosis. Evid Based Complement Alternat Med. 2013:457052. doi: https://doi.org/10.1155/2013/457052
- Kawakita S, Marotta F, Naito Y, Gumaste U, Jain S, Tsuchiya J, Minelli E. 2009. Effect of an isoflavones-containing red clover preparation and alkaline supplementation on bone metabolism in ovariectomized rats. Clin Interv Aging. 4:91–100.
- Kobayashi Y, Udagawa N, Takahashi N. 2009. Action of RANKL and OPG for osteoclastogenesis. Crit Rev Eukaryot Gene Expr. 19(1):61–72. doi: https://doi.org/10.1615/CritRevEukarGeneExpr.v19.i1.30
- Lee KH, Choi EM. 2005. Biochanin A stimulates osteoblastic differentiation and inhibits hydrogen peroxide-induced production of inflammatory mediators in MC3T3-E1 cells. Biol Pharm Bull. 28:1948–1953. doi: https://doi.org/10.1248/bpb.28.1948
- Lems WF, Raterman HG. 2017. Critical issues and current challenges in osteoporosis and fracture prevention. An overview of unmet needs. Ther Adv Musculoskel. 9(12):299–316. doi: https://doi.org/10.1177/1759720X17732562
- Li X, Wang YY, Acero A. 2008. Learning query intent from regularized click graphs. Proceedings of the 31st Annual International ACM SIGIR Conference on Research and Development in Information Retrieval; Singapore.
- Prideaux M, Wijenayaka AR, Kumarasinghe DD, Ormsby RT, Evdokiou A, Findlay DM, Atkins GJ. 2014. SaOS2 osteosarcoma cells as an In vitro model for studying the transition of human osteoblasts to osteocytes. Calcif Tissue Int. 95(2):183–193. doi: https://doi.org/10.1007/s00223-014-9879-y
- Ramos GP, Apel MA, Morais C, Ceolato PC, Schapoval EES, Dall'Agnol M, Zuanazzi JAS. 2012. In vivo and in vitro anti-inflammatory activity of red clover Trifolium pratense dry extract. Rev Bras Farmacogn. 22:176–180. doi: https://doi.org/10.1590/S0102-695X2011005000200
- Sasaki Y. 2007. The truth of the f-measure. Teach Tutor Mater. 1(5):1–5.
- Su SJ, Yeh YT, Shyu HW. 2013. The preventive effect of biochanin a on bone loss in ovariectomized rats: Involvement in regulation of growth and activity of osteoblasts and osteoclasts. Evid Based Complement Alternat Med. 2013:594857.
- Wende K, Krenn L, Unterrieder I, Lindequist U. 2004. Red clover extracts stimulate differentiation of human osteoblastic osteosarcoma HOS58 cells. Planta Med. 70(10):1003–1005. doi: https://doi.org/10.1055/s-2004-832629
- Zakłos-Szyda M, Budryn G, Grzelczyk J, Pérez-Sánchez H, Żyżelewicz D. 2020. Evaluation of isoflavones as bone resorption inhibitors upon interactions with receptor activator of nuclear factor-κb ligand (RANKL). Molecules. 25(1):206. doi: https://doi.org/10.3390/molecules25010206
- Zhang Y, Shen L, Mao Z, Wang N, Wang X, Huang X, Hu Y, Shou D, Wen C. 2017. Icariin enhances bone repair in rabbits with bone infection during post-infection treatment and prevents inhibition of osteoblasts by vancomycin. Front Pharmacol. 8:784. doi: https://doi.org/10.3389/fphar.2017.00784
- Zhang Z, Song C, Fu X, Liu M, Li Y, Pan J, Liu H, Wang S, Xiang L, Xiao GG, et al. 2014. High-dose diosgenin reduces bone loss in ovariectomized rats via attenuation of the RANKL/OPG ratio. Int J Mol. 15(9):17130–17147. doi: https://doi.org/10.3390/ijms150917130
- Zheng X, Lee SK, Chun OK. 2016. Soy isoflavones and osteoporotic bone loss: a review with an emphasis on modulation of bone remodeling. J Med Food. 19(1):1–14. doi: https://doi.org/10.1089/jmf.2015.0045
