ABSTRACT
Synthetic biology (SynBio) is an interdisciplinary field that has developed rapidly in the last two decades. It involves the design and construction of new biological systems and processes from standardized biological components, networks and synthetic pathways. The goal of Synbio is to create logical forms of cellular control. Biological systems and their parts can be redesigned to carry out completely new functions. SynBio is poised to greatly impact human health, the environment, biofuels and chemical production with huge economic benefits. SynBio presents opportunities for the highly agro-based African economies to overcome setbacks that threaten food security: The setbacks are brought about by climate change, land degradation, over-reliance on food imports, global competition, and water and energy security issues among others. With appropriate regulatory frameworks and systems in place, the benefits of harnessing SynBio to boost development in African economies by far potentially outweigh the risks. Countries that are already using GMOs such as South Africa and Kenya should find the application of SynBio seamless, as it would be a matter of expanding the already existing regulations and policies for GMO use.
Introduction
Synthetic biology (SynBio) can be defined as a field of research that seeks to construct new biological parts, devices, and systems, or to redesign systems that are already found in nature control (Lu et al. Citation2009; Liang et al. Citation2011; Pretorius Citation2016; Chen et al. Citation2017; Goñi-Moreno and Nikel Citation2019). The field is interdisciplinary in nature, incorporating a broad range of methods such as molecular biology, systems biology, genetic engineering, computer engineering, biophysics, etc. (König et al. Citation2013; Millar-Haskell et al. Citation2019). SynBio is being propelled into prominence by the ever-decreasing costs of DNA sequencing and DNA synthesis and the increasing speed at which they are being accomplished. This is facilitating a paradigm shift in molecular sciences (Goold et al. Citation2018).
Inspired by computer science and electronics, synthetic gene circuits have been designed to control the flow of information in biological systems. SynBio makes it possible to use interchangeable and standardized ‘biological parts’ to construct complex genetic networks that allow robust and tunable transgene expression in response to changes in signal input (Guiziou et al. Citation2018). Also, existing organisms can be redesigned for new or enhanced purposes to satisfy human needs. Thus, SynBio makes it possible to engineer and redesign biological systems so that they can be used in real-world applications. SynBio projects follow a typical design-build-test cycle (Xiang et al. Citation2018).
The key for the development of biocomputing-based SynBio approaches is in Boolean logic functions design and implementation in cells (normally encoded into genetic material). Logic gates, counters, multiplexers, adders, and memories have been engineered in cells. Through modifying cell–cell communication programs, distributed computations have been designed and built in multicellular systems. Biological systems can solve relatively simple mathematical problems and compute intricate Boolean logic operations. They are a powerful platform for tackling bioproduction, diagnosis and bioremediation that were restricted to silicon-based computer technologies (Goñi-Moreno and Nikel Citation2019). The last few years have witnessed a surge in the availability of tools and components that enable researchers to generate biological modules and genetic devices through modeling and rationalizing via engineering-driven approaches (Amos and Goñi-Moreno Citation2018).
There is tangible evidence demonstrating that SynBio is poised to have major impacts in several fields such as human health, the environment, biofuels and chemical production (McDaniel and Weiss Citation2005; Serrano Citation2007; Khalil and Collins Citation2010; Schmidt Citation2010). Engineering principles are now being applied to complex multigene constructs that include pathways and whole genomes. SynBio offers technologies such as whole-cell biosensors that can be used in environmental monitoring, bioremediation, landmine detection as well as production of safer alternatives such as biodegradable plastics (Teo Citation2014; Belkin et al. Citation2017; Goold et al. Citation2018).
Small-molecule natural products produced by endangered organisms that are on the verge of extinction may need alternative methods of production. This is because the continuation of their extraction from natural sources is not sustainable. Also, the use of plants for the production of high-value compounds such as flavorings, medicines and oils bring caveats such as long generation rates, dependence on arable land and water and seasonality. Genetic engineering of plants is plagued by long generation times and large polyploidy genomes such as in wheat. Using SynBio, multigene pathways can be transplanted to microbes such as yeasts and plant-derived waste materials can be used as feedstocks that are converted to useful metabolites. Yeast can be engineered with ease and has high growth rates. There is an abundance of infrastructure and industries with expertise in yeast fermentation. Thus, together with bacteria, they can be used as hosts for the production of medical and non-medical bio-products. Opioids, cannabinoids, fragrant raspberry ketones and cocoa butter are examples of complex commodities that have been produced in yeast through SynBio techniques (Lee et al. Citation2016; Carvalho et al. Citation2017; Goold et al. Citation2018). This use of microbial hosts in producing plant commodities can thus result in market stability for unstable seasonal plant commodities in Africa.
While SynBio may present benefits for the economy at large, its use in the production of compounds commonly extracted from natural plants could have negative effects on communities that grow/harvest those plants. Common examples include the replacement of the antimalarial artemisinin produced from the plant Artemisia annua with artemisin produced using SynBio and the production of flavors using SynBio as opposed to conventional agriculture (Path Citation2013; Mitchell Citation2018). This could deprive African farmers of income due to reduced demands for their products (Oldham et al. Citation2012; Goold et al. Citation2018). The biosynthetic production of ingredients or chemicals to replace these crops has relevance for Africa. The biosynthetic processes often involve the use of genetically engineered microbes such as yeast or algae, which feed on sugar. Adoption of SynBio in such biosynthesis has the potential for negative effects on biodiversity. The negative effects can include a reduction in demand for natural plants such as shea, cocoa and cassava and a huge demand for sugar which is used to produce the genetically engineered microbes for the production of SynBio products. Sugar is often produced by agribusiness using unsustainable methods and large quantities of water, which is problematic in that water is increasingly becoming scarce as a result of climate change (ETC Group et al. Citation2018).
SynBio can be an investable utility technology capable of ensuring that Africa meets and sustains its food security needs. The far-reaching applications of SynBio in agriculture stretch from farm management to agri-intelligence systems right up to post-harvest stages to reduce risks of product spoilage (Liu and Stewart Citation2015). Implementation of SynBio in agriculture could be hampered by a shortage of adequate tools because agriculture is dominated by higher mammals and plants. However, SynBio in combination with new techniques for genome design and synthesis, more efficient molecular tools that include CRISPR/Cas9 present more opportunities than conventional breeding and cultivar development. They can deliver transformative short to long-term changes to agriculture. These include engineering of biosensors, novel antimicrobials, microbial metabolic engineering, synthetic speciation and mammalian multiplexed CRISPR (Goold et al. Citation2018).
While SynBio applications have generally been predicted to offer great benefits by making products, they have also given rise to concerns about new safety, ethics and socio-economic risks (Dana et al. Citation2012; Edwards Citation2014; Ribeiro and Shapira Citation2018). This review provides an overview of recent progress in the application of SynBio in agriculture as well as on arguments and evidence related to their possible benefits to the African continent while also outlining the risks and governance implications.
Food security issues in Africa and how SynBio can help
Food security issues in Africa
Agriculture, which is one of the bedrock of African economies, faces an increasingly challenging future due to a number of factors, inter alia, climate change, land degradation, over-reliance on food imports, global competition, water and energy security issues (Conceição et al. Citation2016; Ribeiro and Rodriguez Citation2020).
Africa, especially ‘The Horn of Africa’ and Sub-Saharan Africa, is among the most food-insecure regions in the world (FAO Citation2017). The root cause of food insecurity in Africa is the inability to access food due to poverty (FAO, IFAD, UNICEF, WFP, WHO Citation2017). War and political instability also contribute to food insecurity as they disrupt normal economic activities such as agriculture and the distribution of resources. Trade bans and export restrictions connected to the politics of countries also have negative impacts on food security.
With the continent’s population continuing to increase, demand for food will also continue to increase. Hence, food security becomes a paramount economic issue. Africa’s present food production approaches are not capable of providing sufficient food without posing serious adverse environmental impact (Funk et al. Citation2008). This is made worse by climate change which is resulting in severe variations in temperatures, low agricultural production, erratic weather patterns and disease transmission (Ribeiro and Rodriguez Citation2020). Additionally, most African countries do not have sustainable agricultural policies to support food security in the coming years. Farmers have little access to modern sustainable agriculture methods and tools resulting in only a small percentage of the arable land being used for agriculture. Rapid urbanization and population growth also contribute to food insecurity by disrupting agricultural production and increasing food demands (Fawole et al. Citation2005).
Malnutrition observed in some African countries is leaving children weak, vulnerable and less able to fight common childhood illnesses such as diarrhea, acute respiratory infections, malaria and measles. This has more deleterious effects on children living with HIV and AIDS. Adults and adolescents suffer consequences of food insecurity and malnutrition such as decreased energy levels, delayed maturation, growth failure, impaired cognitive ability, diminished capacity to learn, decreased ability to resist infections and illnesses, shortened life expectancy, increased maternal mortality and low birth weight. Individuals experiencing food insecurity are likely to experience and show feelings of alienation, stress and anxiety (Fawole et al. Citation2005; FAO Citation2017).
Potential applications of SynBio to food and agriculture in Africa
SynBio has several applications in the food sector across various sub-sectors (Figure ). SynBio can be applied for the production of metabolites and health products such as vitamins. Artificially produced health products can be packaged as supplements which might be cheaper and more readily available than naturally occurring vitamins and other health products. Another food sector potential application is the production of food derivatives such as nutraceuticals, probiotics and synthetic components used to raise the value of certain foods. Also, SynBio can contribute to the development of nutrient-enriched plants. Such plants are ideal for people in Africa living in poverty as one plant would be able to address several nutritional needs. SynBio is also applicable in the production of preservatives such as nisin and artificial flavors and fragrances. Vanilla has been successfully produced from baker’s yeast (Hansen et al. Citation2009) and synthetic saffron has been produced for commercial use at a fraction of the price of natural saffron (Pretorius Citation2016). Thus, SynBio can potentially reduce the prices of some commodities on the African continent.
Despite advances in technologies for drought monitoring and prediction systems, drought preparedness remains poor in Africa (Hao et al. Citation2017). The development of engineered tomato plants that can activate drought protection mechanisms on the application of fungal spray is one example of how SynBio can help African farmers prepare for drought (Goold et al. Citation2018). This helps abate crop loss due to climate change-induced droughts and ordinary droughts that have been occurring at least once every ten years in many African countries (Hao et al. Citation2017).
There is still low adoption of mineral fertilizer in some African countries due to reasons that include high costs and availability. Using SynBio, non-leguminous crops that can fix atmospheric nitrogen have been developed thereby reducing the need for fertilizers (Goold et al. Citation2018). The technology can be transferred to crops grown on the African continent thereby reducing nutrient-associated losses and also lowering the costs of production for many crops. This is because fertilizer is a major cost driver in agriculture and there are periodic shortages that lead to yield losses. More smart crops with various other advantages such as high yield, drought resistance, and pesticide resistance amongst other adaptations, can be engineered into synthetic plants (Park et al. Citation2015).
The benefit to farm management from SynBio comes through the development of biosensors and the use of agri-intelligence systems that reduce the use of pesticides and fertilizers. The plants will detect when there is a drought or weed threat and activate necessary response mechanisms. This will reduce yield losses and wastage of herbicides that pollute the environment. Food waste processing methods can take advantage of this technology and increase the amount of toxic waste removed from the environment (Pretorius Citation2016).
Given that more than 80% of poor Africans keep livestock (FAO Citation2009), enhancing animal productivity is a noble way of improving livelihoods. Increased productivity is one way of attaining some of the United Nations Sustainable Development Goals, namely (1) no poverty and (2) zero hunger. One of the major challenges in animal production is that the breeds used by most communal and small-scale farmers have not been genetically improved to enhance productivity. Most of the highly efficient breeds in developed countries have been developed over decades of commercial quantitative genetic selection pressure. Major successes include the development of broiler chickens with the ability to attain more than four kilograms at eight weeks coupled with higher feed conversion efficiency (Zuidhof et al. Citation2014). The success that has taken a long time to achieve can be attained in a relatively short time through the application of SynBio. A possible approach is the use of artificial gene synthesis and gene editing techniques to enhance traits of economic importance. Potentially, known major genes can be synthesized de novo and subsequently infused in populations using gene drives (Frieß et al. Citation2019). Major traits that need improvement include growth, feed conversion efficiency, meat quality and prolificacy. Information from known major genes in exotic breeds such as the double muscling gene/myostatin (Kambadur et al. Citation1997), growth hormone (Jomane et al. Citation2015), stearoyl-CoA desaturase and sterol regulatory element-binding protein–1 (Mannen Citation2011) can be used for artificial gene synthesis and infusion into indigenous cattle. In sheep, the booroola gene (Souza et al. Citation2001; Sahu et al. Citation2016) is a good candidate for increasing prolificacy. Other possibilities include whole-genome editing for traits of economic importance. The success recorded in removing all porcine endogenous retroviruses from the pig gene (Niu et al. Citation2017) highlights the practicality of the approach. Apart from productivity, opportunities for improving animal welfare and food safety were highlighted by Goold et al. (Citation2018).
Challenges for the adoption of SynBio in Africa
Risks, advantages and disadvantages of using synbio
SynBio can be misused through compromising computers using malware that can be stored in synthesized DNA, infringing intellectual property rights on biological matter, synthesis of threatening viruses, ‘genetic genocide’ and exploitation of food markets using genetically modified crops inter alia (Elgabry et al. Citation2020). Risks should be thoroughly assessed before large numbers of synthetic organisms are released out of the laboratory, taking into consideration self-replication, crossing over events and recombination. Thus, there is a need for strict monitoring of the technology and its products. Research and development teams should include multiple safeguards in synthetic cells, such as giving them strictly limited life spans or on/off switches, and engineering them to depend on laboratory-specific conditions. They should also keep using unique identifying marks so that products can be traced back to their ‘creators’. The establishment of ethical codes of conduct for biological scientists globally can assist in the control of SynBio (Elgabry et al. Citation2020; Trump et al. Citation2020).
Genome information is normally obtained from active bacteria or viruses which are difficult to get since they are normally under strict laboratory control. However, sequences can be synthesized at multiple locations and assembled using DNA fragment assembly techniques. This can be used to avoid inspection during transportation. A horsepox virus was synthesized this way in 2017. This threat is worsened by an increase in ‘Do-it-Yourself Scientists’ and the use of drones (Wang and Zhang Citation2019).
SynBio offers the advantage of removing the use of selectable markers which are a requirement in many genetic modification applications. This can be achieved by using retargetable mobile group II introns commonly called ‘targetrons’. These have very high efficiency such that there is no requirement for selectable markers (Lambowitz and Zimmerly Citation2004). Targetrons function in a wide variety of bacteria. Beyond suicide plasmids which have low efficiency and are unstable, targetrons are the first genetic tool of significant utility (Heap et al. Citation2007).
One of the biggest challenges with SynBio is the biopiracy of Africa’s vast genetic resources. Biopiracy is ‘the unethical or unlawful appropriation or commercial exploitation of biological materials native to a particular country or territory without providing fair financial compensation to [its] people or government’ (Merriam-Webster). Technologies in DNA synthesis and sequencing now mean that genetic information can be transmitted electronically across borders. There may be no need to transport a physical seed or plant. Currently, in most countries, the laws and policies regulate the transfer of physical material only. Thus, open access to digital sequences can facilitate further biopiracy. It will thus also cause profit extraction of African plant, animal and microbial resources (ETC Group et al. Citation2018).
Groups such as Open Plant (https://www.openplant.org/) and the Joint BioEnergy Institute (https://public-registry.jbei.org/) inter alia, are developing open-source registries for plant-specific DNA parts. This can help with the promotion of access to information. The Open Plant initiative also provided grants to start SynBio labs in Nigeria and Kenya. SynBio Africa is an example of a forum for researchers, students, citizen scientists, policymakers and the public at large. It convenes to strategize and develop pathways for the propagation of SynBio technologies and their resultant products and services throughout Africa. Other initiatives such as the Biomaker Africa program train biologists and non-biologists on SynBio. They also share science hardware which is critical to building SynBio tools (French Citation2019; Ribeiro and Rodriguez Citation2020). The MIT spin out called Amino Labs produces small, cheap, all-in-one kits for doing SynBio to African schools and researchers (French Citation2019). The African Institute of Open Science and Hardware based in Ghana and other citizen initiatives also help train and create awareness on SynBio.
The promotion of the adoption of GMOs needs to include activists as science communicators (Lukanda Citation2020; Trump et al. Citation2020). Activists rarely put scientific evidence to back up their claims and they can polarize the GMO debate using positive and negative emotions based on socio-democratic considerations. This occurred in countries such as Uganda where the government had difficulties passing ‘The National Biotechnology and Biosafety Bill’ (Lukanda Citation2020). Activists argue that the adoption of GMOs will make it illegal for farmers to plant GMO seeds without permission from the patent holders. These include Bayer (which acquired Monsanto), Syngenta, Pioneer (Corteva) and Pannar. Citing perception studies such as Busscher et al. Citation2020 and Rzymski and Królczyk Citation2016, activists argue that GMOs do not increase yield (Dowd-Uribe and Schnurr Citation2016) but cancer (Singh Citation2018). They argue that the technology to produce GMOs can be misused leading to bioterrorism acts. The concerns of activists and other stakeholders need to be considered and brought to test openly. This will help shed light on the concerns and unravel the truth about GMOs and SynBio. Anti-GMO organizations include Route to Food Initiative, Participatory Ecological Land Use Movement (Pelum) Uganda, Food Rights Alliance and Action Aid. Other academics are also part of the anti-GMO movement. There is thus a need to bring the developers of GMOs, farmers, activists and consumers together and avoid the polarization of the GMO debate (Lukanda Citation2020; Beumer and Swart Citation2021).
Regulation of SynBio
The current concerns over SynBio
SynBio is a rapidly evolving, multidisciplinary and promising techno-science field which is anticipated to lead to the 5th industrial revolution (Peccoud Citation2016). Strikingly, the technologies have enormous potential to significantly alter the genomes of viruses, prokaryotes and eukaryotes. It is thought that when these altered organisms are released into the environment, they can become a biodiversity risk as they may become invasive. Biosecurity risks may also arise if biological weapons are made using SynBio (Trump Citation2017). All these concerns raise environmental, health, social, legal and ethical issues (The Parliamentary Office of Science and Technology Citation2015). In light of these concerns, some countries have regulations that govern the use of SynBio. Often where regulatory provisions are non-existent in countries, regulatory authorities have resorted to the use of the precautionary approach principle (UNEP Citation2011).
Regulation of SynBio in Europe
The regulations governing the use of SynBio in Europe exist at various levels of implementation. In the majority of the cases, the regulations originally produced for the regulation of GMOs and their derivatives are revised to suit the current technological innovations. In this regard, for the last two decades, two EU GMO directives namely, the Contained Use Directive (2009/41/EC) and the Deliberate Release Directive (2001/18/EC), have been used for the regulation of SynBio products. There are regulations for laboratory research work and the release of GMOs into the environment. However, in the current EU GMO regulatory framework, the genetically modified (GM) organism is compared with an equivalent non-GMO. However, as the number of traits and sources of genetic materials increases, finding a comparator organism becomes a daunting task. Since these complex organisms are developed in a step by step manner and regulatory approvals are sought at each stage, the EU Scientific Committees have suggested that a complex organism developed earlier in the chain could be used as a comparator if it has a history of safe use (The Parliamentary Office of Science and Technology Citation2015).
Regulation of SynBio in the United States of America
The United States of America (USA) is using the same regulatory frameworks for GMOs for the regulation of SynBio. The present state and form of the legal regulatory framework for GMOs are applied to SynBio and products derived thereof. Agencies involved in the implementation of the regulatory system are the U.S. Department of Agriculture’s Animal and Plant Health Inspection Service (APHIS), the U.S. Environmental Protection Agency (EPA), and the U.S. Food and Drug Administration (FDA) (Carter et al. Citation2014). However, some stakeholders are of the opinion that these bodies have limited regulatory authority to regulate some SynBio products. For instance, APHIS regulates organisms in which plant pests or components thereof have been used to modify the plant. It is most likely that the development methods of SynBio derived organisms will not be covered by these regulations (Carter et al. Citation2014). Thus, the products will go without regulatory oversight because they are not explicitly covered by the existing statutes. The responsible and enforcing authority is thus rendered ‘powerless’. In the case of EPA, as modified microbes become more complex, risk assessments will become more difficult, requiring more financial resources and expertise.
Regulation of SynBio in Africa
Despite the potential positive impact of SynBio, it is important to note that the regulatory frameworks for SynBio products including synthetic organisms are yet to be developed in some African countries. Even African countries with well-established systems for regulation of genetically modified organisms (GMOs) such as Kenya, and South Africa, are yet to put in place regulations that are specifically meant for SynBio (Rhodes and Mandivenyi Citation2020).
It is worth noting that certain provisions currently contained in the GMO regulations of many African countries may be extended to SynBio since some SynBio approaches build on modern biotechnology methodologies and techniques. There is a growing number of African countries that are now adopting GMOs. These include Uganda, Ghana, Nigeria, Cameroon, Ethiopia, Malawi, Mozambique, and eSwatini. These might find it easy to adopt SynBio. As more complex organisms are produced by SynBio there will be a need to develop regulations for more comprehensive risk analysis (The Parliamentary Office of Science and Technology Citation2015). This is because the comparative principle used for the regulation of GMOs may not be applicable if organisms that are fundamentally different from natural organisms are produced using SynBio.
Nigeria amended the National Biosafety Management Agency Act of 2015 in 2019 to cater to the regulation of SynBio. A new section was inserted (NBMA Citation2019) and it reads as follows:
25A. Application of gene drive, gene editing and synthetic biology
‘No person, institution or body shall carry out gene drive, gene editing and synthetic biology except with the approval of the Agency’. Supporting regulations however are still to be developed.
In Zimbabwe SynBio is regulated through the National Biotechnology Authority Act [Chap.14.31] of Citation2006. Subsection 3 (2) c of the Act states that the Act shall apply to – (c) any activity involving biological and molecular engineering technologies such as metabolic engineering, proteomics, metabolomics, nanotechnology, genetic modification, cloning, DNA-chip technology and bioinformatics; and such other technologies as may be declared by the authority to constitute potentially harmful research or undertaking. SynBio products are currently classified as genetically engineered (GE) and are covered under the current regulations hence the NBA Act of 2006 is used. However, although a comprehensive National Biosafety Framework exists for effective regulation of biotechnology, future reviews of regulations to accommodate complex SynBio processes cannot be ruled out. Furthermore, future reviews of the regulations will be motivated by the fact that globally the definition of SynBio and products is ambiguous and SynBio produces more complex products which potentially present legal issues. The NBA Act does not make specific reference to SynBio and to avoid any ambiguity, a statutory instrument that supports the NBA Act requires gazetting.
International treaties
The Biological Weapons Convention (BWC) bans the development, production and stockpiling of all weapons of mass destruction (United Nations Office for Disarmament Affairs and Biological Weapons Citation2018). This includes microbial, other biological agents and toxins for which there is no justified use for preventative, protective, or other peaceful cause. The BWC provides for any unforeseen misuse of SynBio techniques (The Parliamentary Office of Science and Technology Citation2015). The United Nations Security Council Resolution 1540 of April 2004 requires all UN member states to refrain from supporting terrorists to make, obtain, transport, develop, possess, use or transfer any nuclear, chemical, or biological weapons (UN Office for Disarmament Affairs and Biological Weapons Citation2018). This resolution and the BWC provide a safeguard measure for guiding against the misuse of SynBio.
The Convention on Biological Diversity (CBD) at its 13th meeting held in Mexico in 2016 invited parties to take a precautionary approach on SynBio. It noted that current living organisms developed using SynBio and those in early stages of research and development fall within the Cartagena Protocol on Biosafety (CPB) definition for living modified organisms (LMOs). As such, the current risks assessment methodologies under the CPB can be applied for these. However, they may need to be reviewed as the technology advances. CBD parties also noted that it is not clear whether the final products of the early stages of SynBio research and development would fall under the CPB LMO definition.
There are many capacity limitations and challenges that need to be addressed globally if the countries are to effectively regulate SynBio products. Addressing these issues would go a long way in ensuring that countries benefit from these technologies whilst protecting human and animal health and the environment. SynBio is rapidly advancing and current regulations may not adequately cover future products of the technologies. Taking cognizance of both benefits and risks of the technology (Goo et al. Citation2018), countries need to come up with all-encompassing regulatory frameworks which will not stifle development, at the same time making sure that adequate biosafety and biosecurity measures are put in place to prevent misuse of the technologies. In the case of Africa, it is worth noting that the judicious application of synthetic biology can alleviate food and energy security problems, reduce poverty, boost industrial growth, reduce greenhouse gases and promote environmental conservation (Garang and Onkware Citation2016).
Most SynBio outputs can be protected using patents. However, their patentability differs depending on individual countries’ laws. Copyright protection of SynBio outputs is currently unlikely. The Nagoya Protocol sets minimum obligations for members but does not constrain maximalist national ABS/PIC/ DOO measures. It however does not pose a direct conflict with the WTO TRIPS Agreement. While many countries ratified the Nagoya Protocol, the enactment of the required implementing legislation and provision of the necessary infrastructure is slow in many countries. WIPO made efforts to have a binding treaty obligating countries to require that patent applicants disclose the origin of genetic resources used in creating a claimed invention. This might reduce biopiracy and ensure intangible genetic information is taken in compliance with the domestic laws of a provider country (Bagley Citation2016).
Future perspectives and conclusion
The adoption of SynBio has the potential to improve food security and livelihoods in Africa. Considering that most African countries are yet to accept genetically modified organisms, the adoption of SynBio might seem arduous. However, there are countries such as Zimbabwe where the growing of GMOs plants for agricultural purposes is not permitted but controlled research on the GMOs is permissible. Stakeholders’ perspectives on GMOs must be investigated: the understanding of GMOs definition(s), methods employed in obtaining GMOs, knowledge of SynBio, source of information and willingness to fund research of GMOs. This will improve platforms for knowledge transfer, identifying key challenges and mapping solutions. It is the knowledge that will assist in developing informed policies that have a meaningful impact on the socio-economic factors.
Without a clear policy on GMOs, public funds will not be readily channeled towards research on such organisms. In countries that are leading research and adoption of SynBio and GMOs, their governments have made significant investments of more than US$30 billion. However, most African countries are lagging behind (World Bank Citation2015). The government, industry and academia in Africa should work together in improving the knowledge, adoption and safety of SynBio and GMOs. The industry will only fund research where there is a guaranteed return on investment. With the current policy in most African nations where GMOs knowledge is limited, the industry is less likely to invest in research on GE’s. It becomes a chain: No good policy → no funding → no research → no information → no good policy. This cycle needs to be broken. Though SynBio presents a golden opportunity for improving livelihoods in Africa, its success can only be realized if the policy and legislative environment are conducive.
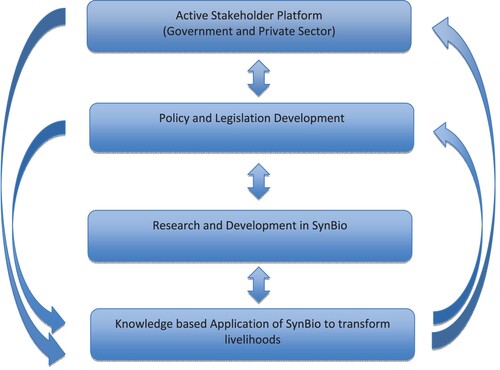
There are critical activities that the majority of African countries need to consider in making SynBio a success in transforming livelihoods (Figure ).
Figure 2. Flow chart of critical activities to be adopted by the majority of African countries to make the adoption of SynBio successful.
In conclusion, we are of the view that SynBio offers great opportunities to improve livelihoods, especially food security, in Africa. However, widespread and unregulated adoption of SynBio can result in many adverse effects on the environment and economies in Africa. Its adoption is, however, still low. African governments need to assume a harmonized position on regulation and governance of SynBio, whether it should be case-specific or not, process-based or product-based. These gaps in the regulatory frameworks need to be addressed if Africa is to derive maximum benefit from SynBio whilst minimizing the risks associated with the technology. This is critical given that some African countries such as South Africa are among the leading researchers of SynBio (Oldham et al. Citation2012).
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
References
- Amos M, Goñi-Moreno A. 2018. Cellular computing and synthetic biology. In: Stepney S., Rasmussen S., Amos M., editors. Computational matter. Berlin: Springer; p. 93–110.
- Bagley MA. 2016. Digital DNA: The Nagoya protocol, intellectual property treaties, and synthetic biology. University of Virginia School of Law Public Law and Legal Theory Research Paper Series 2016. Available from: https://ssrn.com/abstract=2725986. doi:10.2139/ssrn.2725986.
- Belkin S, Yagur-Kroll S, Kabessa Y, Korouma V, Septon T, Anati Y, Zohar-Perez C, Rabinovitz Z, Nussinovitch A. 2017. Remote detection of buried landmines using a bacterial sensor. Nat Biotechnol. 35(4):308–310. doi:10.1038/nbt.3791.
- Beumer K, Swart JAA. 2021. Who is the African farmer? The importance of actor representations in the debate about Biotechnology crops in Africa. J Agric Environ Ethics. 34:1. doi:10.1007/s10806-021-09841-8.
- Busscher N, Colombo EL, van der Ploeg L, Gabella JI, Leguizamón A. 2020. Civil society challenges the global food system: the International Monsanto tribunal. Globalizations. 17(1):16–30. doi:10.1080/14747731.2019.1592067.
- Carter SR, Rodemeyer M, Garfinkel MS, Friedman RM. 2014. Synthetic biology and the U.S. Biotechnology Regulatory System: challenges and options. Available from: http://www.jcvi.org/cms/research/projects/synthetic-biology-and-the-us-biotechnology-regulatory-system/overview/.
- Carvalho Â, Hansen EH, Kayser O, Carlsen S, Stehle F. 2017. Designing microorganisms for heterologous biosynthesis of cannabinoids. FEMS Yeast Res. 17:1–11. doi:10.1093/femsyr/fox037.
- Chen L, Blanchard AE, Ting L. 2017. An integrative circuit–host modeling framework for predicting synthetic gene network behaviors. Nat Microbiol. doi:10.1038/s41564-017-0022-5.
- Conceição P, Levine S, Lipton M, Warren-Rodríguez A. 2016. Toward a food secure future: ensuring food security for sustainable human development in Sub-Saharan Africa. Food Policy. 60:1–9. www.sciencedirect.com/article/pii/S030691921600021X.
- Dana GV, Kuiken T, Rejeski D, Snow AA. 2012. Four steps to avoid a synthetic-biology disaster. Nature. 29(483):29. doi:10.1038/483029a.
- Dowd-Uribe B, Schnurr MA. 2016. ‘Burkina Faso’s Bt cotton reversal: why Africa’s largest producer of GM cotton is phasing out production and what this means for GM crops in Africa’. Food, Farming and Biotechnology. Available from: https://fieldquestions.com/2016/08/06/bt-cotton-in-africa-what-happened-in-burkina-faso/.
- Edwards B. 2014. Taking stock of security concerns related to synthetic biology in an age of responsible innovation. Front Public Health. 2:79. doi:10.3389/fpubh.2014.00079.
- Elgabry M, Nesbeth D, Johnson SD. 2020. A systematic review protocol for crime trends facilitated by synthetic biology. Syst Rev. 9:22. doi:10.1186/s13643-020-1284-1.
- ETC Group, Third World Network, African Centre for Biodiversity. 2018. What does synthetic biology mean for Africa? Available from: https://www.acbio.org.za/sites/default/files/documents/What%20does%20Synthetic%20Biology%20mean%20for%20Africa.pdf.
- FAO. 2009. The state of food and agriculture. Livestock in the balance. Rome. Available from: www.fao.org/docrep/012/i0680e/i0680e00.htm
- FAO. 2017. Hunger and food security. State of Food Insecurity (SOFI 2017). Available from: www.fao.org/3/a-I7695e.pdf.
- FAO, IFAD, UNICEF, WFP, WHO. 2017. The state of food security and nutrition in the world 2017. Rome: FAO. Available from: www.data.unicef.org/wp-content/uploads/2017/12/web_I7787EN_SOFI2017_InBrief.pdf.
- Fawole WO, Ozkan B, Ilbasmis E. 2005. Food insecurity in Africa in terms of causes, effects and solutions: A case study of Nigeria. Konya ResearchGate. doi:10.24925/turjaf.v5i6.629–636.1113.
- French KE. 2019. Harnessing synthetic biology for sustainable development. Nat Sustain. 2:250–252. doi:10.1038/s41893-019-0270-x.
- Frieß JL, von Gleich A, Giese B. 2019. Gene drives as a new quality in GMO releases – a comparative technology characterization. PeerJ. 7:e6793. doi:10.7717/peerj.6793.
- Funk C, Dettinger MD, Michaelsen JC, Verdin JP, Brown ME, Barlow M, Hoell A. 2008. Warming of the Indian ocean threatens eastern and southern African food security but could be mitigated by agricultural development. PNAS. 105:11081–11086. doi:10.1073/pnas.0708196105.
- Garang B, Onkware A. 2016. Redirecting the wheels of natural progression: review of synthetic biology and the African Biotechnology revolution. Bioeng Biosci. 4(2):11–19. [accessed 6 November 2019]. https://pdfs.semanticscholar.org/81bc/f384afb50e07ed842693de2a9ceeb1d1f26b.pdf?_ga=2.255418056.1331119020.1573054035-240779808.1573054035.
- Goñi-Moreno A, Nikel PI. 2019. High-performance biocomputing in synthetic biology-integrated transcriptional and metabolic circuits. Front Bioeng Biotechnol. 7:40. doi:10.3389/fbioe.2019.00040.
- Goold HD, Wright P, Hailstones H. 2018. Emerging opportunities for synthetic biology in agriculture. Genes (Basel). 9:341. doi:10.3390/genes.9070341.
- Guiziou S, Bonnet J, Moreau V, Ulliana F, Lechere M. 2018. An automated design framework for multicellular recombinanse logic. ACS Synth Biol. 7:1406–1412. doi:10.1012/acssynbio.8b00016.
- Hansen EH, Moller BL, Kock GR, Bunner CM, Kristensen C, Jensen OR, Okkels FT, Olsen CE, Motawaia MS, Hansen J. 2009. De novo biosynthesis of vanillin in fission yeast (Schizosaccharomyces pombe) and baker's yeast(Saccharomyces cerevisiae). Appl Environ Microbiol. 75:2765–2774. doi:10.1128/AEM.02681-08.
- Hao Z, Yuan X, Xia Y, Hao F, Singh PV. 2017. An overview of drought monitoring and prediction systems at regional and global scales. Am Meteorol Soc. 1879–1896. doi:10.1175/BAMS-D-15-00149.1.
- Heap JT, Pennington OJ, Cartman ST, Carter GP, Minton NP. 2007. The ClosTron: a universal gene knock-out system for the genus Clostridium. J Microbiol Methods. 70:452–464. doi:10.1016/j.mimet.2007.05.021.
- Jomane FN, Ishida T, Morimoto K, Fujishita N, Tokunaga T, Hararada H, Morita T. 2015. Genetic Polymorphisms and their Association with growth and carcass traits in Japanese black steer under progeny testing. J Warm Reg Soc Anim Sci. 58:217–224.
- Kambadur R, Sharma M, Smith TPL, Bass JJ. 1997. Mutations in myostatin (GDF8) in double-muscled Belgian Blue and Piedmontese Cattle. Genome Res. 7:910–915.
- Khalil A, Collins J. 2010. Synthetic biology: applications come of age. Nat Rev Genet. 11:367–379.
- König H, Frank D, Heil R, Coenen C. 2013. Synthetic Genomics and Synthetic biology applications between hopes and concerns. Curr Genomics. 14:11–24.
- Lambowitz AM, Zimmerly S. 2004. Mobile group II introns. Annual Review of Genetics. 38:1–35. doi:10.1146/annurev.genet.38.072902.091600.
- Lee D, Lloyd NDR, Pretorius IS, Borneman AR. 2016. Heterologous production of raspberry ketone in the wine yeast Saccharomyces cerevisiae via pathway engineering and synthetic enzyme fusion. Microb Cell Fact. 15:49. doi:10.1186/s12934-016-0446-2.
- Liang J, Luo Y, Zhao H. 2011. Synthetic biology: putting synthesis into biology. Wiley Interdisc Rev Syst Biol Med. 3:7–20. doi:10.1002/wsbm.104.
- Liu W, Stewart CN. 2015. Plant synthetic biology. Trends Plant Sci. 20:309–317.
- Lu TK, Khalil AS, Collins JJ. 2009. Next-generation synthetic gene networks. Nat Biotechnol. 27:1139–1150.
- Lukanda IN. 2020. Activists as strategic science communicators on the adoption of GMOs in Uganda. JCOM. 19(06):C06. doi:10.22323/2.19060306.
- Mannen H. 2011. Identification and utilization of genes associated with beef qualities. Anim Sci. 82:1–7.
- McDaniel R, Weiss R. 2005. Advances in synthetic biology : on the path from prototypes to applications. Curr Opin Biotechnol. 16:476–483.
- Millar-Haskell CS, Dang AM, Gleghorn JP. 2019. Coupling synthetic biology and programmable materials to construct complex tissue ecosystems. MRS Commun. 9(2):421–432. doi:10.1557/mrc.2019.69.
- Mitchell J. 2018. Life 2.0: inside the synthetic biology revolution. COSMOS 78: The Science of Everything.
- National Biosafety Management Agency (NBMA). 2019. Available from: https://nbma.gov.ng/wp-content/uploads/2017/06/Amendment-of-NBMA-Act-2019.pdf [Accessed 6 Nov. 2019].
- National Biotechnology Authority Act. 2006. [Chap.14:31].
- Niu D, Wei HJ, Lin L, George H, Wang TB, Lee IH, Zhao HY, Wang Y, Kan Y, Shrock E. 2017. Inactivation of porcine endogenous retrovirus in pigs using CRISPR-Cas9. Science. 357:1303–1307.
- Oldham P, Hall S, Burton G. 2012. Synthetic biology: mapping the scientific landscape. PLoS ONE. 7:e34368. doi:10.1371/journal.pone.0034368.
- Otono BF, Mwaku IM, Ndofunsu DA, Ndiku LS. 2020. Democratic Republic of the Congo – GMOs/Synthetic biology rules/regulations and biodiversity: A legal perspective. In: Chaurasia A, Hawksworth DL, Pessoa de Miranda M, editors. GMOs. Topics in biodiversity and conservation, vol 19. Cham: Springer. doi:10.1007/978-3-030-53183-6_22.
- Park SY, Peterson FC, Mosquna A, Yao J, Volkman BF, Cutler SR. 2015. Agrochemical control of plant water use using engineered abscisic acid receptors. Nature. 520:545–548.
- Path. 2013. Sanofi and PATH announce the launch of large-scale production of semisynthetic artemisinin against malaria. [online]. [accessed October 23, 2018]. Available from: https://path.org/media-center/sanofi-and-path-announce-the-launch-of-large-scale-production-of-semisynthetic-artemisinin-against-malaria/.
- Peccoud J. 2016. Synthetic biology: fostering the cyber-biological revolution. Synth Biol. 1:1–7.
- Polo M. 2020. Botswana – genetically modified organisms (GMOs) and synthetic biology: their potential applications and the legal perspectives. In: Chaurasia A, Hawksworth DL, Pessoa de Miranda M, editors. GMOs. Topics in biodiversity and conservation. Cham: Springer; p. 19. doi:10.1007/978-3-030-53183-6_21.
- Pretorius S. 2016. Application of synthetic biology to increased agricultural productivity in Australia. Agric Innov. 69:2.
- Rhodes JI, Mandivenyi W. 2020. South Africa – synthetic biology regulatory considerations and biodiversity – a legal perspective for South Africa. In: Chaurasia A, Hawksworth DL, Pessoa de Miranda M, editors. GMOs. Topics in biodiversity and conservation. Cham: Springer; p. 19. doi:10.1007/978-3-030-53183-6_24.
- Ribeiro B, Shapira P. 2018. Anticipating governance challenges in synthetic biology: insights from biosynthetic menthol. Elsevier. 139:311–320.
- Ribeiro FP, Rodriguez CAV. 2020. Emerging advanced technologies to mitigate the impact of climate change in Africa. Plants. 9(3):381. doi:10.3390/plants9030381.
- Rzymski P, Królczyk A. 2016. Attitudes toward genetically modified organisms in Poland: to GMO or not to GMO? Food Secur. 8 (3):689–697. doi:10.1007/s12571-016-0572-z.
- Sahu AR, Jeichitra V, Ramanujan R, Angamuthu R. 2016. Genetic Polymorphisms of myostatin (MSTN) gene in sheep breeds. J Anim Res. 6:18. doi:10.5958/2277-940X.2016.00013.9.
- Schmidt C. 2010. Environmental health implications of a new field. Environ Health Perspect Synth Biol. 118:118–123.
- Serrano L. 2007. Synthetic biology: promises and challenges. NCBI. 3:157–158.
- Singh A. 2018. Cancer! Roots in our foods. Gut Gastroenterol. 1(1):1–2.
- Souza CJ, MacDougall C, Campbell BK, McNeilly AS, Baird DT. 2001. The booroola (FecB) phenotype is associated with a mutation in the bone morphogenetic receptor type 1B (BMPR1B) gene. J Endocrinol. 169:R1–R6.
- Teo SC. 2014. Whole cell-based biosensors for environmental heavy metals detection. Ann Res Rev Biol. 4(17):2663–2674.
- The Parliamentary Office of Science and Technology. 2015. Regulation of synthetic biology. London: PostNote.
- Trump BD. 2017. Synthetic biology regulation and governance: lessons from TAPIC for the United States, European Union, and Singapore. Health Policy. 121:1139–1146.
- Trump BD, Galaitsi SE, Appleton E, Bleijs DA, Florin MV, Gollihar JD, Hamilton RA, Kuiken T, Lentzos F, Mampuys R, et al. 2020. Building biosecurity for synthetic biology. Mol Syst Biol. 16:e9723.
- UNEP. 2011. Food security in the horn of Africa: the implications of a drier, hotter and more crowded future, s.l.: unep.org/GEAS/.
- United Nations Office for Disarmament Affairs and Biological Weapons. 2018. United Nations. [accessed Feb 25]. Available from: https://www.un.org/disarmament/wmd/bio/
- Wang F, Zhang W. 2019. Synthetic biology: recent progress, biosafety and biosecurity concerns, and possible solutions. Journal of Biosafety and Biosecurity. 1(1):22–30. doi:10.1016/j.jobb.2018.12.003.
- World Bank. 2015. Research and development expenditure (% GPD). Available from: www.data.worldbank.org.
- Xiang Y, Dalchau N, Wang B. 2018. Scaling up genetic circuit design for cellular computing: advances and prospects. Nat Comput. 17(4):833–853. doi:10.1007/s11047-018-9715-9.
- Zuidhof MJ, Schneider BL, Carney VL, Korver DR, Robinson FE. 2014. Growth, efficiency, and yield of commercial broilers from 1957, 1978, and 2005. Poult Sci. 93:1–13.